Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents
Abstract
1. Introduction
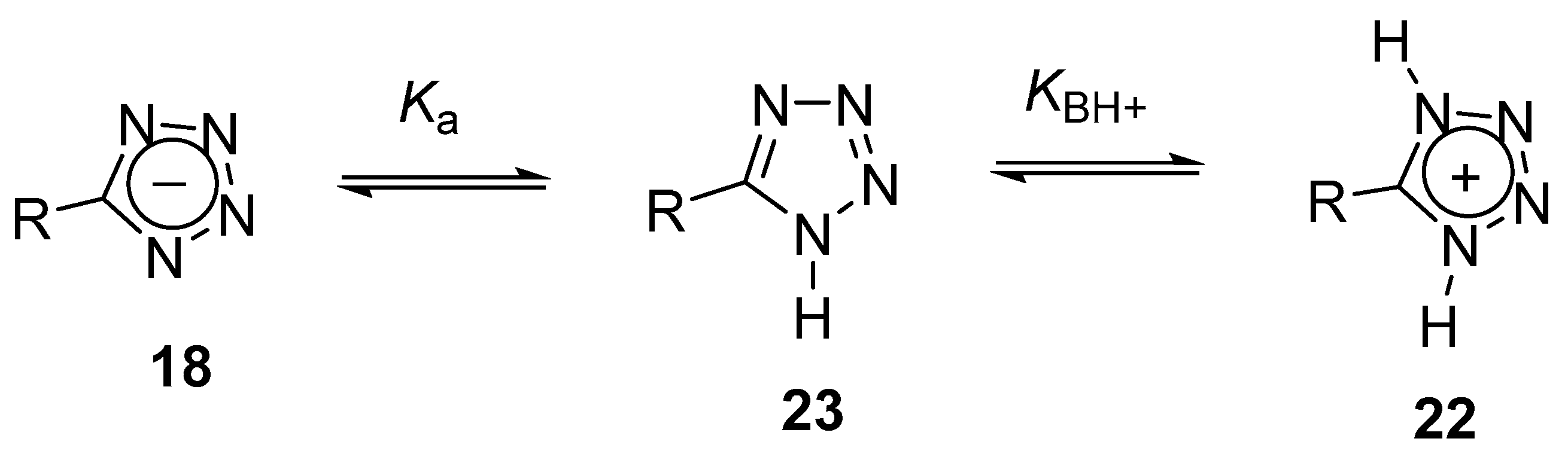
2. Tetrazoles for Biomedicine
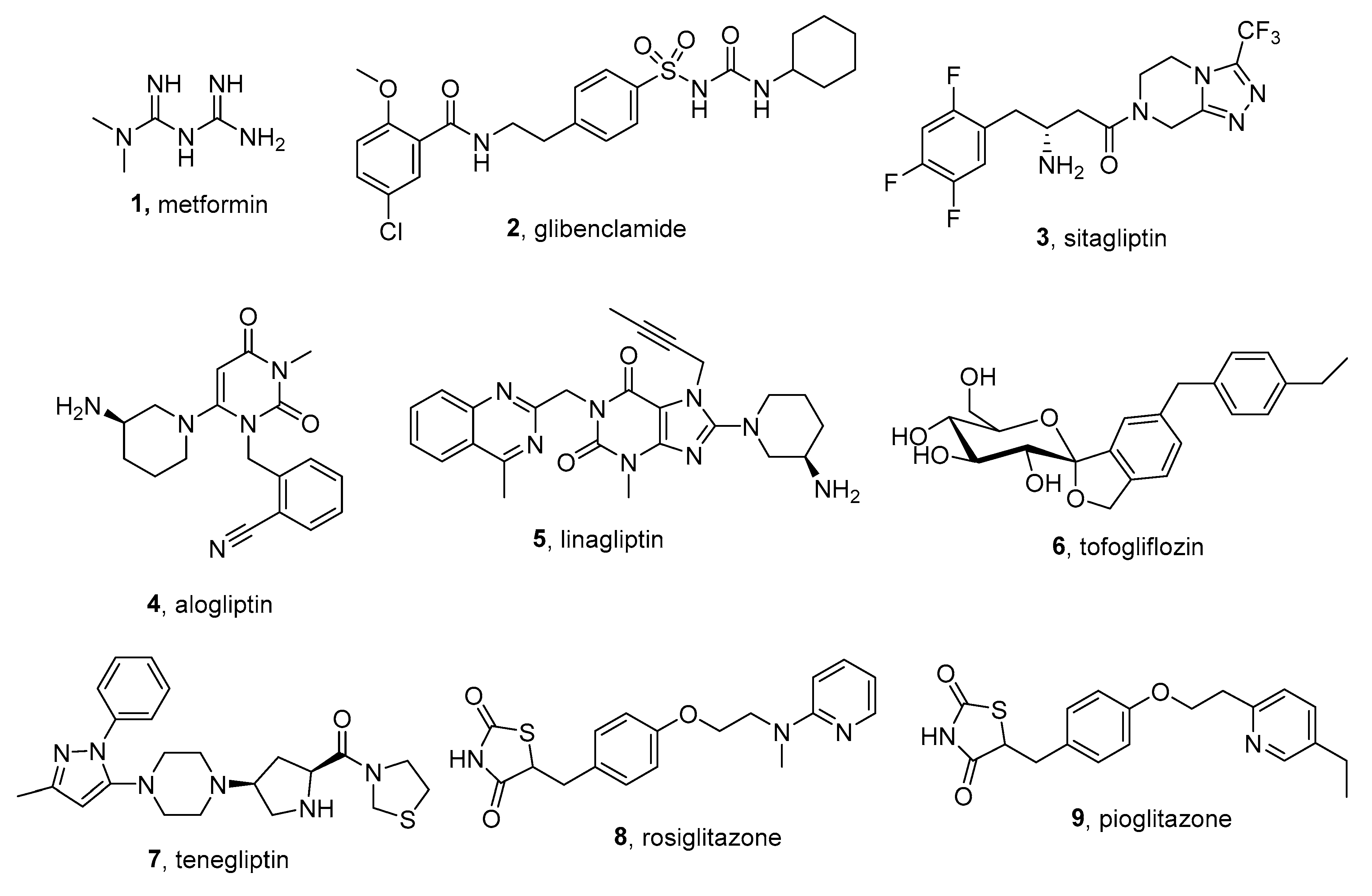
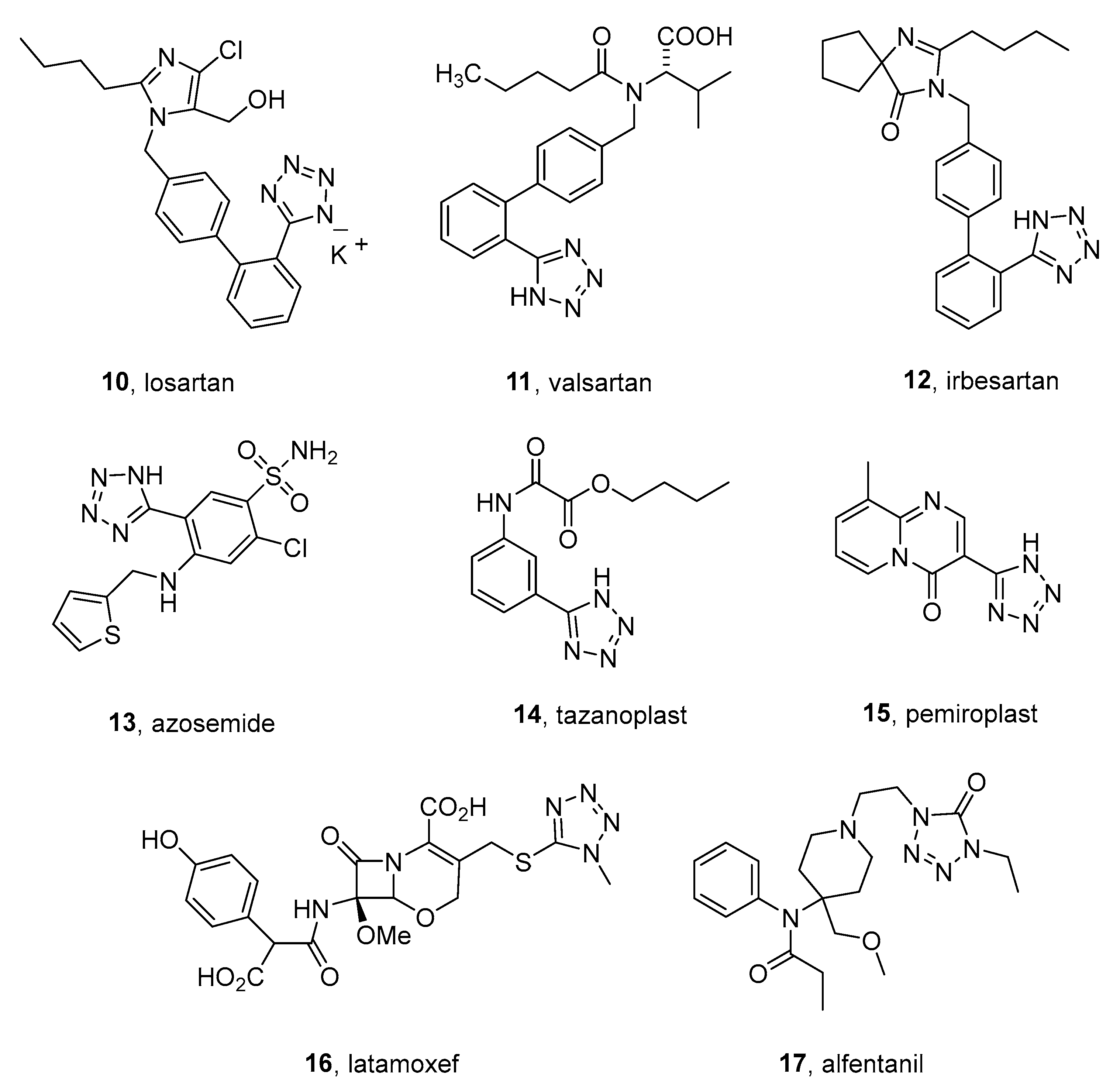
3. Low-Molecular Antidiabetic Agents Containing Tetrazolyl Moiety
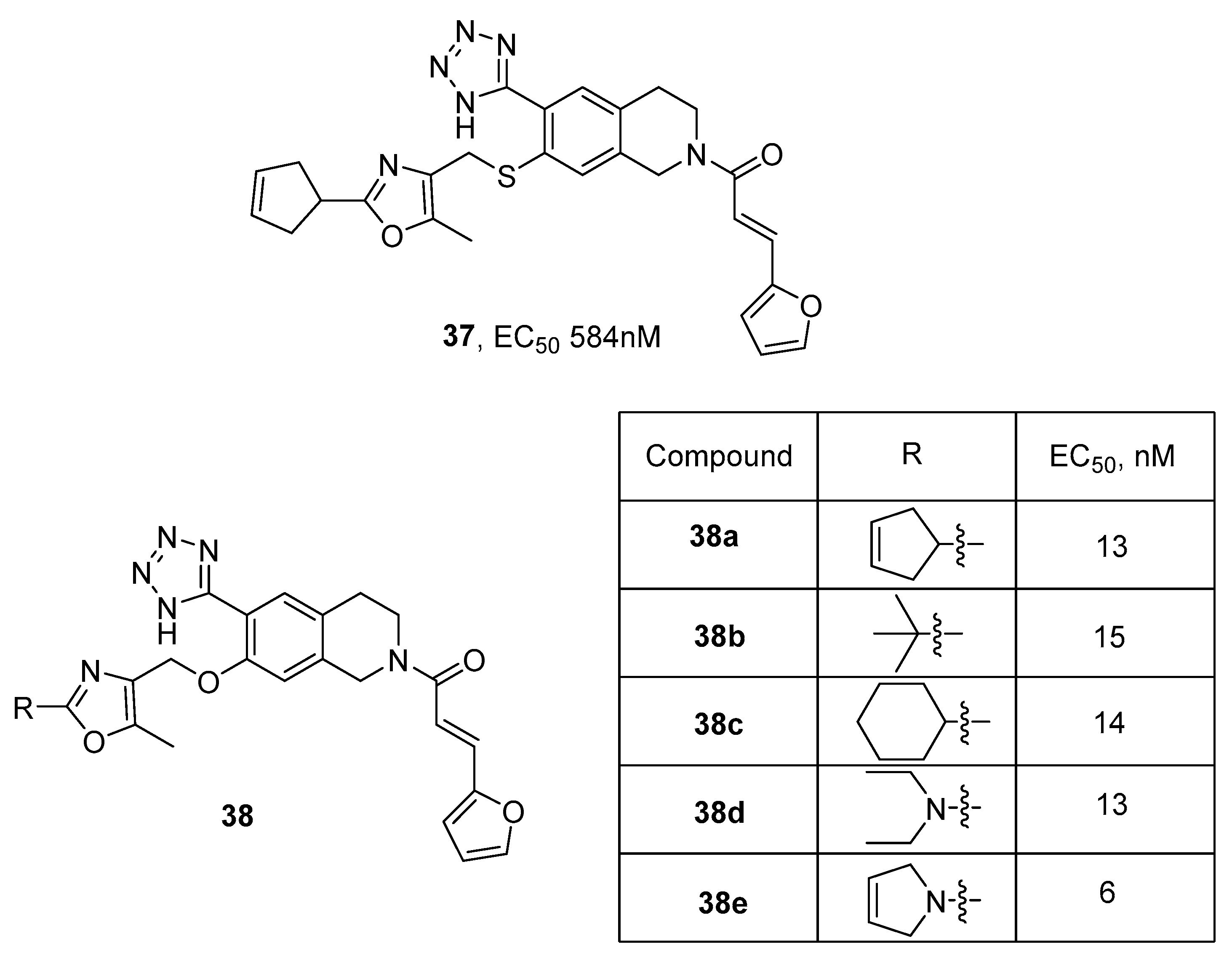
3.1. Peroxisome Proliferator-Activated Receptors (PPARs) Agonists
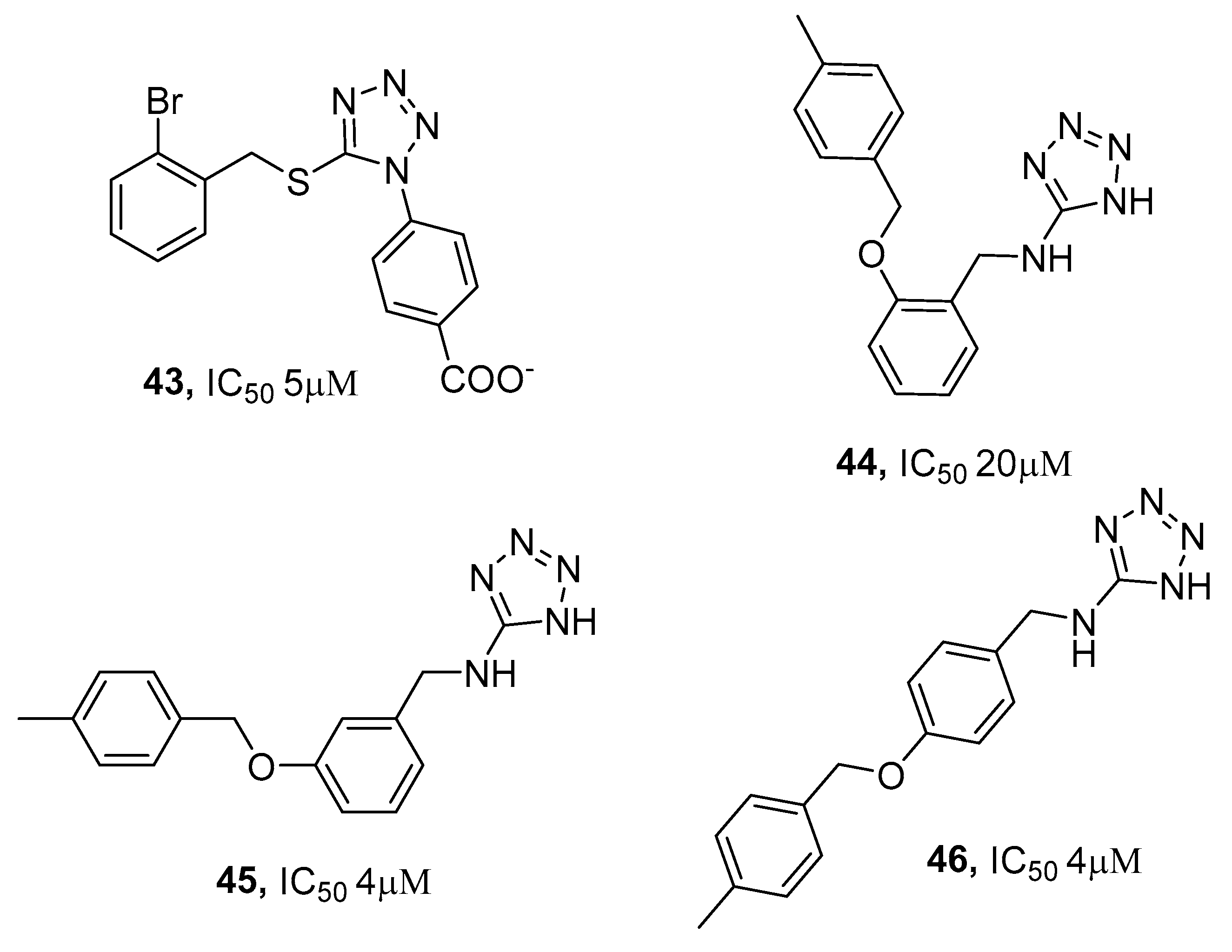
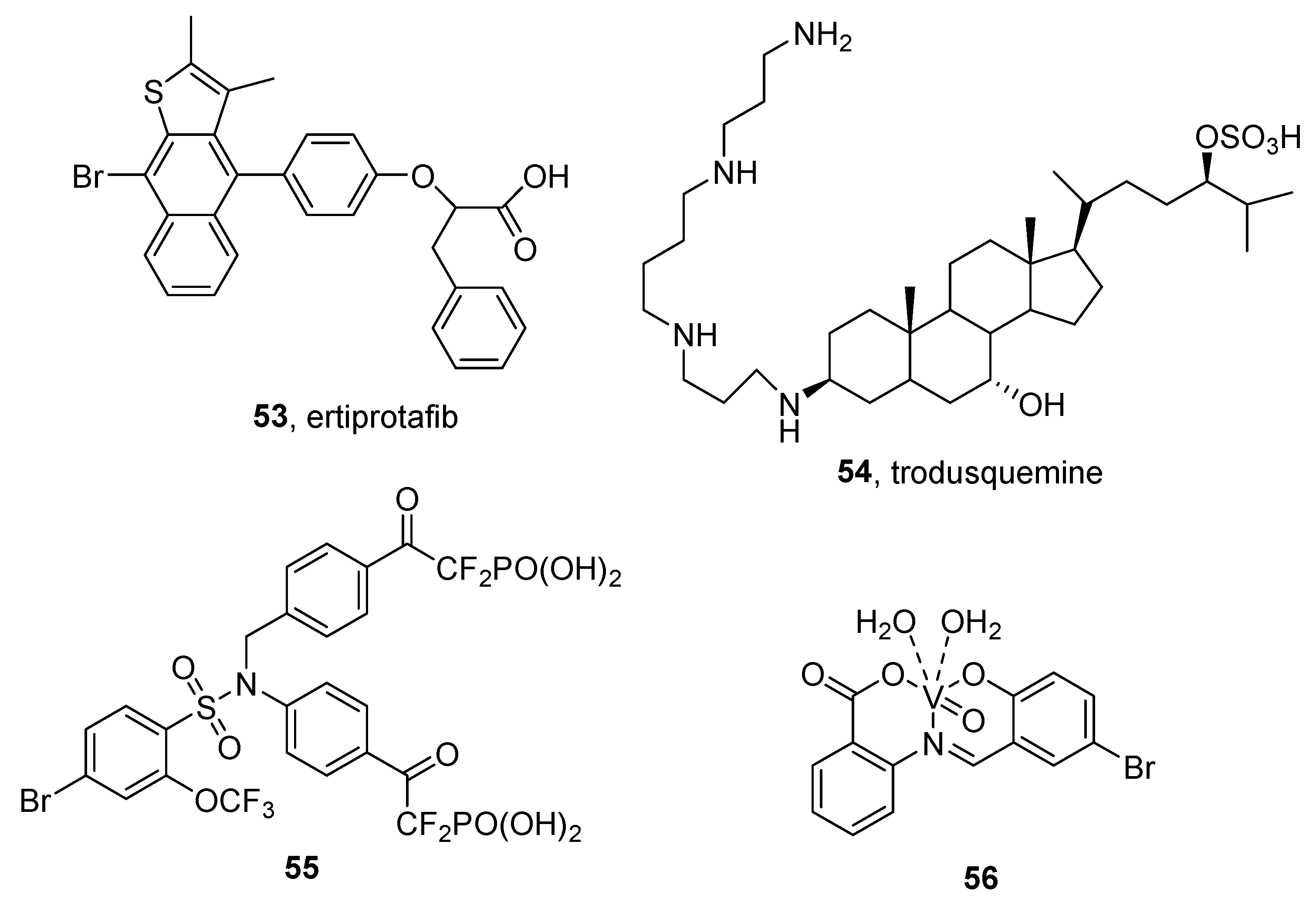
3.2. Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitors
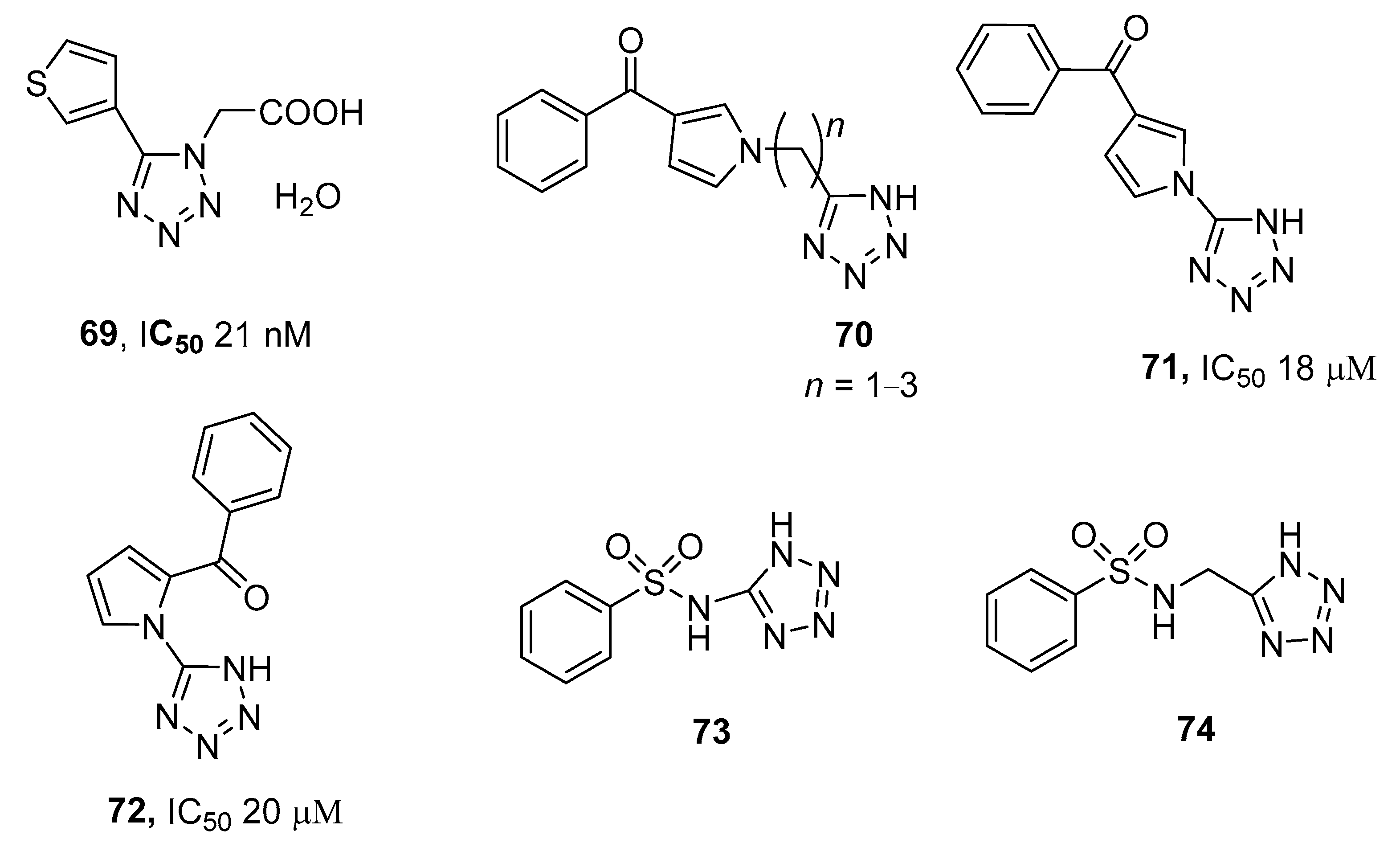
3.3. Aldose Reductase (AR) Inhibitors
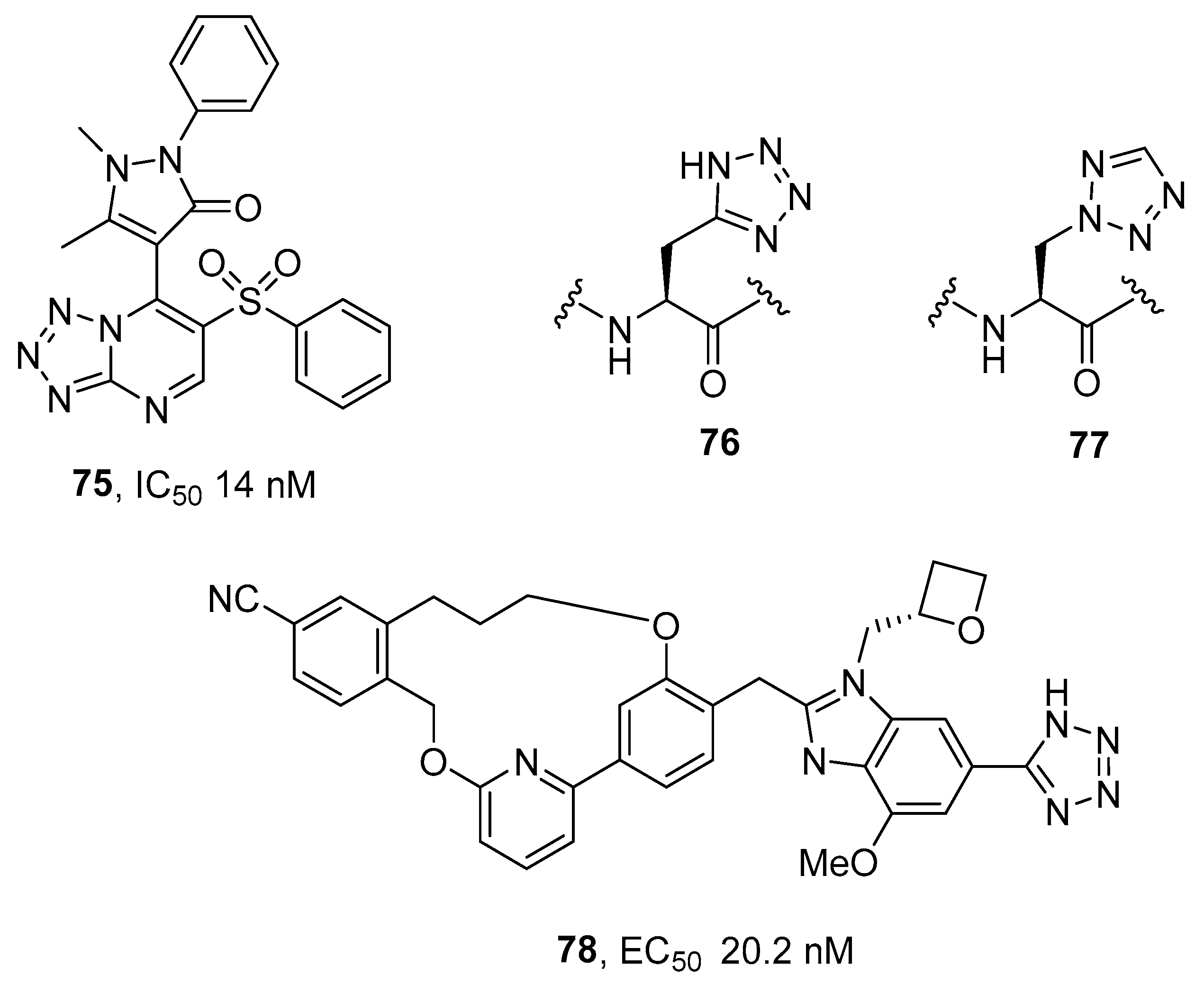
3.4. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors and Glucagon-like Peptide 1 (GLP-1) Agonists
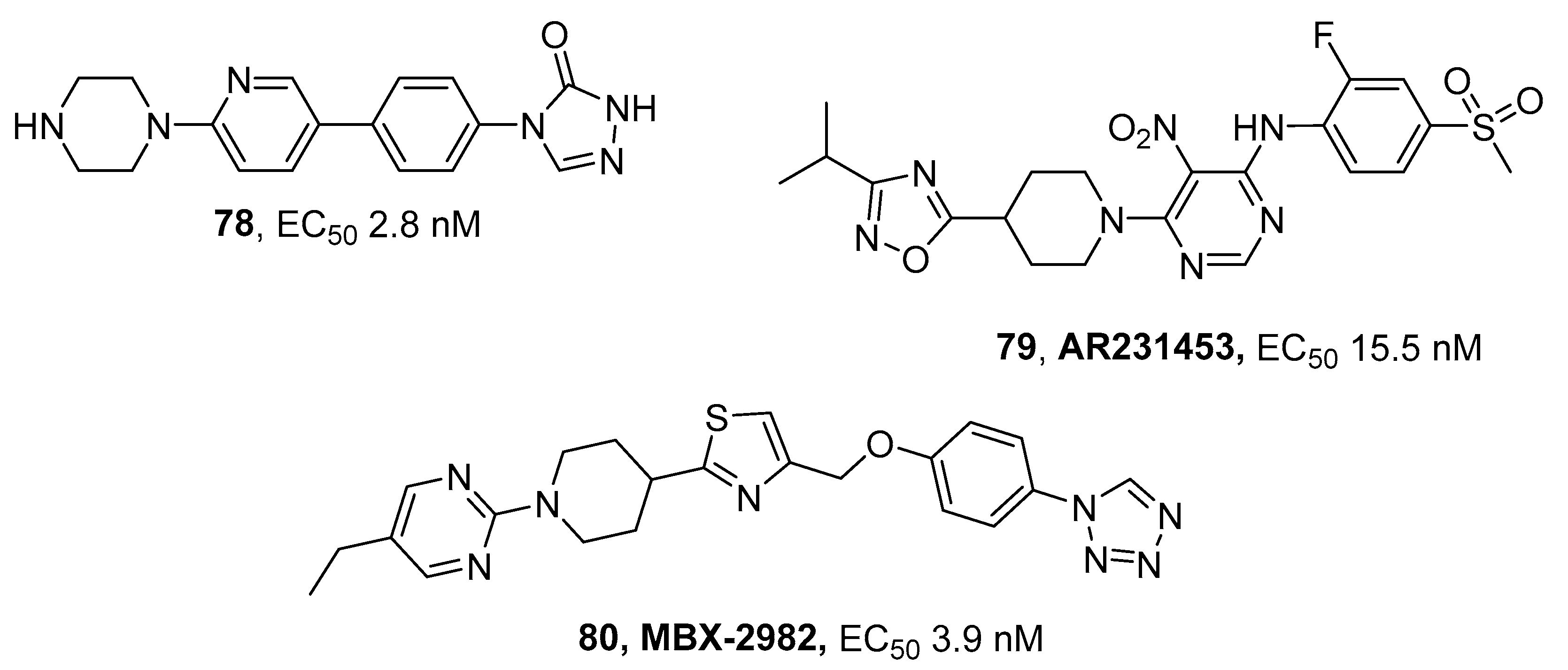
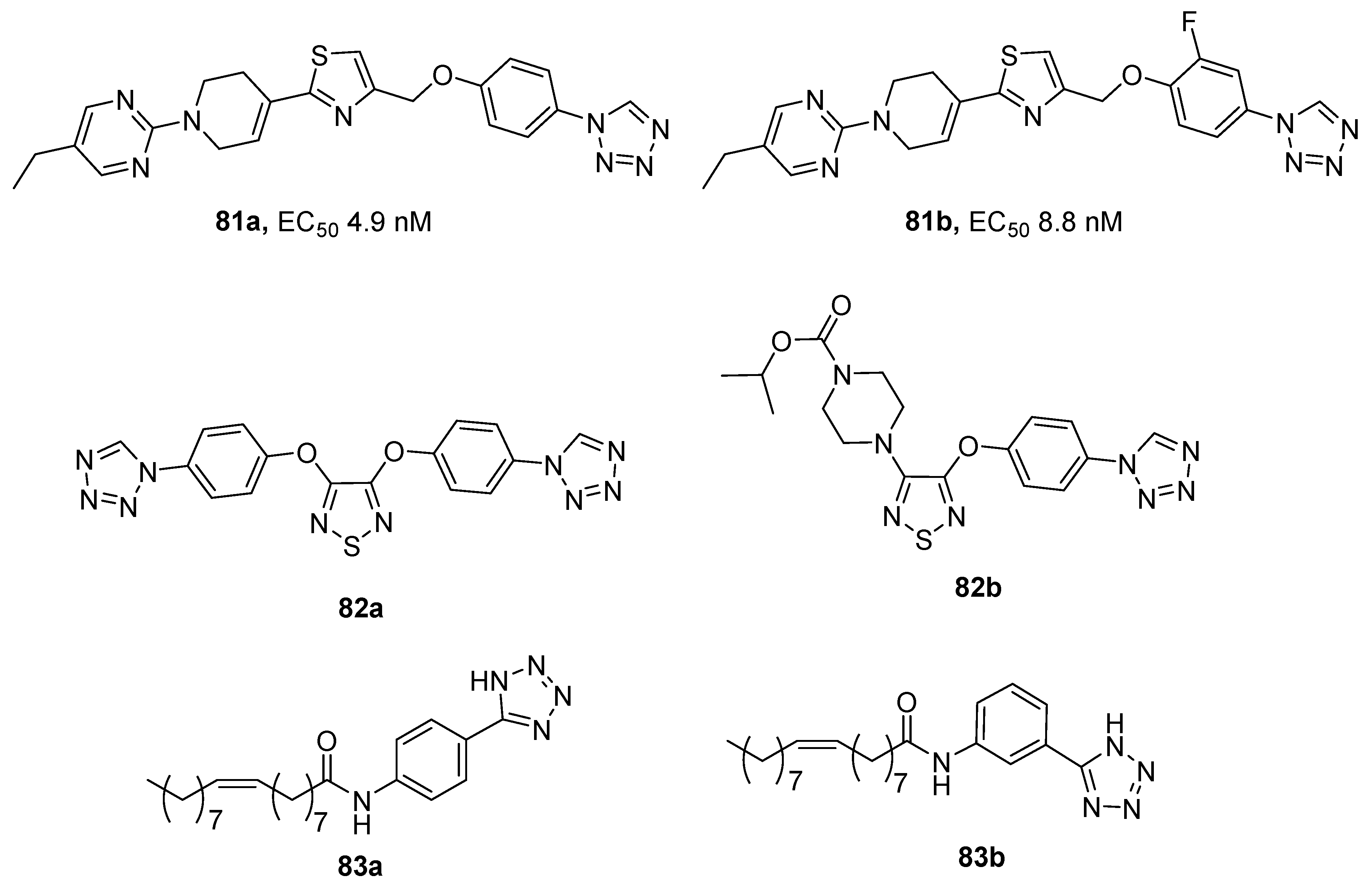
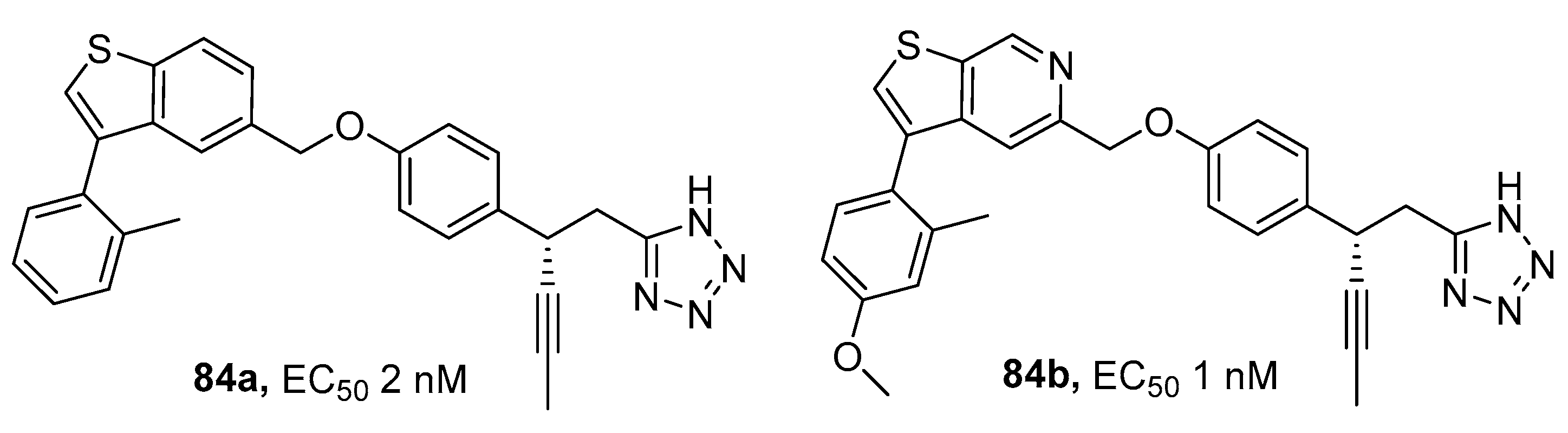
3.5. G Protein-Coupled Receptor (GPCR) Agonists
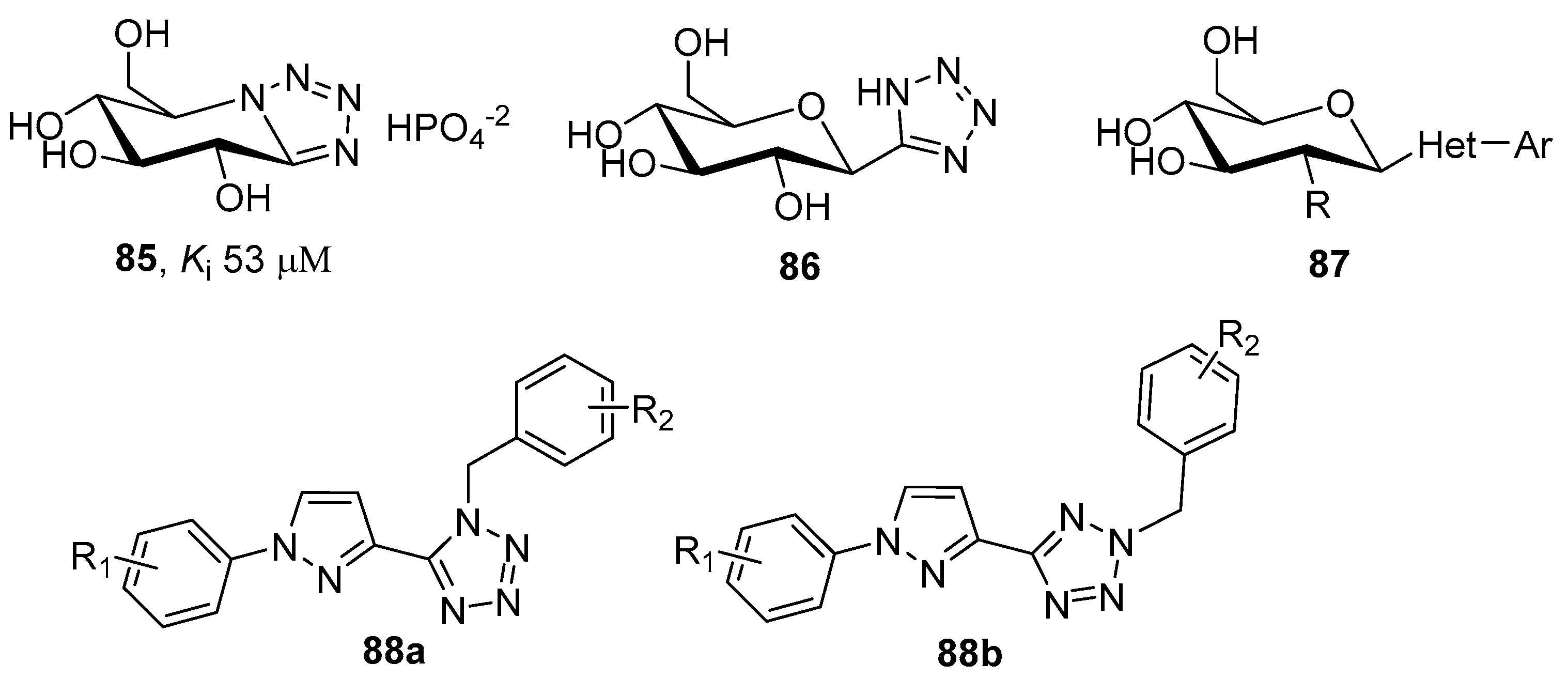
3.6. Glycogen Phosphorylases (GP) Inhibitors
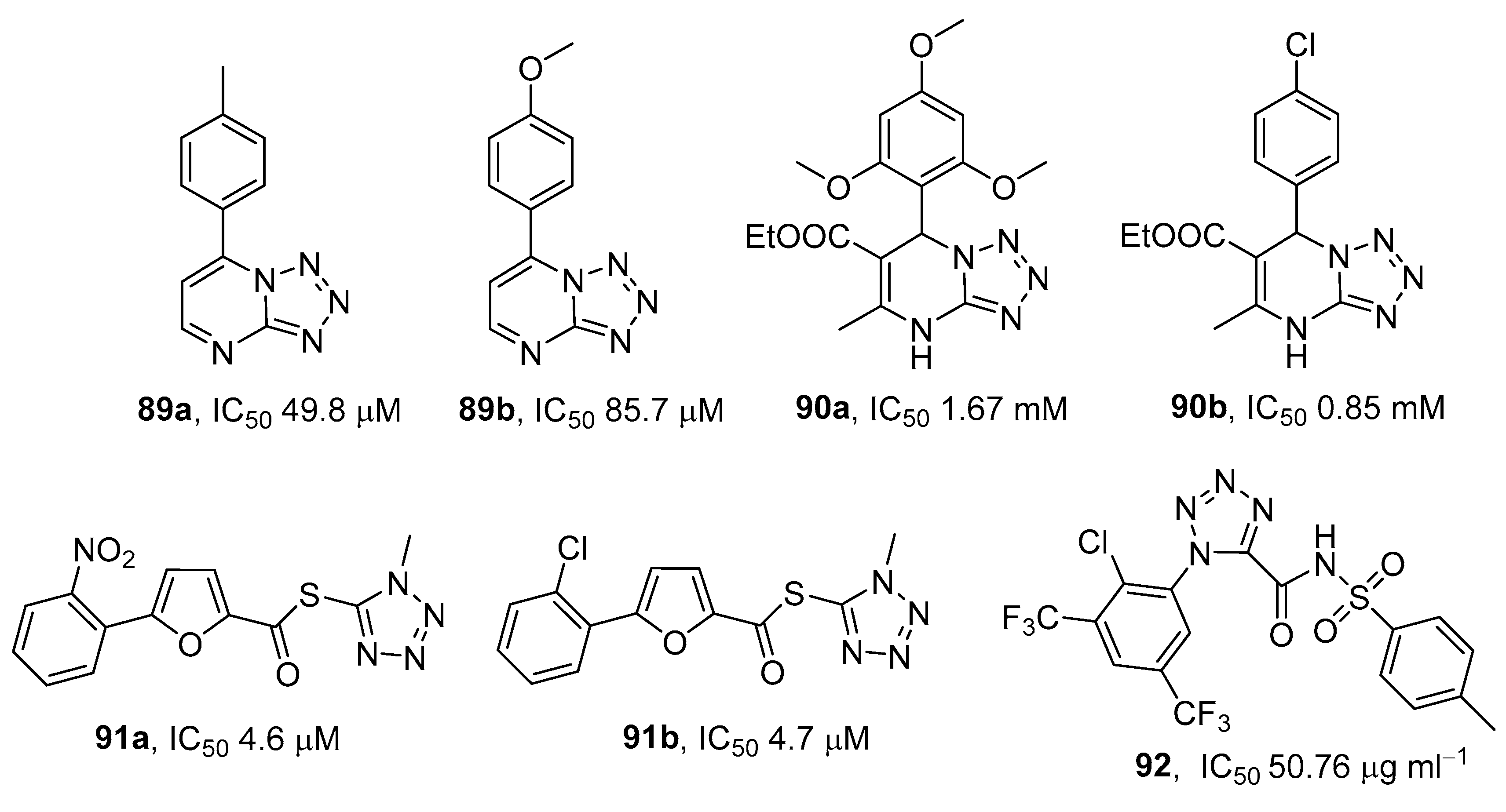
3.7. α-Glycosidase (AG) Inhibitors
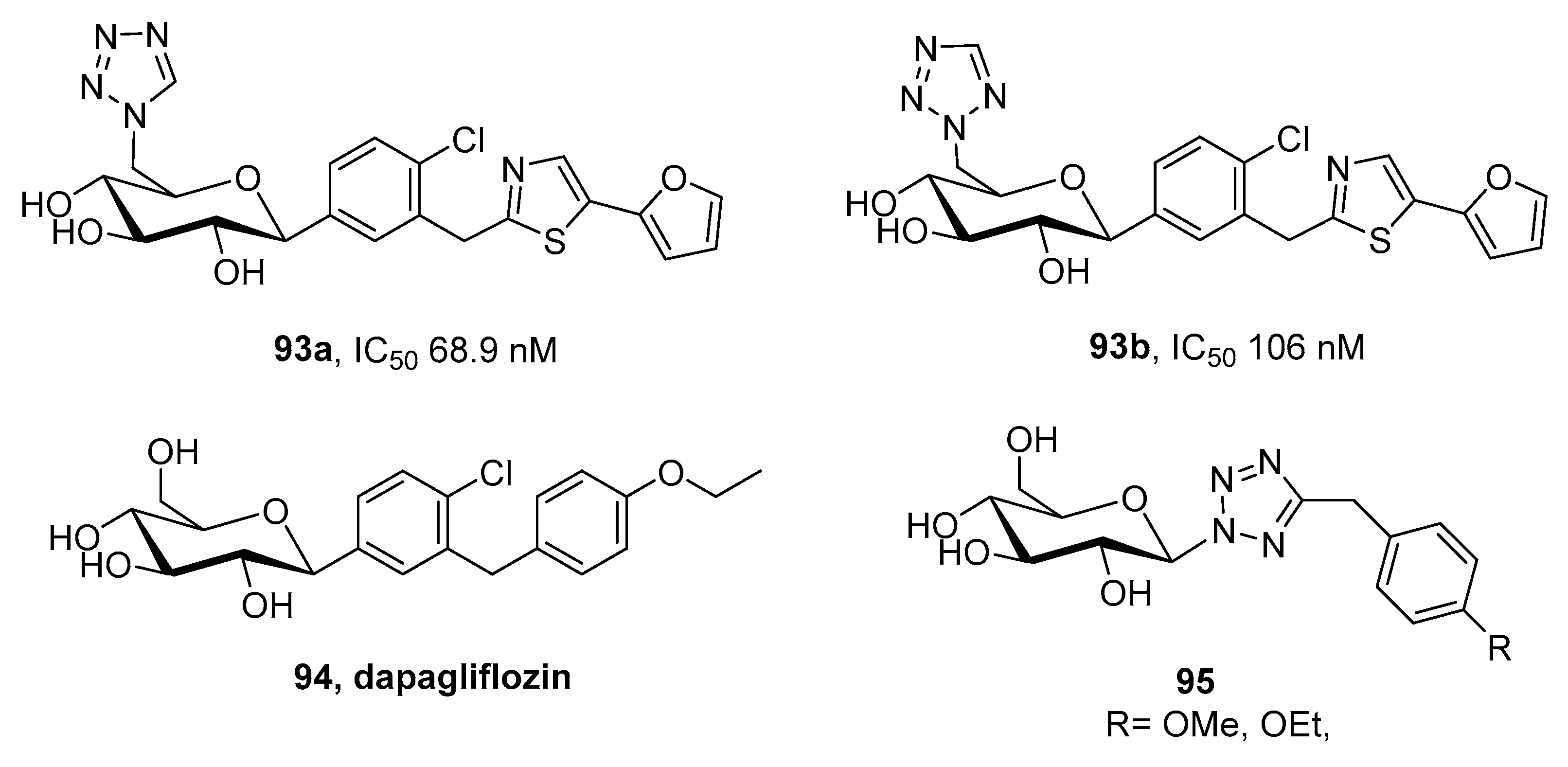
3.8. Sodium Glucose Co-Transporter (SGLT) Inhibitors
3.9. Other Types of Activities
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas. International Diabetes Federation Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Salmen, T.; Serbanoiu, L.-I.; Bica, I.-C.; Serafinceanu, C.; Muzurovic, E.; Janez, A.; Busnatu, S.; Banach, M.; Rizvi, A.A.; Rizzo, M.; et al. Review on critical view over newest antidiabetic molecules in light of efficacy. Int. J. Mol. Sci. 2023, 24, 9760. [Google Scholar] [CrossRef]
- Shi, Y.; Si, D.; Chen, D.; Zhang, X.; Han, Z.; Yu, Q.; Liu, J.; Si, J. Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives. Food Chem. 2023, 408, 135183. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z.; Zeng, T.; Zhan, J.; Wang, S.; Ho, C.-T.; Li, S. Bioactives of Momordica charantia as Potential Anti-Diabetic/Hypoglycemic Agents. Molecules 2022, 27, 2175. [Google Scholar] [CrossRef]
- Su, J.; Luo, Y.; Hu, S.; Tang, L.; Ouyang, S. Advances in Research on Type 2 Diabetes Mellitus Targets and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 13381. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; Ali, I.I.; Adeghate, J.O.; Tekes, K.; Kalasz, H.; Adeghate, E.A. An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 9328. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Rusinov, V.L.; Sapozhnikova, I.M.; Spasov, A.A.; Chupakhin, O.N. Fused azoloazines with antidiabetic activity. Russ. Chem. Bull. 2022, 71, 2561–2594. [Google Scholar] [CrossRef]
- Mohammad, B.D.; Baig, M.S.; Bhandari, N.; Siddiqui, F.A.; Khan, S.L.; Ahmad, Z.; Khan, F.S.; Tagde, P.; Jeandet, P. Heterocyclic Compounds as Dipeptidyl Peptidase-IV Inhibitors with Special Emphasis on Oxadiazoles as Potent Anti-Diabetic Agents. Molecules 2022, 27, 6001. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Butler, R.N. Tetrazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK; New York, NY, USA, 1996; Volume 4, pp. 621–678. [Google Scholar]
- Ostrovskii, V.A.; Koldobskii, G.I.; Trifonov, R.E. Tetrazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Zhdankin, V.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, pp. 259–423. [Google Scholar]
