Effects of Anxious Depression on Antidepressant Treatment Response
Abstract
:1. Introduction
2. Results
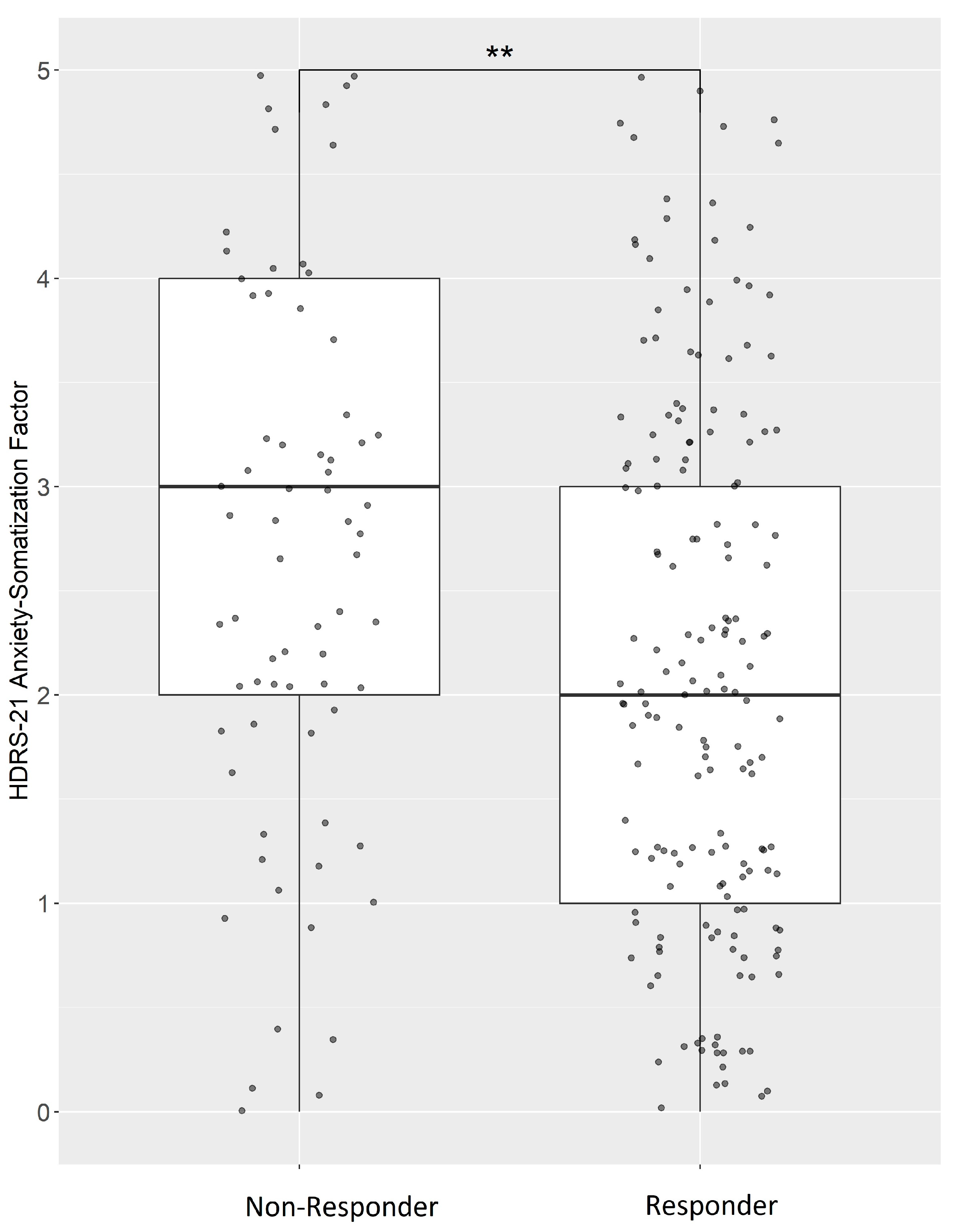
2.1. Treatment Response in Anxious Depression
2.2. Drug Doses and Serum Concentrations in Anxious Depression
2.3. Drug-Specific Treatment Response in Anxious Depression
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Definition of Anxious Depression
4.3. Definition of Treatment Response
4.4. Drug Doses and Serum Concentrations
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S.; National Comorbidity Survey, R. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Spencer, D.; Fava, M. The STAR*D study: Treating depression in the real world. Cleve Clin. J. Med. 2008, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, M.C.; Van Buel, E.M.; Bosker, F.J.; Gladkevich, A.V.; Klein, H.C.; Oude Voshaar, R.C.; Ruhe, E.G.; Eisel, U.L.; Schoevers, R.A. Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark. Med. 2015, 9, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.N.; Nguyen, N.P.K.; Nguyen, L.T.H.; Shin, H.M.; Yang, I.J. Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines 2023, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Editorial: Case reports in neuroimaging and stimulation. Front. Psychiatry 2023, 14, 1264669. [Google Scholar] [CrossRef]
- Fava, M.; Alpert, J.E.; Carmin, C.N.; Wisniewski, S.R.; Trivedi, M.H.; Biggs, M.M.; Shores-Wilson, K.; Morgan, D.; Schwartz, T.; Balasubramani, G.K.; et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol. Med. 2004, 34, 1299–1308. [Google Scholar] [CrossRef]
- Fava, M.; Rush, A.J.; Alpert, J.E.; Balasubramani, G.K.; Wisniewski, S.R.; Carmin, C.N.; Biggs, M.M.; Zisook, S.; Leuchter, A.; Howland, R.; et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A STAR*D report. Am. J. Psychiatry 2008, 165, 342–351. [Google Scholar] [CrossRef]
- Wurst, C.; Schiele, M.A.; Stonawski, S.; Weiss, C.; Nitschke, F.; Hommers, L.; Domschke, K.; Herrmann, M.J.; Pauli, P.; Deckert, J.; et al. Impaired fear learning and extinction, but not generalization, in anxious and non-anxious depression. J. Psychiatr. Res. 2021, 135, 294–301. [Google Scholar] [CrossRef]
- VanValkenburg, C.; Akiskal, H.S.; Puzantian, V.; Rosenthal, T. Anxious depressions. Clinical, family history, and naturalistic outcome—Comparisons with panic and major depressive disorders. J. Affect. Disord. 