The Impact of Various Organic Phosphorus Carriers on the Uptake and Use Efficiency in Barley
Abstract
:1. Introduction
2. Results
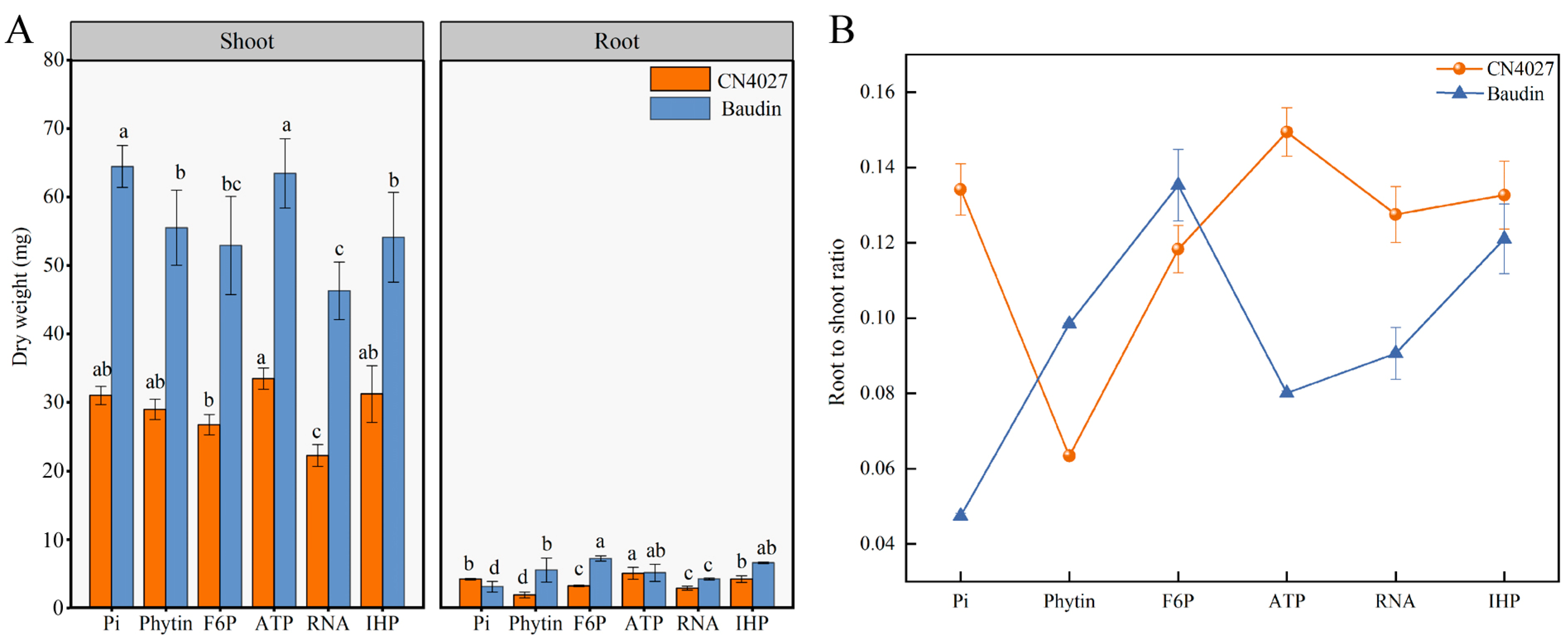
2.1. The Growth of Barley under Diverse OP Treatments
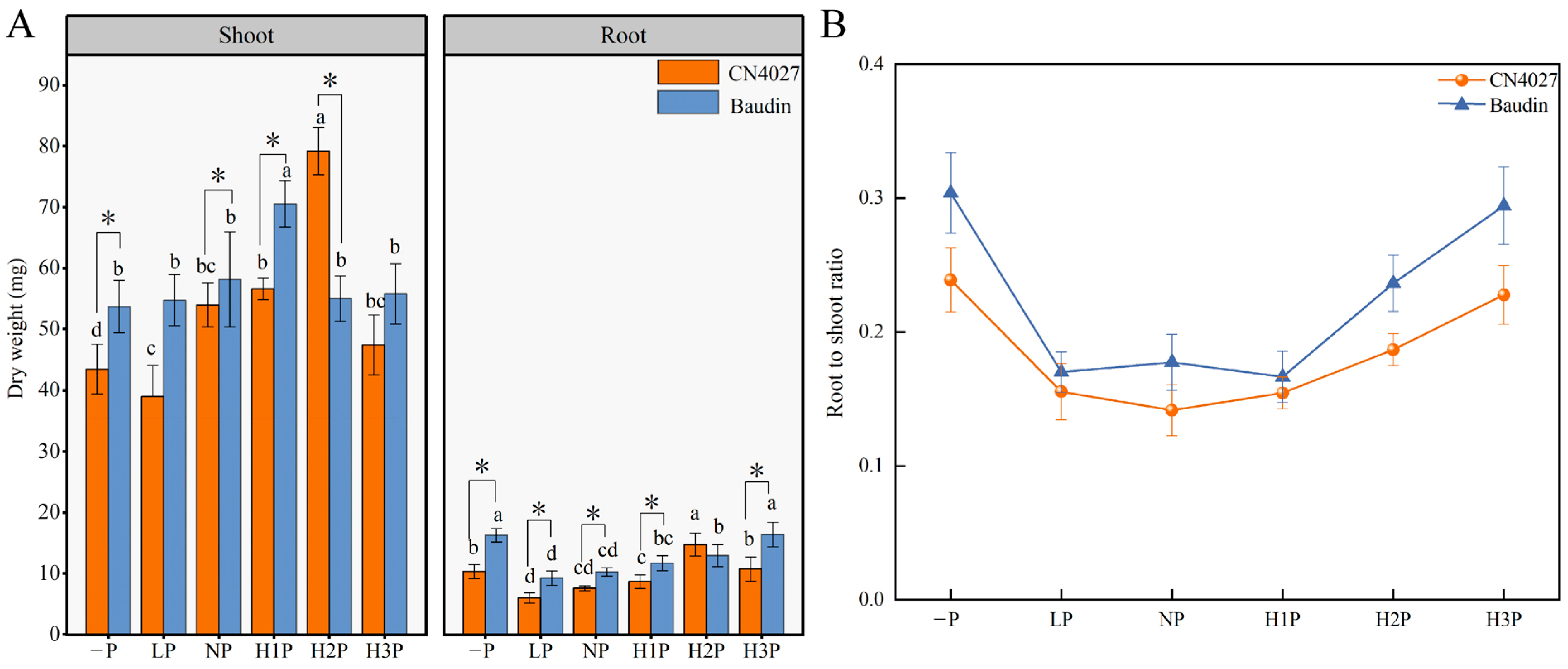
2.2. The Growth of Barley Nourished with Different Concentrations of Phytin
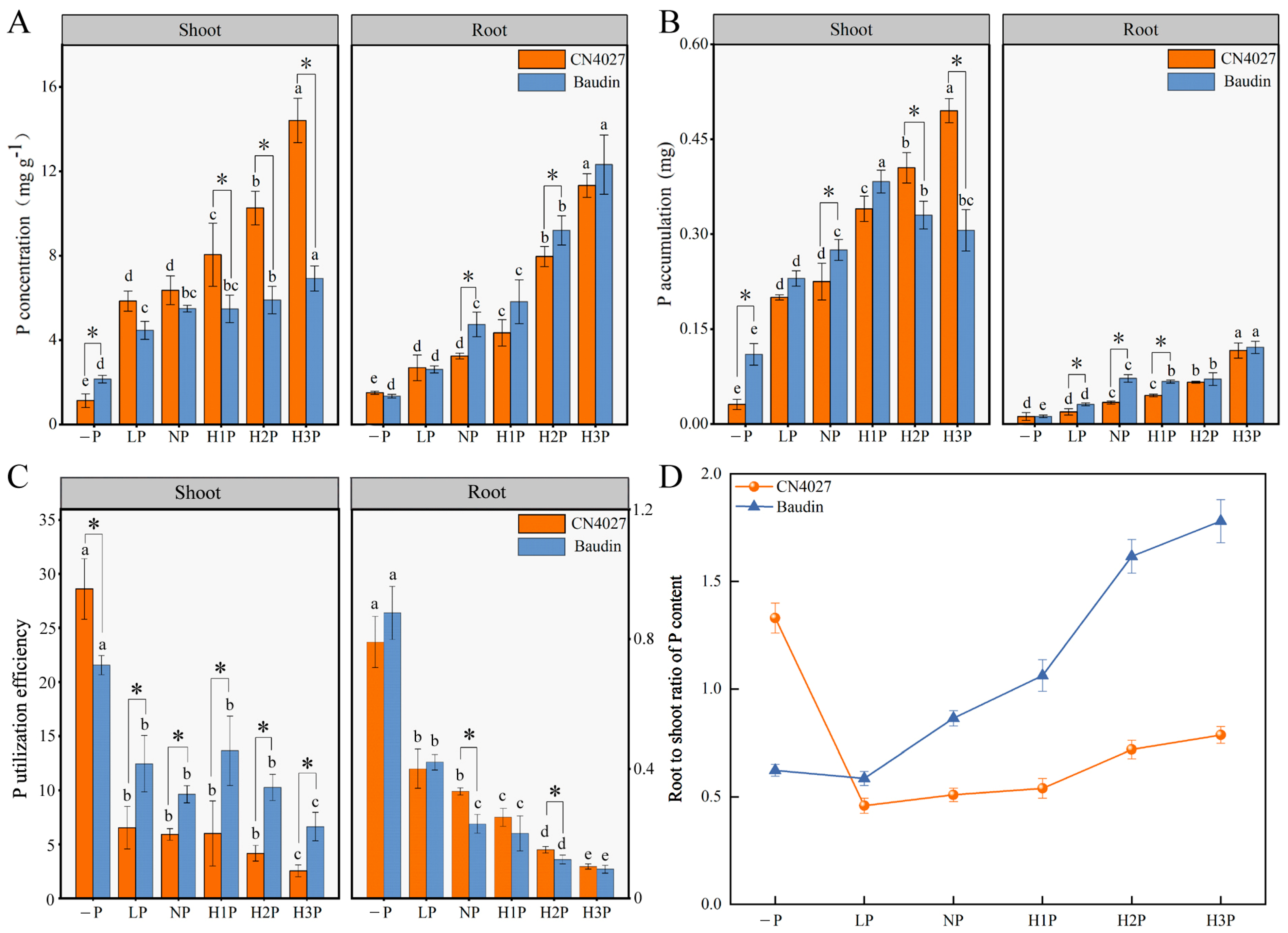
2.3. Utilization of P in Barley Nourished with Different Concentrations of Phytin
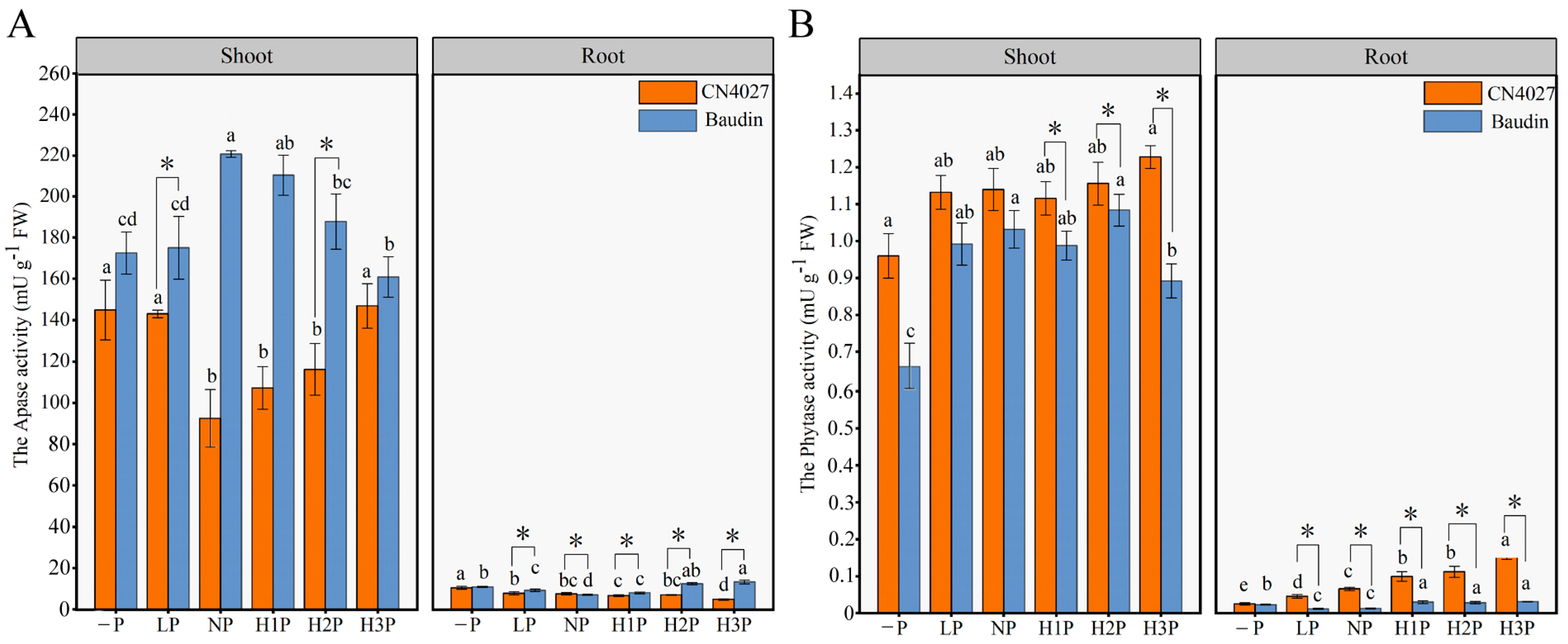
2.4. Acid Phosphatase Activity and Phytase Activity of Barley
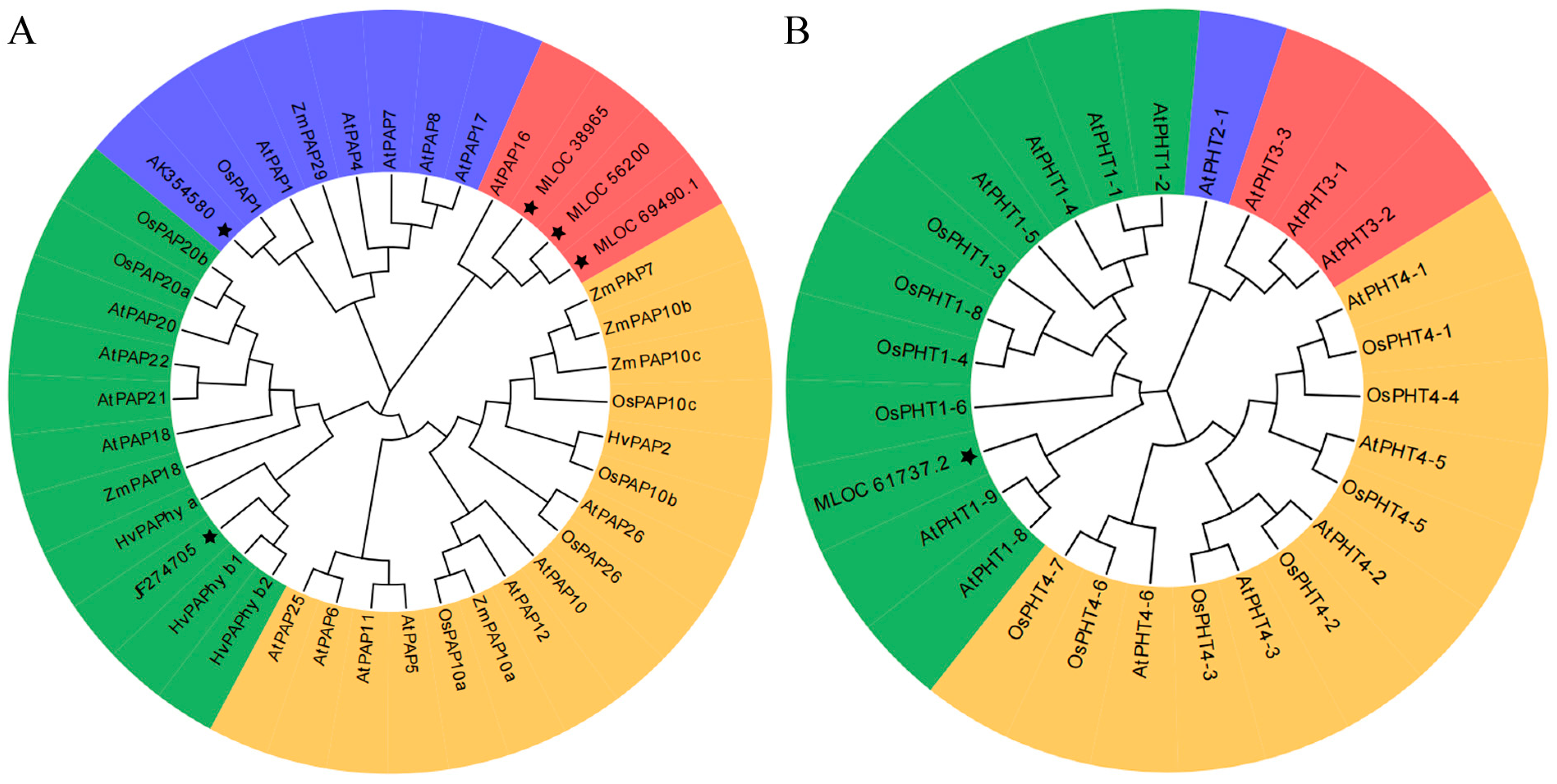
2.5. Phylogenetic Analysis of PAPs and PHTs in Barley
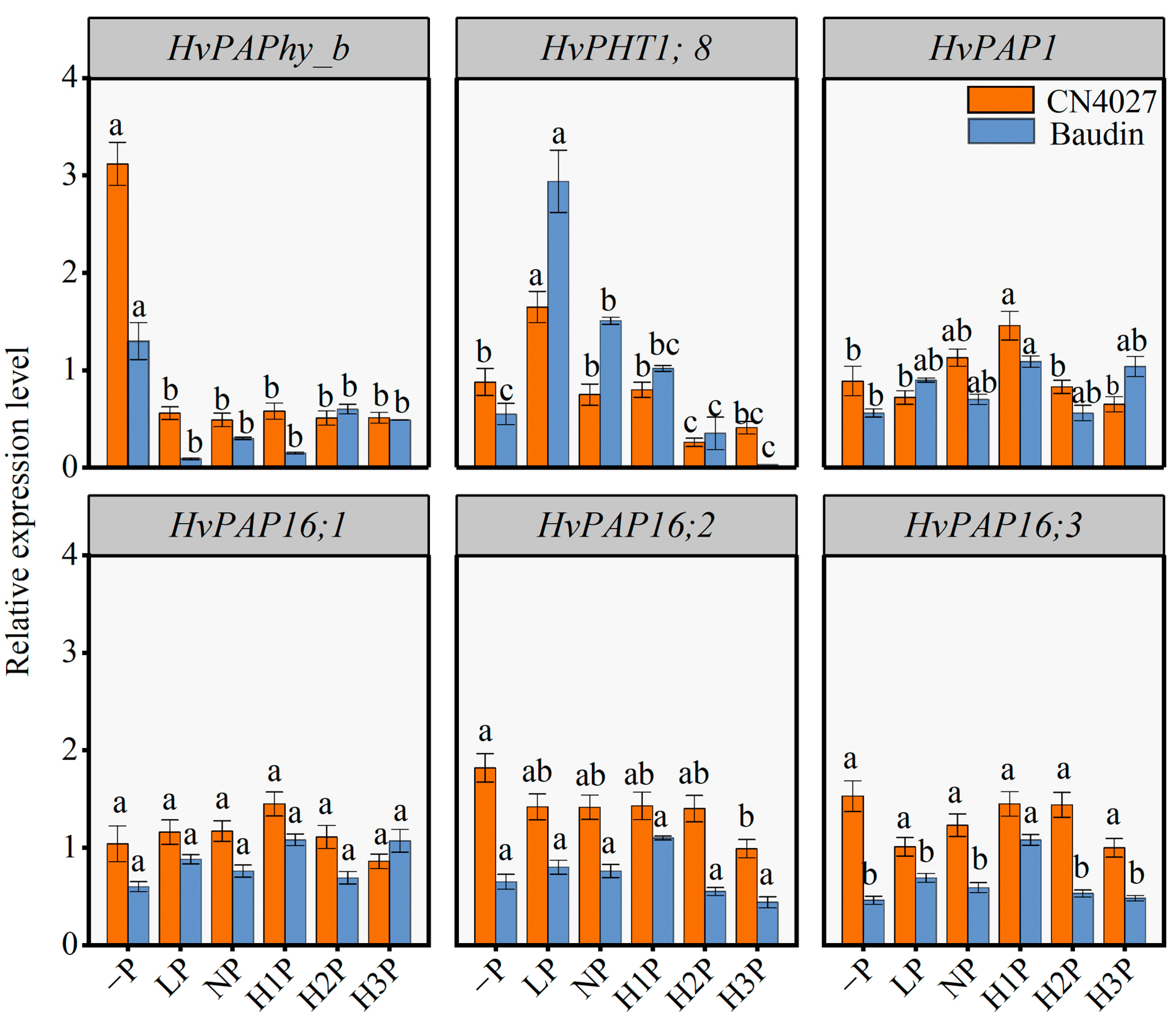
2.6. Expression of P-Utilization-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Analysis of Plant Growth
4.4. Determination of P Content and Enzyme Activity
4.5. Phylogenetic Analysis
4.6. Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Annu. Rev. Plant Biol. 2018, 48, 3–22. [Google Scholar]
- Sulieman, S.; Mühling, K.H. Game changer in plant nutrition utilization of soil organic phosphorus as a strategic approach. J. Plant Nutr. Soil Sci. 2021, 184, 311–319. [Google Scholar] [CrossRef]
- Huang, L.M.; Jia, X.X.; Zhang, G.L.; Shao, M.A. Soil organic phosphorus transformation during ecosystem development: A review. Plant Soil 2017, 417, 17–42. [Google Scholar] [CrossRef]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants-facing phosphorus scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Ramiro, R.; Barbara, J.; Antonio, D. Organic phosphorus forms in agricultural soils under mediterranean climate. Soil Sci. Soc. Am. J. 2018, 82, 783–795. [Google Scholar]
- Pedersen, E.J.N. On phytin phosphorus in the soil. Plant Soil 1953, 4, 252–266. [Google Scholar] [CrossRef]