- Ostrovskii, V.A.; Popova, E.A.; Trifonov, R.E. Tetrazoles. In Comprehensive Heterocyclic Chemistry IV; Black, D., Cossy, J., Stevens, C., Eds.; Elsevier: Oxford, UK, 2022; Volume 6, pp. 182–232. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Popova, E.A.; Trifonov, R.E. Developments in tetrazole chemistry (2009–16). Adv. Heterocycl. Chem. 2017, 123, 1–62. [Google Scholar] [CrossRef]
- Popova, E.A.; Trifonov, R.E. Synthesis and biological properties of amino acids and peptides containing a tetrazolyl moiety. Russ. Chem. Rev. 2015, 84, 891–916. [Google Scholar] [CrossRef]
- Popova, E.A.; Trifonov, R.E.; Ostrovskii, V.A. Tetrazoles for biomedicine. Russ. Chem. Rev. 2019, 88, 644–676. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Trifonov, R.E.; Popova, E.A. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. 2012, 61, 768–780. [Google Scholar] [CrossRef]
- Popova, E.A.; Protas, A.V.; Trifonov, R.E. Tetrazole Derivatives as Promising Anticancer Agents. Anti-Cancer Agents Med. Chem. 2017, 17, 1856–1868. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kumar, A.; Singh, U.K. Tetrazoles: Synthesis and Biological Activity. Immunol. Endocr. Metab. Agents Med. Chem. 2018, 18, 3–21. [Google Scholar] [CrossRef]
- Myznikov, L.V.; Hrabalek, A.; Koldobskii, G.I. Drugs in the tetrazole series. Chem. Heterocycl. Compd. 2007, 43, 1–9. [Google Scholar] [CrossRef]
- Popova, E.A.; Trifonov, R.E.; Ostrovskii, V.A. Advances in the synthesis of tetrazoles coordinated to metal ions. ARKIVOC 2012, 45–65. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Kriminskaya, Z.K. Radiolysis of tetrazolium salt solutions. Russ. Chem. Rev. 1998, 67, 745–754. [Google Scholar] [CrossRef]
- Trifonov, R.E.; Alkorta, I.; Ostrovskii, V.A.; Elguero, J. A theoretical study of the tautomerism and ionization of 5-substituted NH-tetrazoles. J. Mol. Struct. THEOCHEM 2004, 668, 123–132. [Google Scholar] [CrossRef]
- Trifonov, R.E.; Ostrovskii, V.A. Protolytic equilibria in tetrazoles. Russ. J. Org. Chem. 2006, 42, 1585–1605. [Google Scholar] [CrossRef]
- Kaplanskiy, M.V.; Faizullina, O.E.; Trifonov, R.E. Experimental and Theoretical Quantitative Studies of the Hydrogen Bonding Basicity of 2,5-Disubstituted Tetrazoles and Some Related Heterocycles. J. Phys. Chem. A 2023, 127, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Groom, C.R.; Liebeschuetz, J.W.; Bardwell, D.A.; Olsson, T.S.G.; Wood, P.A. The Hydrogen Bond Environments of 1 H -Tetrazole and Tetrazolate Rings: The Structural Basis for Tetrazole–Carboxylic Acid Bioisosterism. J. Chem. Inf. Model. 2012, 52, 857–866. [Google Scholar] [CrossRef]
- Modafferi, S.; Ries, M.; Calabrese, V.; Schmitt, C.P.; Nawroth, P.; Kopf, S.; Peters, V. Clinical Trials on Diabetic Nephropathy: A CrossSectional Analysis. Diabetes Ther. 2019, 10, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef]
- Youssef, J.; Badr, M. Peroxisome Proliferator-Activated Receptors: Discovery and Recent Advances; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–137. [Google Scholar]