1984, 6, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Joffe, R.T.; Bagby, R.M.; Levitt, A. Anxious and nonanxious depression. Am. J. Psychiatry 1993, 150, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, R.A.; Deeg, D.J.; van Tilburg, W.; Beekman, A.T. Depression and generalized anxiety disorder: Co-occurrence and longitudinal patterns in elderly patients. Am. J. Geriatr. Psychiatry 2005, 13, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.J.; Grove, W.M.; Coryell, W.; Keller, M.; Hirschfeld, R.; Fawcett, J. Follow-up and family study of anxious depression. Am. J. Psychiatry 1991, 148, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.R.; Meoni, P.; Haudiquet, V.; Cantillon, M.; Hackett, D. Achieving remission with venlafaxine and fluoxetine in major depression: Its relationship to anxiety symptoms. Depress. Anxiety 2002, 16, 4–13. [Google Scholar] [CrossRef]
- Domschke, K.; Deckert, J.; Arolt, V.; Baune, B.T. Anxious versus non-anxious depression: Difference in treatment outcome. J. Psychopharmacol. 2010, 24, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Uebelacker, L.A.; Alpert, J.E.; Nierenberg, A.A.; Pava, J.A.; Rosenbaum, J.F. Major depressive subtypes and treatment response. Biol. Psychiatry 1997, 42, 568–576. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart’s tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatr. Scand. 2023, 148, 463–466. [Google Scholar] [CrossRef]
- Nelson, J.C. Anxious depression and response to treatment. Am. J. Psychiatry 2008, 165, 297–299. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 244–254. [Google Scholar] [CrossRef]
- Zimmerman, M.; Kerr, S.; Kiefer, R.; Balling, C.; Dalrymple, K. What is anxious depression? Overlap and agreement between different definitions. J. Psychiatr. Res. 2019, 109, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Blashfield, R.K.; Morey, L.C. The classification of depression through cluster analysis. Compr. Psychiatr. 1979, 20, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Shafer, A.B. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J. Clin. Psychol. 2006, 62, 123–146. [Google Scholar] [CrossRef] [PubMed]
- West, E.D.; Dally, P.J. Effects of iproniazid in depressive syndromes. Br. Med. J. 1959, 1, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Paykel, E.S.; Rowan, P.R.; Parker, R.R.; Bhat, A.V. Response to phenelzine and amitriptyline in subtypes of outpatient depression. Arch. Gen. Psychiatry 1982, 39, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Ravaris, C.L.; Robinson, D.S.; Ives, J.O.; Nies, A.; Bartlett, D. Phenelzine and amitriptyline in the treatment of depression. A comparison of present and past studies. Arch. Gen. Psychiatry 1980, 37, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Rosenbaum, J.F.; Hoog, S.L.; Tepner, R.G.; Kopp, J.B.; Nilsson, M.E. Fluoxetine versus sertraline and paroxetine in major depression: Tolerability and efficacy in anxious depression. J. Affect. Disord. 2000, 59, 119–126. [Google Scholar] [CrossRef]