- Mohsin, S.; Maqbool, A.; Ashraf, M.; Malik, K.A. Extracellular secretion of phytase from transgenic wheat roots allows utilization of phytate for enhanced phosphorus uptake. Mol. Biotechnol. 2017, 59, 334–342. [Google Scholar] [CrossRef]
- Aslam, M.M.; Pueyo, J.J.; Pang, J.; Yang, J.; Chen, W.; Chen, H.; Waseem, M.; Li, Y.; Zhang, J.; Xu, W. Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, J.D.; Zhang, Y.C. Root growth and phosphorus efficiency among sweet potato genotypes under low phosphorus. J. Plant Nutr. 2020, 43, 1320–1330. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Hocking, P.; Richardson, A.E. Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environ. Exp. Bot. 2008, 64, 239–249. [Google Scholar] [CrossRef]
- Ciereszko, I.; Żebrowska, E.; Ruminowicz, M. Acid phosphatases and growth of barley (Hordeum vulgare L.) cultivars under diverse phosphorus nutrition. Acta Physiol. Plant. 2011, 33, 2355–2368. [Google Scholar] [CrossRef]
- Waddell, H.A.; Simpson, R.J.; Ryan, M.H. Root morphology and its contribution to a large root system for phosphorus uptake by rytidosperma species (wallaby grass). Plant Soil 2017, 412, 7–19. [Google Scholar] [CrossRef]
- Gianfreda, L. Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nutr. 2015, 15, 283–306. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D. Functions and regulation of phosphate starvation-induced secreted acid phosphatases in higher plants. Plant Sci. 2018, 271, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 2006, 29, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.J.; Fang, Y.L.; Liu, S.; Wang, H.; Xu, B.C.; Zhang, S.Q.; Deng, X.P.; Palta, J.A.; Siddique, K.H.M.; Chen, Y.L. Root morphology and rhizosheath acid phosphatase activity in legume and graminoid species respond differently to low phosphorus supply. Rhizosphere 2021, 19, 100391. [Google Scholar] [CrossRef]
