Benzodiazepine Delorazepam Induces Locomotory Hyperactivity and Alterations in Pedal Mucus Texture in the Freshwater Gastropod Planorbarius corneus
Abstract
:1. Introduction
2. Results
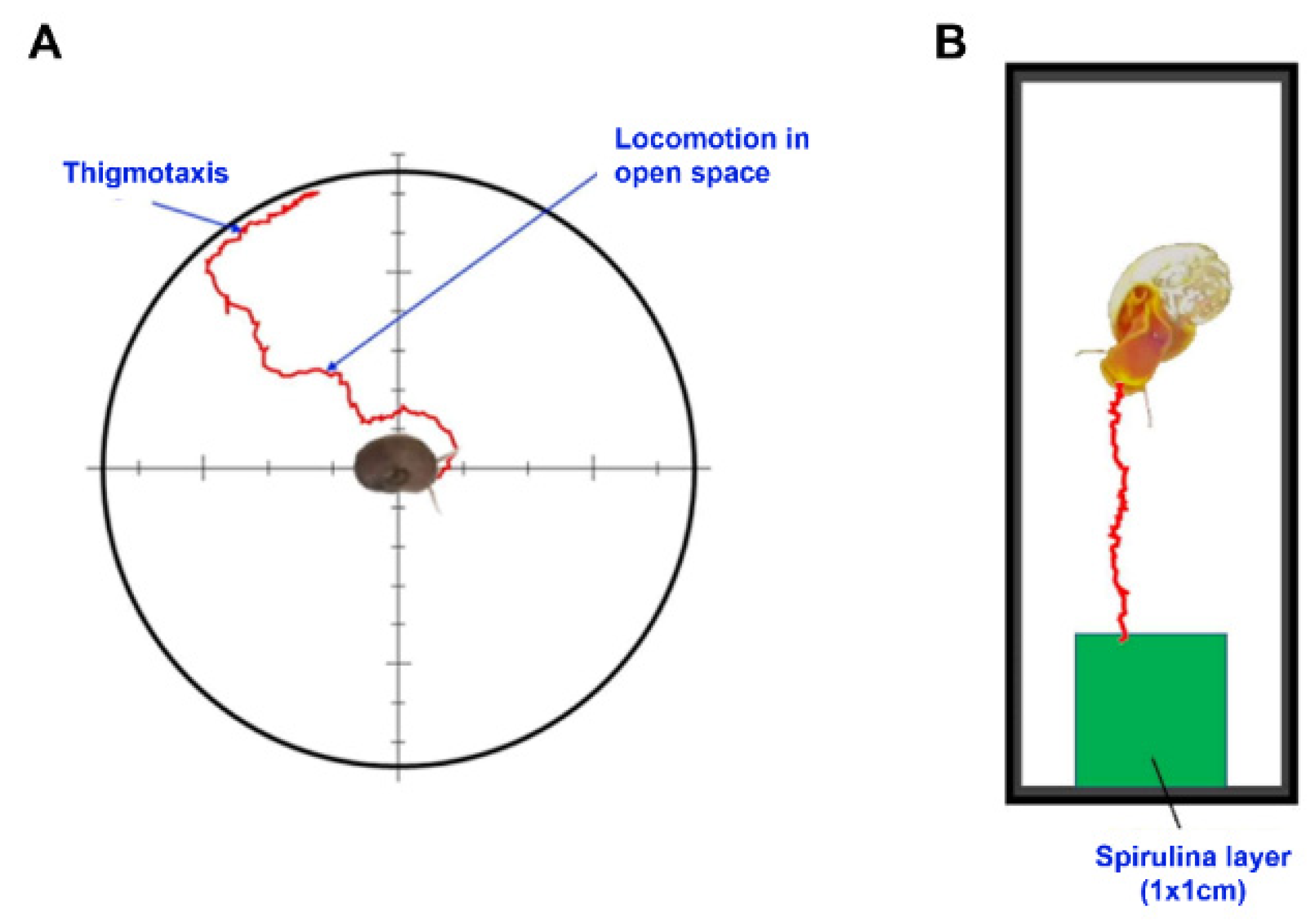