- Tollefson, G.D.; Holman, S.L.; Sayler, M.E.; Potvin, J.H. Fluoxetine, placebo, and tricyclic antidepressants in major depression with and without anxious features. J. Clin. Psychiatry 1994, 55, 50–59. [Google Scholar]
- Nelson, J.C. Anxiety does not predict response to duloxetine in major depression: Results of a pooled analysis of individual patient data from 11 placebo-controlled trials. Depress. Anxiety 2010, 27, 12–18. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Trivedi, M.H.; Alpert, J.E.; Seifert, C.A.; Krishen, A.; Goodale, E.P.; Tucker, V.L. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: A meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J. Psychiatr. Res. 2008, 42, 134–140. [Google Scholar] [CrossRef]
- Russell, J.M.; Koran, L.M.; Rush, J.; Hirschfeld, R.M.; Harrison, W.; Friedman, E.S.; Davis, S.; Keller, M. Effect of concurrent anxiety on response to sertraline and imipramine in patients with chronic depression. Depress. Anxiety 2001, 13, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gaspersz, R.; Nawijn, L.; Lamers, F.; Penninx, B. Patients with anxious depression: Overview of prevalence, pathophysiology and impact on course and treatment outcome. Curr. Opin. Psychiatry 2018, 31, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Fava, M.; Wisniewski, S.R.; Lavori, P.W.; Trivedi, M.H.; Sackeim, H.A.; Thase, M.E.; Nierenberg, A.A.; Quitkin, F.M.; Kashner, T.M.; et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Control Clin. Trials 2004, 25, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Lichter, K.; Klupfel, C.; Stonawski, S.; Hommers, L.; Blickle, M.; Burschka, C.; Das, F.; Heissler, M.; Hellmuth, A.; Helmel, J.; et al. Deep phenotyping as a contribution to personalized depression therapy: The GEParD and DaCFail protocols. J. Neural. Transm. 2023, 130, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Rabe-Jablonska, J.; Bienkiewicz, W. Anxiety disorders in the fourth edition of the classification of mental disorders prepared by the American Psychiatric Association: Diagnostic and statistical manual of mental disorders (DMS-IV—Options book. Psychiatr. Pol. 1994, 28, 255–268. [Google Scholar] [PubMed]
- Cleary, P.; Guy, W. Factor analysis of the Hamilton depression scale. Drugs Exp. Clin. Res. 1977, 1, 115–120. [Google Scholar]
- Rush, A.J.; Kraemer, H.C.; Sackeim, H.A.; Fava, M.; Trivedi, M.H.; Frank, E.; Ninan, P.T.; Thase, M.E.; Gelenberg, A.J.; Kupfer, D.J.; et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006, 31, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, e1. [Google Scholar] [CrossRef]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://www.R-project.org/ (accessed on 1 February 2023).