- Lu, L.H.; Qiu, W.M.; Gao, W.W.; Tyerman, S.D.; Shou, H.X.; Wang, C. OsPAP10c, a novel secreted acid phosphatase in rice, plays an important role in the utilization of external organic phosphorus. Plant Cell Environ. 2016, 39, 2247–2259. [Google Scholar] [CrossRef]
- Fei, D. Identification of a phytase gene in barley (Hordeum vulgare L.). PLoS ONE 2011, 6, e18829. [Google Scholar]
- Wang, Y.; Yang, Z.; Kong, Y.; Li, X.; Li, W.; Du, H.; Zhang, C. GmPAP12 is required for nodule development and nitrogen fixation under phosphorus starvation in soybean. Front. Plant Sci. 2020, 11, 450. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, D. Arabidopsis phosphatase under-producer mutants pup1 and pup3 contain mutations in the AtPAP10 and AtPAP26 genes. Plant Signal. Behav. 2015, 10, e10358. [Google Scholar] [CrossRef]
- Gao, S.Q.; Chen, G.D.; Hu, D.Y.; Zhang, X.Z.; Li, T.X.; Liu, S.H.; Liu, C.J. A major quantitative trait locus controlling phosphorus utilization efficiency under different Phytin conditions at vegetative stage in barley. J. Integr. Agric. 2018, 17, 285–296. [Google Scholar] [CrossRef]
- Yang, M.; Yang, H. Utilization of soil residual phosphorus and internal reuse of phosphorus by crops. PeerJ 2021, 15, e11704. [Google Scholar] [CrossRef] [PubMed]
- Belinque, H.; Pucheu, N.; Kerber, N.; Rubiol, G. Utilization of organic phosphorus sources by oilseed rape, sunflower, and soybean. J. Plant Nutr. Soil Sci. 2015, 178, 339–344. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Claassen, N. Organic phosphorus utilization by wheat plants under sterile conditions. Biol. Fertil. Soils 2003, 39, 25–29. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Cai, X.; Wan, Y.T.; Fu, Y.F.; Yang, X.Y.; Zhang, Z.W.; Yuan, S. Relatively low light intensity promotes phosphorus absorption and enhances the ethylene signaling component EIN3in maize, wheat, and oilseed rape. Agronomy 2022, 12, 427. [Google Scholar] [CrossRef]
- Priya, P.; Sahi, S.V. Influence of phosphorus nutrition on growth and metabolism of Duo grass (Duo festulolium). Plant Physiol. Biochem. 2009, 47, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ. 2000, 23, 397–405. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V. Enhanced organic phosphorus assimilation promoting biomass and shoot P hyperaccumulations in Lolium multiflorum grown under sterile conditions. Environ. Sci. Technol. 2011, 45, 10531–10537. [Google Scholar] [CrossRef]
- Han, Y.; White, P.J.; Cheng, L. Mechanisms for improving phosphorus utilization efficiency in plants. Annu. Rev. Plant Biol. 2022, 129, 247–258. [Google Scholar] [CrossRef]
- Montalvo, D.; Degryse, F.; McLaughlin, M.J. Natural colloidal P and its contribution to plant P uptake. Environ. Sci. Technol. 2015, 49, 3427–3434. [Google Scholar] [CrossRef]
- Amadou, I.; Faucon, M.; Houben, D. Role of soil minerals on organic phosphorus availability and phosphorus uptake by plants. Geoderma 2022, 428, 116125. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Nobile, C. Tradeoffs among phosphorus-acquisition root traits of crop species for agroecological intensification. Plant Soil 2021, 461, 137–150. [Google Scholar] [CrossRef]
- Ayalew, H.; Ma, X.; Yan, G. Screening wheat (Triticum spp.) genotypes for root length under contrasting water regimes: Potential sources of variability for drought resistance breeding. J. Agron. Crop Sci. 2015, 201, 189–194. [Google Scholar] [CrossRef]