- Dutta, D.; Bhattacharya, S.; Surana, V.; Aggarwal, S.; Singla, R.; Khandelwal, D.; Sharma, M. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: A meta-analysis. Diabetes Metab. Syndr. 2020, 14, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Kees, K.L.; Cheesemant, R.S.; Prozialeck, D.H.; Steiner, K.E. Perfluoro-N-[4-(1H-tetrazol-5-ylmethyl)phenyl]alkanamides. A new class of oral antidiabetic agents. J. Med. Chem. 1989, 32, 11–13. [Google Scholar] [CrossRef]
- Kees, K.L.; Smith, T.M.; McCaleb, M.L.; Prozialeck, D.H.; Cheeseman, R.S.; Christos, T.E.; Patt, W.C.; Steiner, K.E. Perfluorocarbon-based antidiabetic agents. J. Med. Chem. 1992, 35, 944. [Google Scholar] [CrossRef]
- Kees, K.L.; Caggiano, T.J.; Steiner, K.E.; Fitzgerald, J.J., Jr.; Kates, M.J.; Christos, T.E.; Kulishoff, J.M.; Moore, R.D.; McCaleb, M.L. Studies on New Acidic Azoles as Glucose-Lowering Agents in Obese, Diabetic db/db Mice. J. Med. Chem. 1995, 38, 617. [Google Scholar] [CrossRef]
- Otake, K.; Azukizawa, S.; Takeda, S.; Fukui, M.; Kawahara, A.; Kitao, T.; Shirahase, H. Novel 2,7-Substituted -1,2,3,4-Tetrahydroisoquinoline-3-carboxylic Acids: Peroxisome Proliferator-Activated Receptor γ Partial Agonists with Protein–Tyrosine Phosphatase 1B Inhibition. Chem. Pharm. Bull. 2015, 63, 998–1014. [Google Scholar] [CrossRef][Green Version]
- Morishita, K.; Miike, T.; Takeda, S.; Fukui, M.; Ito, Y.; Kitao, T.; Ozawa, S.-I.; Hirono, S.; Shirahase, H. (S)-1,2,3,4-tetrahydroisoquinoiine derivatives substituted with an acidic group at the 6-position as a selective peroxisome proliferator-activated receptor γ partial agonist. Chem. Pharm. Bull. 2019, 67, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Ito, Y.; Otake, K.; Takahashi, K.; Yamamoto, M.; Kitao, T.; Ozawa, S.-I.; Hirono, S.; Shirahase, H. Synthesis and evaluation of a novel series of 2,7-substituted-6-tetrazolyl-1,2,3,4-tetrahydroisoquinoline derivatives as selective peroxisome proliferator-activated receptor γ partial agonists. Chem. Pharm. Bull. 2021, 69, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yamamoto, M.; Furukawa, S.; Fukui, M.; Morishita, K.; Kitao, T.; Shirahase, H. Effects of KY-903, a Novel Tetrazole-Based Peroxisome Proliferator-Activated Receptor γ Modulator, in Male Diabetic Mice and Female Ovariectomized Rats. Biol. Pharm. Bull. 2021, 44, 659–668. [Google Scholar] [CrossRef]
- Momose, Y.; Maekawa, T.; Odaka, H.; Ikeda, H.; Sohda, T. Novel 5-substituted-1H-tetrazole derivatives as potent glucose and lipid lowering agents. Chem. Pharm. Bull. 2002, 50, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.R.; Bhosle, M.R.; Khillare, L.D.; Dhumal, S.T.; Mishra, A.; Srivastava, A.K.; Mane, R.A. New tetrazoloquinolinyl methoxyphenyl-4-thiazolidinones: Synthesis and antihyperglycemic evaluation. Res. Chem. Intermed. 2017, 43, 1107–1120. [Google Scholar] [CrossRef]
- Sharon, A.; Pratap, R.; Tiwari, P.; Srivastava, A.; Maulik, P.R.; Ram, V.J. Synthesis and in vivo antihyperglycemic activity of 5-(1H-pyrazol-3-yl)methyl-1H-tetrazoles. Bioorg. Med. Chem. Lett. 2005, 15, 2115–2117. [Google Scholar] [CrossRef]
- da Silva, F.M.C.; dos Santos, J.C.; Campos, J.L.O.; Mafud, A.C.; Polikarpov, I.; Figueira, A.C.M.; Nascimento, A.S. Structure-based identification of novel PPAR gamma ligands. Bioorg. Med. Chem. Lett. 2013, 23, 5795–5802. [Google Scholar] [CrossRef]