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]



| N | Mean ± SD (Range) %/% | |
|---|---|---|
| Included patients | 346 | |
| Age [years] | 346 | 45.58 ± 15.31 (18–80) |
| Male/Female | 146/200 | 42.2/57.8 |
| Duration of disorder [years] | 339 | 13.62 ± 12.31 (0–60) |
| HDRS-21 Baseline | 346 | 25.06 ± 7.48 (4–46) |
| HDRS-21 Out | 340 | 10.61 ± 6.81 (0–38) |
| Responder/Nonresponder | 220/109 | 66.9/33.1 |
| HDRS anxiety-somatization factor Baseline | 342 | 6.68 ± 2.92 (0–15) |
| HDRS anxiety-somatization factor Out | 332 | 2.8 ± 2.4 (0–10) |
| aMDD/naMDD Baseline | 183/159 | 53.5/46.5 |
| aMDD/naMDD Out | 31/304 | 9.3/90.7 |
| Week 1 | Week 7 | |||||||
|---|---|---|---|---|---|---|---|---|
| Dimensional (ASF-Score) | Categorical (aMDD vs. naMDD) | Dimensional (ASF-Score) | Categorical (aMDD vs. naMDD) | |||||
| Beta | p (Adjusted) | Beta | p (Adjusted) | Beta | p (Adjusted) | Beta | p (Adjusted) | |
| Treatment response in anxious depression | ||||||||
| Complete sample | 0.05 | 0.27 | −0.04 | 0.87 | −0.32 | 4.67 × 10−8 (1.21 × 10−6) | −2.96 | 6.78 × 10−7 (1.76 × 10−5) |
| Drug doses in anxious depression | ||||||||
| Amitriptyline | 0.01 | 0.30 (1) | 0.01 | 0.44 (1) | −0.02 | 0.07 (1) | −0.01 | 0.26 (1) |
| Mirtazapine | 0.02 | 0.53 (1) | 0.00 | 0.92 (1) | −0.01 | 0.79 (1) | −0.02 | 0.63 (1) |
| Sertraline | −0.02 | 0.19 (1) | −0.02 | 0.13 (1) | −0.01 | 0.31 (1) | −0.27 | 1.00 (1) |
| Venlafaxine | 0.00 | 0.17 (1) | 0.00 | 0.09 (1) | 0.00 | 0.92 (1) | 0.00 | 0.95 (1) |
| Serum concentrations in anxious depression | ||||||||
| Amitriptyline | 0.01 | 0.41 (1) | 0.00 | 0.60 (1) | 0.01 | 0.31 (1) | 0.01 | 0.51 (1) |
| Mirtazapine | −0.01 | 0.69 (1) | 0.00 | 0.86 (1) | 0.00 | 0.88 (1) | 0.01 | 0.47 (1) |
| Sertraline | 0.02 | 0.30 (1) | 0.18 | 0.48 (1) | 0.02 | 0.03 (0.72) | 0.03 | 0.02 (0.62) |
| Venlafaxine | 0.00 | 0.34 (1) | 0.00 | 0.09 (1) | 0.00 | 0.09 (1) | 0.00 | 0.21 (1) |
| Drug-specific treatment response in anxious depression | ||||||||
| Amitriptyline | 0.08 | 0.45 (1) | −0.26 | 0.66 (1) | −0.30 | 6.98 × 10−3 (0.18) | −1.99 | 0.03 (1) |
| Mirtazapine | 0.05 | 0.49 (1) | 0.31 | 0.53 (1) | −0.53 | 1.76 × 10−4 (4.58 × 10−3) | −20.86 | 0.99 (1) |
| Sertraline | 0.03 | 0.87 (1) | 0.64 | 0.55 (1) | −0.55 | 0.05 (1) | −21.12 | 1.00 (1) |
| Venlafaxine | 0.03 | 0.72 (1) | −0.53 | 0.20 (1) | −0.40 | 7.98 × 10−6 (2.07 × 10−4) | −3.11 | 2.89 × 10−4 (7.51 × 10−3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hampf, C.; Scherf-Clavel, M.; Weiß, C.; Klüpfel, C.; Stonawski, S.; Hommers, L.; Lichter, K.; Erhardt-Lehmann, A.; Unterecker, S.; Domschke, K.; et al. Effects of Anxious Depression on Antidepressant Treatment Response. Int. J. Mol. Sci. 2023, 24, 17128. https://doi.org/10.3390/ijms242417128
Hampf C, Scherf-Clavel M, Weiß C, Klüpfel C, Stonawski S, Hommers L, Lichter K, Erhardt-Lehmann A, Unterecker S, Domschke K, et al. Effects of Anxious Depression on Antidepressant Treatment Response. International Journal of Molecular Sciences. 2023; 24(24):17128. https://doi.org/10.3390/ijms242417128
Chicago/Turabian StyleHampf, Chantal, Maike Scherf-Clavel, Carolin Weiß, Catherina Klüpfel, Saskia Stonawski, Leif Hommers, Katharina Lichter, Angelika Erhardt-Lehmann, Stefan Unterecker, Katharina Domschke, and et al. 2023. "Effects of Anxious Depression on Antidepressant Treatment Response" International Journal of Molecular Sciences 24, no. 24: 17128. https://doi.org/10.3390/ijms242417128
APA StyleHampf, C., Scherf-Clavel, M., Weiß, C., Klüpfel, C., Stonawski, S., Hommers, L., Lichter, K., Erhardt-Lehmann, A., Unterecker, S., Domschke, K., Kittel-Schneider, S., Menke, A., Deckert, J., & Weber, H. (2023). Effects of Anxious Depression on Antidepressant Treatment Response. International Journal of Molecular Sciences, 24(24), 17128. https://doi.org/10.3390/ijms242417128