- Silberbush, M.; Shomer-Ilan, A.; Waisel, Y. Root surfacephosphatase activity in ecotypes of Aegilops peregrina. Plant Physiol. 1981, 53, 501–504. [Google Scholar] [CrossRef]
- Pandey, B.K.; Mehra, P.; Verma, L.; Bhadouria, J.; Giri, J. OsHAD1, a haloacid dehalogenase-like APase, enhances phosphate accumulation. Plant Physiol. 2017, 174, 2316–2332. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, Y.Y.; Zhao, X.Q.; He, X.; Ma, W.Y.; Deng, Y.; Chen, X.P.; Tong, Y.P. Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front. Plant Sci. 2017, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kuo, H.F.; Chiou, T.J. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef]
- Dionisio, G.; Madsen, C.K.; Holm, P.B.; Welinder, K.G.; Jorgensen, M.; Stoger, E.; Arcalis, E.; Brinch-Pedersen, H. Cloning and characterization of purple acid phosphatase phytases from wheat, barley, maize, and rice. Plant Physiol. 2011, 156, 1087–1100. [Google Scholar] [CrossRef]
- Lapis-Gaza, H.R.; Jost, R.; Finnegan, P.M. Arabidopsis PHOSPHATE TRANSPORTER1 genes PHT1;8 and PHT1;9 are involved in root-to-shoot translocation of orthophosphate. BMC Plant Biol. 2014, 27, 334. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Teng, W.; Tong, Y.; Chen, X.; Zou, C. Phosphorus efficiency mechanisms of two wheat cultivars as affected by a range of phosphorus levels in the field. Front. Plant Sci. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Guo, J.Y.; Ye, D.H.; Chen, G.D.; Yu, H.Y.; Zhang, X.Z.; Li, T.X. Coincidence of QTLs determining foliar phosphorus fractionation patterns and phosphorus utilization efficiency in barley under low phosphorus stress. Plant Soil 2019, 44, 349–362. [Google Scholar] [CrossRef]