- de Paula, K.; Santos, J.C.; Mafud, A.C.; Nascimento, A.S. Tetrazoles as peroxisome proliferator-activated receptor gamma ligands: A structural and computational investigation. J. Mol. Graph. Modell. 2021, 106, 107932. [Google Scholar] [CrossRef]
- Pattana, S.R.; Kekareb, P.; Patil, A.; Nikalje, A.; Kittur, B.S. Studies on the synthesis of novel 2,4-thiazolidinedione derivatives with antidiabetic activity. Iran. J. Pharm. Sci. 2009, 5, 225–230. [Google Scholar]
- Antypenko, O.M.; Kovalenko, S.I.; Zhernova, G.O. Search for compounds with hypoglycemic activity in the series of 1-[2-(1H-tetrazol-5-yl)-R1-phenyl]-3-R2-phenyl(ethyl)ureas and R1-tetrazolo[1,5-c]quinazolin-5(6H)-ones. Sci. Pharm. 2016, 84, 233–254. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Alaniz-Palacios, A.; Hidalgo-Figueroa, S.; Gonzalez-Acevedo, C.; Avila-Villarreal, G.; Estrada-Soto, S.; Webster, S.P.; Medina-Franco, J.L.; Lopez-Vallejo, F.; Guerrero-Alvarez, J.; et al. Discovery, synthesis and in combo studies of a tetrazole analogue of clofibric acid as a potent hypoglycemic agent. Bioorg. Med. Chem. Lett. 2013, 23, 3244–3247. [Google Scholar] [CrossRef]
- Vara-Gama, N.; Valladares-Mendez, A.; Navarrete-Vazquez, G.; Estrada-Soto, S.; Orozco-Castellanos, L.M.; Rivera-Leyva, J.C. Biopharmaceutical characterization and bioavailability study of a tetrazole analog of clofibric acid in rat. Molecules 2017, 22, 282. [Google Scholar] [CrossRef] [PubMed]
- Cho, H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam. Horm. 2013, 91, 405–424. [Google Scholar] [CrossRef]
- Tyurenkov, I.N.; Kurkin, D.V.; Volotova, E.V.; Bakulin, D.A.; Lomkina, E.M. Drug discovery for type 2 diabetes mellitus and metabolic syndrome: Ten novel biological targets. Diabetes Mellit. 2015, 18, 101–109. [Google Scholar] [CrossRef]
- Olloquequi, J.; Cano, A.; Sanchez-López, E.; Carrasco, M.; Verdaguer, E.; Fortuna, A.; Folch, J.; Bulló, M.O.; Auladell, C.; Camins, A.; et al. Protein tyrosine phosphatase 1B (PTP1B) as a potential therapeutic target for neurological disorders. Biomed. Pharmacother. 2022, 155, 113709. [Google Scholar] [CrossRef]
- Sun, J.; Qu, C.; Wang, Y.; Huang, H.; Zhang, M.; Zou, W. Type 2 diabetes mellitus and protein-tyrosine phosphatase 1b. J. Diabetes Metab. Disord. Control 2016, 3, 180–183. [Google Scholar] [CrossRef]
- Deshpande, T.A.; Isshak, M.; Priefer, R. PTP1B Inhibitors as Potential Target for Type II Diabetes. Curr. Res. Diabetes Obes. J. 2020, 14, 555876. [Google Scholar] [CrossRef]
- Murthy, V.S.; Kulkarni, V.M. Molecular Modeling of Protein Tyrosine Phosphatase 1B (PTP 1B) Inhibitors. Bioorg. Med. Chem. 2002, 10, 897–906. [Google Scholar] [CrossRef]
- Liljebris, C.; Larsen, S.D.; Ogg, D.; Palazuk, B.J.; Bleasdale, J.E. Investigation of Potential Bioisosteric Replacements for the Carboxyl Groups of Peptidomimetic Inhibitors of Protein Tyrosine Phosphatase 1B: Identification of a Tetrazole-Containing Inhibitor with Cellular Activity. J. Med. Chem. 2002, 45, 1785–1798. [Google Scholar] [CrossRef]
- Maheshwari, N.; Karthikeyan, C.; Bhadada, S.V.; Sahi, C.; Verma, A.K.; Hari Narayana Moorthy, N.S.; Trivedi, P. Synthesis and biological evaluation of some N-(3-(1H-tetrazol-5-yl) phenyl)acetamide derivatives as novel non-carboxylic PTP1B inhibitors designed through bioisosteric modulation. Bioorg. Chem. 2018, 80, 145–150. [Google Scholar] [CrossRef]
- Maheshwari, N.; Karthikeyan, C.; Bhadada, S.V.; Verma, A.K.; Sahi, C.; Moorthy, N.S.H.N.; Trivedi, P. Design, synthesis and biological evaluation of some tetrazole acetamide derivatives as novel non-carboxylic PTP1B inhibitors. Bioorg. Chem. 2019, 92, 103221. [Google Scholar] [CrossRef]
- Ghareb, N.; El-Sayed, N.M.; Abdelhameed, R.; Yamada, K.; Elgawish, M.S. Toward a treatment of diabesity: Rational design, synthesis and biological evaluation of benzene-sulfonamide derivatives as a new class of PTP-1B inhibitors. Bioorg. Chem. 2019, 86, 322–338. [Google Scholar] [CrossRef]
- Garcia-Marin, J.; Griera, M.; Alajarin, R.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D.; Vaquero, J.J. A Computer-Driven Scaffold-Hopping Approach Generating New PTP1B Inhibitors from the Pyrrolo[1,2-a]quinoxaline Core. ChemMedChem 2021, 16, 2895–2906. [Google Scholar] [CrossRef]
- Balestri, F.; Moschini, R.; Mura, U.; Cappiello, M.; Del Corso, A. In Search of Differential Inhibitors of Aldose Reductase. Biomolecules 2022, 12, 485. [Google Scholar] [CrossRef]
- Inukai, S.; Agata, M.; Sato, M.; Naitou, A.; Matsukawa, H.; Goto, M. Characterization of a novel aldose reductase inhibitor, TAT, and its effects of streptozotocin-induced diabetic neuropathy in rats. Jpn. J. Pharmacol. 1993, 61, 221. [Google Scholar] [CrossRef]
- Hotta, N.; Kakuta, H.; Fukasawa, H.; Koh, N.; Sakakibara, F.; Nakamura, J.; Hamada, Y.; Wakao, T.; Hara, T.; Mori, K.; et al. Effect of a potent new aldose reductase inhibitor, (5-(3-thienyl)tetrazol-1-yl)acetic acid (TAT), on diabetic neuropathy in rats. Diabetes Res. Clin. Pract. 1995, 27, 107. [Google Scholar] [CrossRef]
- Hotta, N.; Koh, N.; Sakakibara, F.; Nakamura, J.; Hara, T.; Hamada, Y.; Fukasawa, H.; Kakuta, H.; Sakamoto, N. Effect of an aldose reductase inhibitor on abnormalities of electroretinogram and vascular factors in diabetic rats. Eur. J. Pharmacol. 1997, 326, 45–51. [Google Scholar] [CrossRef]
- Nakamura, J.; Hamada, Y.; Sakakibara, F.; Hara, T.; Wakao, T.; Mori, K.; Nakashima, E.; Naruse, K.; Kamijo, M.; Koh, N.; et al. Physiological and morphometric analyses of neuropathy in sucrose-fed OLETF rats. Diabetes Res. Clin. Pract. 2001, 51, 9–20. [Google Scholar] [CrossRef]
- Nakamura, J.; Koh, N.; Sakakibara, F.; Hamada, Y.; Hara, T.; Sasaki, H.; Chaya, S.; Komori, T.; Nakashima, E.; Naruse, K.; et al. Polyol pathway hyperactivity is closely related to carnitine deficiency in the pathogenesis of diabetic neuropathy of streptozotocin-diabetic rats. J. Pharmacol. Exp. Ther. 1998, 287, 897–902. [Google Scholar]
- Pegklidou, K.; Koukoulitsa, C.; Nicolaou, I.; Demopoulos, V.J. Design and synthesis of novel series of pyrrole based chemotypes and their evaluation as selective aldose reductase inhibitors. A case of bioisosterism between a carboxylic acid moiety and that of a tetrazole. Bioorg. Med. Chem. 2010, 18, 2107–2114. [Google Scholar] [CrossRef]
- Alexiou, P.; Demopoulos, V.J. A Diverse Series of Substituted Benzenesulfonamides as Aldose Reductase Inhibitors with Antioxidant Activity: Design, Synthesis, and in vitro Activity. J. Med. Chem. 2010, 53, 7756–7766. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Gomha, S.M.; Eldebss, T.M.A.; Badrey, M.G.; Abdulla, M.M.; Mayhoub, A.S. Novel 4-Heteroaryl-Antipyrines as DPP-IV Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1292–1303. [Google Scholar] [CrossRef]