- Lung, S.C.; Leung, A.; Kuang, R.; Wang, Y.; Leung, P.; Lim, B.L. Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase. Phytochemistry 2008, 69, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.K.; Brinch-Pedersen, H. Molecular advances on phytases in barley and wheat. Int. J. Mol. Sci. 2019, 20, 2459. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Feng, L.Y.; Wang, J.H.; Fu, Y.F.; Cai, X.; Wang, C.Q.; Du, J.B.; Yuan, M.; Chen, Y.E.; Xu, P.Z.; et al. Two-factor ANOVA of SSH and RNA-seq analysis reveal development-associated Pi-starvation genes in oilseed rape. Planta 2019, 250, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
| OP | Length (cm) | Surface Area (cm2) | Average Diam. (mm) | Volume (mm3) | ||||
|---|---|---|---|---|---|---|---|---|
| CN4027 | Baudin | CN4027 | Baudin | CN4027 | Baudin | CN4027 | Baudin | |
| Pi | 29.5 ± 0.6c | 19.3 ± 1.3d | 4.23 ± 0.50bc | 4.24 ± 0.68e | 0.38 ± 0.01a | 0.38 ± 0.01bc | 40.3 ± 3.9b | 24.5 ± 4.5c |
| Phytin | 30.8 ± 0.1c | 49.5 ± 2.9b | 3.72 ± 0.32c | 7.00 ± 0.73bc | 0.36 ± 0.03a | 0.44 ± 0.02b | 36.0 ± 6.0b | 58.5 ± 8.5b |
| ATP | 42.2 ± 3.6b | 60.2 ± 3.7ab | 5.43 ± 0.33ab | 9.37 ± 0.33a | 0.40 ± 0.03a | 0.35 ± 0.02c | 23.5 ± 0.5c | 57.8 ± 1.0b |
| RNA | 31.9 ± 1.5c | 27.1 ± 5.1cd | 3.34 ± 0.39cd | 4.85 ± 0.46de | 0.37 ± 0.04a | 0.72 ± 0.02a | 34.0 ± 3.2bc | 84.3 ± 6.8a |
| F6P | 33.6 ± 3.7c | 63.8 ± 3.2a | 4.02 ± 0.57c | 8.79 ± 0.48a | 0.38 ± 0.02a | 0.44 ± 0.03b | 43.5 ± 2.5b | 89.5 ± 8.5a |
| IHP | 18.1 ± 0.3d | 53.3 ± 4.1ab | 2.35 ± 0.16d | 7.99 ± 0.80ab | 0.39 ± 0.04a | 0.45 ± 0.03b | 24.7 ± 3.4c | 95.7 ± 8.0a |
| Treatment | Length | Surface Area | Volume | |||
|---|---|---|---|---|---|---|
| CN4027 | Baudin | CN4027 | Baudin | CN4027 | Baudin | |