- Andrews, S.; Congreve, M.S.; Bortolato, A.; Mason, J.S. Preparation of Novel GLP-1 Receptor Agonist Peptides for Treating Diabetes Mellitus and Related Diseases. Patent GB2551945, 10 January 2018. [Google Scholar]
- Agejas Chicharro, F.J.; Bauer, R.A.; Bell, M.G.; Chen, Q.; Cumming, G.R.; Fields, T.; Gernert, D.L.; Ho, J.D.; Kaoudi, T.A.; Masquelin, T.J.; et al. Preparation of Macrocyclic Glucagon-like Peptide 1 Receptor Agonists. Patent WO2022246019, 24 November 2022. [Google Scholar]
- Li, H.; Fang, Y.; Guo, S.; Yang, Z. GPR119 agonists for the treatment of type 2 diabetes: An updated patent review (2014–present). Expert Opin. Ther. Pat. 2021, 31, 795–808. [Google Scholar] [CrossRef]
- Jones, R.M.; Leonard, J.N.; Buzard, D.J.; Lehmann, J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin. Ther. Pat. 2009, 19, 1339–1359. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, J.; Yang, L.; Liu, Y.; Wang, L.; Liu, W.; Lin, Y.; Yang, H.; Ma, L.; Ye, S.; et al. Activation and signaling mechanism revealed by GPR119-Gs complex structures. Nat. Commun. 2022, 13, 7033. [Google Scholar] [CrossRef]
- Spasov, A.A.; Kosolapov, V.A.; Babkov, D.A. Effect of GRP119 receptor agonist, compound MBX-2982, on activity of human glucokinase. Bull. Exp. Biol. Med. 2017, 163, 695–698. [Google Scholar] [CrossRef]
- Zuo, Z.; Chen, M.; Shao, X.; Qian, X.; Liu, X.; Zhou, X.; Xiang, J.; Deng, P.; Li, Y.; Jie, H.; et al. Design and biological evaluation of tetrahydropyridine derivatives as novel human GPR119 agonists. Bioorg. Med. Chem. Lett. 2020, 30, 126855. [Google Scholar] [CrossRef]
- Patil, R.I.; Gunjal, A.P.; Verma, J.; Kumar, P.; Rai, S.K.; Rai, H.; Kumar, A. Preparation of Heterocyclic Compounds as GPR119 Agonist for Treatment of Metabolic Disorders. Patent WO2017175066, 12 October 2017. [Google Scholar]
- Paternoster, S.; Simpson, P.V.; Kokh, E.; Kizilkaya, H.S.; Rosenkilde, M.M.; Mancera, R.L.; Keating, D.J.; Massi, M.; Falasca, M. Pharmacological and structure-activity relationship studies of oleoyl-lysophosphatidylinositol synthetic mimetics. Pharmacol. Res. 2021, 172, 105822. [Google Scholar] [CrossRef]
- Huang, H.; Lanter, J.C.; Meegalla, S.K.; Player, M.R. Preparation of Cyclohexyl Derivatives as GPR40 Agonists for the Treatment of Type II Diabetes. Patent WO2018081047, 3 May 2018. [Google Scholar]
- Huang, H.; Winters, M.P.; Meegalla, S.K.; Arnoult, E.; Lee, S.P.; Zhao, S.; Martin, T.; Rady, B.; Liu, J.; Towers, M.; et al. Discovery of novel benzo[b]thiophene tetrazoles as non-carboxylate GPR40 agonists. Bioorg. Med. Chem. Lett. 2018, 28, 429–436. [Google Scholar] [CrossRef]
- Meegalla, S.; Player, M.R.; Huang, H.; Winters, M.P. Preparation of Substituted Benzothiophenyl Derivatives as GPR40 Agonists for the Treatment of Type II Diabetes. U.S. Patent US20170291908, 12 October 2017. [Google Scholar]
- Staehr, P.; Hother-Nielsen, O.; Beck-Nielsen, H. Hepatic glucose production: Therapeutic target in type 2 diabetes? Diabetes Obes. Metab. 2002, 4, 215–223. [Google Scholar] [CrossRef]
- Mitchell, E.P.; Withers, S.G.; Ermert, P.; Vasella, A.T.; Garman, E.F.; Oikonomakos, N.G.; Johnson, L.N. Ternary Complex Crystal Structures of Glycogen Phosphorylase with the Transition State Analogue Nojirimycin Tetrazole and Phosphate in the T and R States. Biochemistry 1996, 35, 7341–7355. [Google Scholar] [CrossRef]
- Heightman, T.D.; Vasella, A.; Tsitsanou, K.E.; Zographos, S.E.; Skamnaki, V.T.; Oikonomakos, N.G. Cooperative Interactions of the Catalytic Nucleophile and the Catalytic Acid in the Inhibition of β-Glycosidases. Calculations and their validation by comparative kinetic and structural studies of the inhibition of glycogen phosphorylase b. Helv. Chim. Acta 1998, 81, 853–864. [Google Scholar] [CrossRef]
- Bokor, É.; Kyriakis, E.; Solovou, T.G.A.; Koppány, C.; Kantsadi, A.L.; Szabó, K.E.; Szakács, A.; Stravodimos, G.A.; Docsa, T.; Skamnaki, V.T.; et al. Nanomolar Inhibitors of Glycogen Phosphorylase Based on β-d-Glucosaminyl Heterocycles: A Combined Synthetic, Enzyme Kinetic, and Protein Crystallography Study. J. Med. Chem. 2017, 60, 9251–9262. [Google Scholar] [CrossRef]
- Somsak, L.; Nagy, V.; Hadady, Z.; Docsa, T.; Gergely, P. Glucose analog inhibitors of glycogen phosphorylases as potential antidiabetic agents: Recent developments. Curr. Pharm. Des. 2003, 9, 1177–1189. [Google Scholar] [CrossRef]
- Toth, M.; Kun, S.; Bokor, E.; Benltifa, M.; Tallec, G.; Vidal, S.; Docsa, T.; Gergely, P.; Somsak, L.; Praly, J.-P. Synthesis and structure-activity relationships of C-glycosylated oxadiazoles as inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. 2009, 17, 4773–4785. [Google Scholar] [CrossRef]
- Somsak, L.; Bokor, E.; Czifrak, K.; Konya, B.; Kun, S.; Toth, M. Glucose-analog inhibitors of glycogen phosphorylase as potential antidiabetic agents. Magy. Kem. Foly. Kem. Kozl. 2010, 116, 19–30. [Google Scholar]
- Kun, S.; Bokor, E.; Varga, G.; Szocs, B.; Pahi, A.; Czifrak, K.; Toth, M.; Juhasz, L.; Docsa, T.; Gergely, P.; et al. New synthesis of 3-(β-D-glucopyranosyl)-5-substituted-1,2,4-triazoles, nanomolar inhibitors of glycogen phosphorylase. Eur. J. Med. Chem. 2014, 76, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, P.P.; Somagond, S.M.; Bayannavar, P.K.; Kamble, R.R.; Bijjaragi, S.C.; Hunnur, R.K.; Joshi, S.D. Novel 5-(1-aryl-1H-pyrazol-3-yl)-1H-tetrazoles as glycogen phosphorylase inhibitors: An in vivo antihyperglycemic activity study. Drug Dev. Res. 2020, 81, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Onkara, P.; Kumar, P.S.V.; Pydisetty, Y.; Chandramouli, G.V.P. Ionic liquid-promoted multicomponent synthesis of fused tetrazolo[1,5-a]pyrimidines as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4007–4014. [Google Scholar] [CrossRef]
- Suwito, H.; Kurnyawaty, N.; Haq, K.U.; Ramadhan, R.; Abdulloh, A.; Hardiyanti, H.D.; Phuwapraisirisan, P. Synthesis of dihydrotetrazolopyrimidine derivatives as anticancer agents and inhibitor of α-glucosidase. Rasayan J. Chem. 2023, 16, 147–158. [Google Scholar] [CrossRef]
- He, M.; Li, Y.-J.; Shao, J.; Fu, C.; Li, Y.-S.; Cui, Z.-N. 2,5-Disubstituted furan derivatives containing imidazole, triazole or tetrazole moiety as potent α-glucosidase inhibitors. Bioorg. Chem. 2023, 131, 106298. [Google Scholar] [CrossRef] [PubMed]