| Pi | 4.68 ± 0.36b | 2.97 ± 0.40c | 1.06 ± 0.09a | 0.63 ± 0.16c | 17.7 ± 1.4a | 11.8 ± 2.6e |
| Phytin | 3.92 ± 0.23c | 10.3 ± 0.1b | 0.68 ± 0.14c | 3.35 ± 0.52ab | 12.4 ± 1.7b | 42.0 ± 5.6bc |
| ATP | 3.29 ± 0.05d | 10.6 ± 0.5b | 0.76 ± 0.01bc | 2.92 ± 0.24ab | 17.1 ± 0.5a | 31.9 ± 2.8d |
| RNA | 3.95 ± 0.30c | 11.0 ± 1.1b | 0.51 ± 0.06d | 3.01 ± 0.58ab | 13.8 ± 0.4ab | 47.7 ± 3.0ab |
| F6P | 2.83 ± 0.22d | 13.9 ± 0.9a | 0.74 ± 0.09bc | 3.70 ± 0.35a | 13.6 ± 1.2ab | 58.0 ± 4.6a |
| IHP | 3.41 ± 0.03cd | 14.5 ± 0.2a | 0.75 ± 0.06bc | 3.78 ± 0.27a | 12.3 ± 2.6b | 57.9 ± 5.7a |
| Treatment | Length | Surface Area | Volume | |||
|---|---|---|---|---|---|---|
| CN4027 | Baudin | CN4027 | Baudin | CN4027 | Baudin | |
| Pi | 24.8 ± 0.2b | 16.3 ± 0.9cd | 3.17 ± 0.56c | 3.55 ± 0.52c | 22.6 ± 2.6c | 12.7 ± 1.9d |
| Phytin | 26.8 ± 0.1b | 39.3 ± 2.8b | 3.04 ± 0.18c | 3.65 ± 0.21c | 23.6 ± 4.3bc | 16.5 ± 2.9d |
| ATP | 38.9 ± 3.6a | 49.6 ± 3.2a | 4.67 ± 0.34a | 6.45 ± 0.09a | 26.4 ± 4.6d | 26.0 ± 1.7c |
| RNA | 27.9 ± 1.3b | 16.1 ± 4.0d | 2.88 ± 0.33c | 1.84 ± 0.33d | 20.2 ± 2.9c | 36.7 ± 3.9ab |
| F6P | 30.8 ± 3.6b | 49.9 ± 2.6a | 3.28 ± 0.47bc | 5.10 ± 0.12b | 29.9 ± 1.3b | 31.5 ± 3.9abc |
| IHP | 14.7 ± 0.3c | 38.8 ± 3.9b | 1.60 ± 0.11d | 4.20 ± 0.53bc | 12.2 ± 1.5d | 37.7 ± 2.6a |
| Phytin Treatments | Length (cm) | Surface Area (cm2) | Average Diam (mm) | Volume (mm3) | ||||
|---|---|---|---|---|---|---|---|---|
| CN4027 | Baudin | CN4027 | Baudin | CN4027 | Baudin | Baudin | CN4027 | |
| −P | 56.6 ± 1.6c | 78.2 ± 3.8b | 9.37 ± 0.24b | 12.38 ± 0.56b | 0.49 ± 0.04a | 0.51 ± 0.03a | 127 ± 7a | 148 ± 13ab |
| LP | 48.4 ± 2.4d | 51.9 ± 2.1c | 6.08 ± 0.50d | 7.31 ± 0.53d | 0.39 ± 0.01b | 0.36 ± 0.01c | 58.7 ± 6.7d | 61.5 ± 1.8d |
| NP | 36.9 ± 2.5e | 50.9 ± 0.3c | 4.86 ± 0.19e | 8.74 ± 0.05cd | 0.38 ± 0.01b | 0.41 ± 0.01bc | 46.7 ± 2.7e | 67.3 ± 1.3d |
| H1P | 64.7 ± 3.2b | 72.8 ± 2.0b | 8.23 ± 0.35bc | 10.34 ± 0.30c | 0.41 ± 0.00b | 0.47 ± 0.04ab | 83.7 ± 2.8c | 142 ± 12b |
| H2P | 58.2 ± 3.0bc | 78.7 ± 2.2b | 7.55 ± 0.53c | 9.76 ± 0.52c | 0.41 ± 0.01b | 0.41 ± 0.03bc | 72.5 ± 4.0c | 93.7 ± 5.6c |
| H3P | 80.7 ± 1.2a | 122 ± 5a | 10.6 ± 0.5a | 17.5 ± 1.0a | 0.42 ± 0.01b | 0.46 ± 0.01ab | 104 ± 2b | 171 ± 10a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Y.; Wang, J.; Xu, Y.; Hu, D.; Zhang, K.; Chen, B.; Wu, Y.; Liu, J.; Yan, T.; Li, Y.; et al. The Impact of Various Organic Phosphorus Carriers on the Uptake and Use Efficiency in Barley. Int. J. Mol. Sci. 2023, 24, 17191. https://doi.org/10.3390/ijms242417191
Huo Y, Wang J, Xu Y, Hu D, Zhang K, Chen B, Wu Y, Liu J, Yan T, Li Y, et al. The Impact of Various Organic Phosphorus Carriers on the Uptake and Use Efficiency in Barley. International Journal of Molecular Sciences. 2023; 24(24):17191. https://doi.org/10.3390/ijms242417191
Chicago/Turabian StyleHuo, Yuanfeng, Jingyue Wang, Yinggang Xu, Deyi Hu, Kexian Zhang, Bingjie Chen, Yueyi Wu, Jiaxin Liu, Tianlang Yan, Yang Li, and et al. 2023. "The Impact of Various Organic Phosphorus Carriers on the Uptake and Use Efficiency in Barley" International Journal of Molecular Sciences 24, no. 24: 17191. https://doi.org/10.3390/ijms242417191
APA StyleHuo, Y., Wang, J., Xu, Y., Hu, D., Zhang, K., Chen, B., Wu, Y., Liu, J., Yan, T., Li, Y., Yan, C., Gao, X., Yuan, S., & Chen, G. (2023). The Impact of Various Organic Phosphorus Carriers on the Uptake and Use Efficiency in Barley. International Journal of Molecular Sciences, 24(24), 17191. https://doi.org/10.3390/ijms242417191