- Selvarasu, S.; Srinivasan, P.; Mannathusamy, G.; Maria Susai, B. Synthesis, characterization, in silico molecular modeling, anti-diabetic and antimicrobial screening of novel 1-aryl-N-tosyl-1H-tetrazole-5-carboxamide derivatives. Chem. Data Collect. 2021, 32, 100648. [Google Scholar] [CrossRef]
- Gao, Y.L.; Zhao, G.L.; Liu, W.; Shao, H.; Wang, Y.L.; Xu, W.R.; Tang, L.D.; Wang, J.W. Design, synthesis and in vivo hypoglycemic activity of tetrazole-bearing N-glycosides as SGLT2 inhibitors. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2010, 49B, 1499–1508. [Google Scholar] [CrossRef]
- Ho, L.T.; Kulkarni, S.S.; Lee, J.C. Development of sodium-dependent glucose co-transporter 2 inhibitors as potential anti-diabetic therapeutics. Curr. Top. Med. Chem. 2011, 11, 1476–1512. [Google Scholar] [CrossRef]
- Kumari, B.; Chetia, D. In-silico docking studies of selected N-glycoside bearing tetrazole ring in the treatment of hyperglycemia showing inhibitory activity on SGLT. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 2), 633–638. [Google Scholar]
- Reddy, B.P.; Reddy, K.R.; Sharma, V.M.; Reddy, M.M.; Poshala, R. Preparation of Tetrazole Glycosides for Treating Diseases Related SGLT2 Inhibition. Patent WO2010018438, 20 January 2010. [Google Scholar]
- Liao, B.-R.; He, H.-B.; Yang, L.-L.; Gao, L.-X.; Chang, L.; Tang, J.; Li, J.-Y.; Li, J.; Yang, F. Synthesis and structure-activity relationship of non-phosphorus-based fructose-1,6-bisphosphatase inhibitors: 2,5-Diphenyl-1,3,4-oxadiazoles. Eur. J. Med. Chem. 2014, 83, 15–25. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Dong, J.; Cheng, S.; Li, G.; Wang, C.; Ouyang, D.; Leung, C.-H.; Lin, L. Artificial intelligence-aided discovery of prolyl hydroxylase 2 inhibitors to stabilize hypoxia inducible factor-1α and promote angiogenesis. Chin. Chem. Lett. 2023, 34, 107514. [Google Scholar] [CrossRef]
- Beyett, T.S.; Gan, X.; Reilly, S.M.; Chang, L.; Gomez, A.V.; Saltiel, A.R.; Showalter, H.D.; Tesmer, J.J.G. Carboxylic acid derivatives of amlexanox display enhanced potency toward TBK1 and IKKe and reveal mechanisms for selective inhibition. Mol. Pharmacol. 2018, 94, 1210–1219. [Google Scholar] [CrossRef]
- Wu, A.-D.; Lu, H.-L. A new Cu (II)-coordination polymer: Inhibiting bcl-2 gene expression in retinal cells and exerting treatment activity on diabetic retinopathy. Inorg. Nano-Met. Chem. 2021, 51, 1453–1458. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonov, R.E.; Ostrovskii, V.A. Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents. Int. J. Mol. Sci. 2023, 24, 17190. https://doi.org/10.3390/ijms242417190
Trifonov RE, Ostrovskii VA. Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents. International Journal of Molecular Sciences. 2023; 24(24):17190. https://doi.org/10.3390/ijms242417190
Chicago/Turabian StyleTrifonov, Rostislav E., and Vladimir A. Ostrovskii. 2023. "Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents" International Journal of Molecular Sciences 24, no. 24: 17190. https://doi.org/10.3390/ijms242417190
APA StyleTrifonov, R. E., & Ostrovskii, V. A. (2023). Tetrazoles and Related Heterocycles as Promising Synthetic Antidiabetic Agents. International Journal of Molecular Sciences, 24(24), 17190. https://doi.org/10.3390/ijms242417190


_Putnam.png)