2.1. Effects of Delorazepam on Locomotor Activity
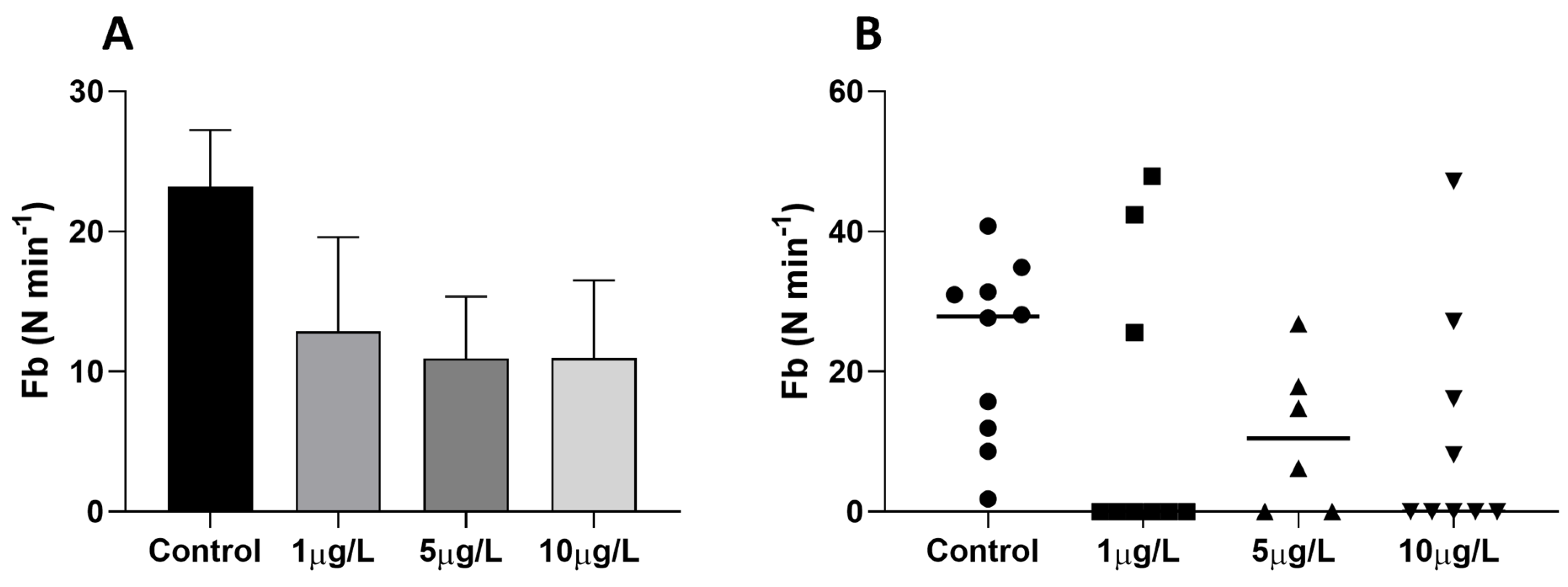
2.2. Effects of Delorazepam on Feeding Behavior Response
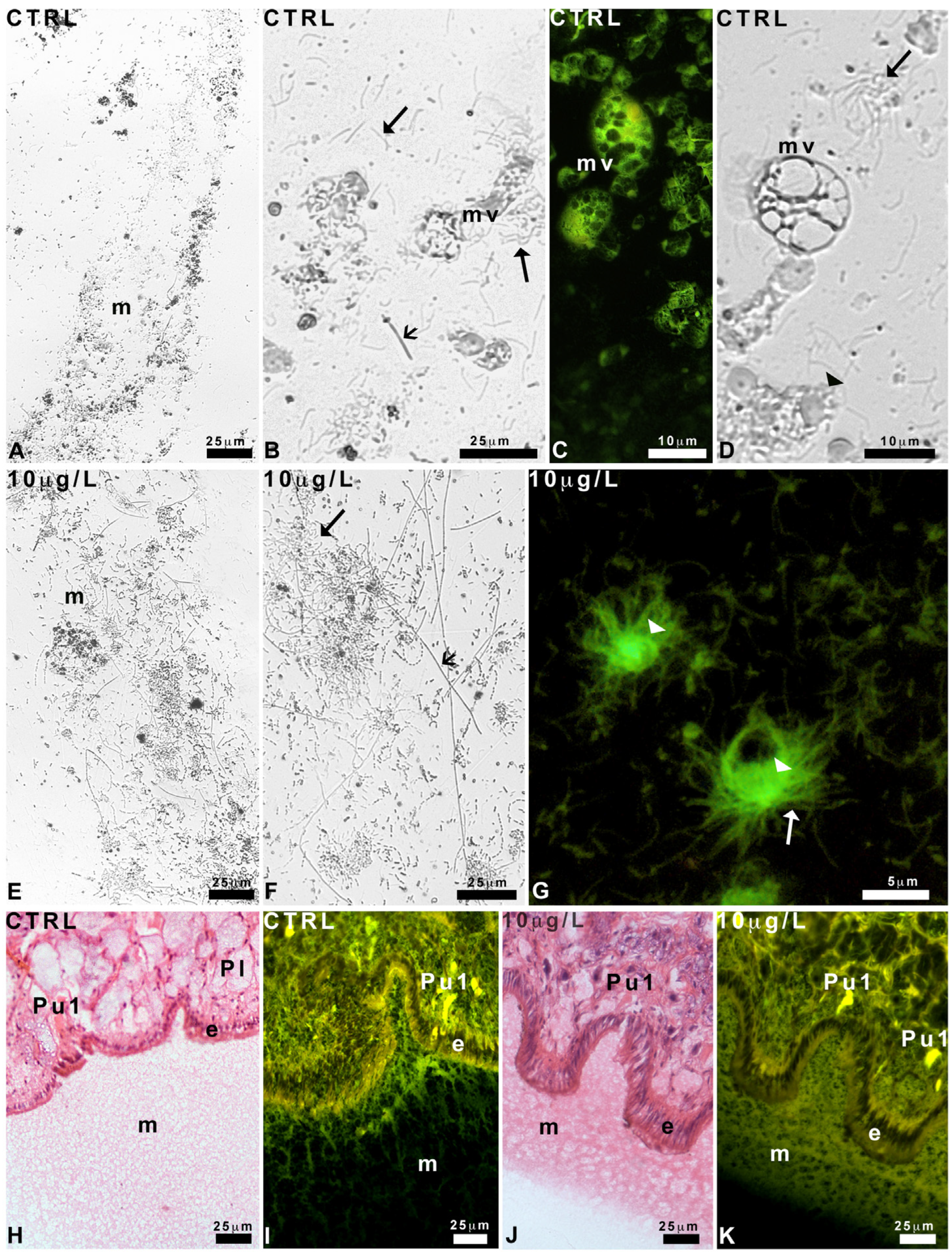
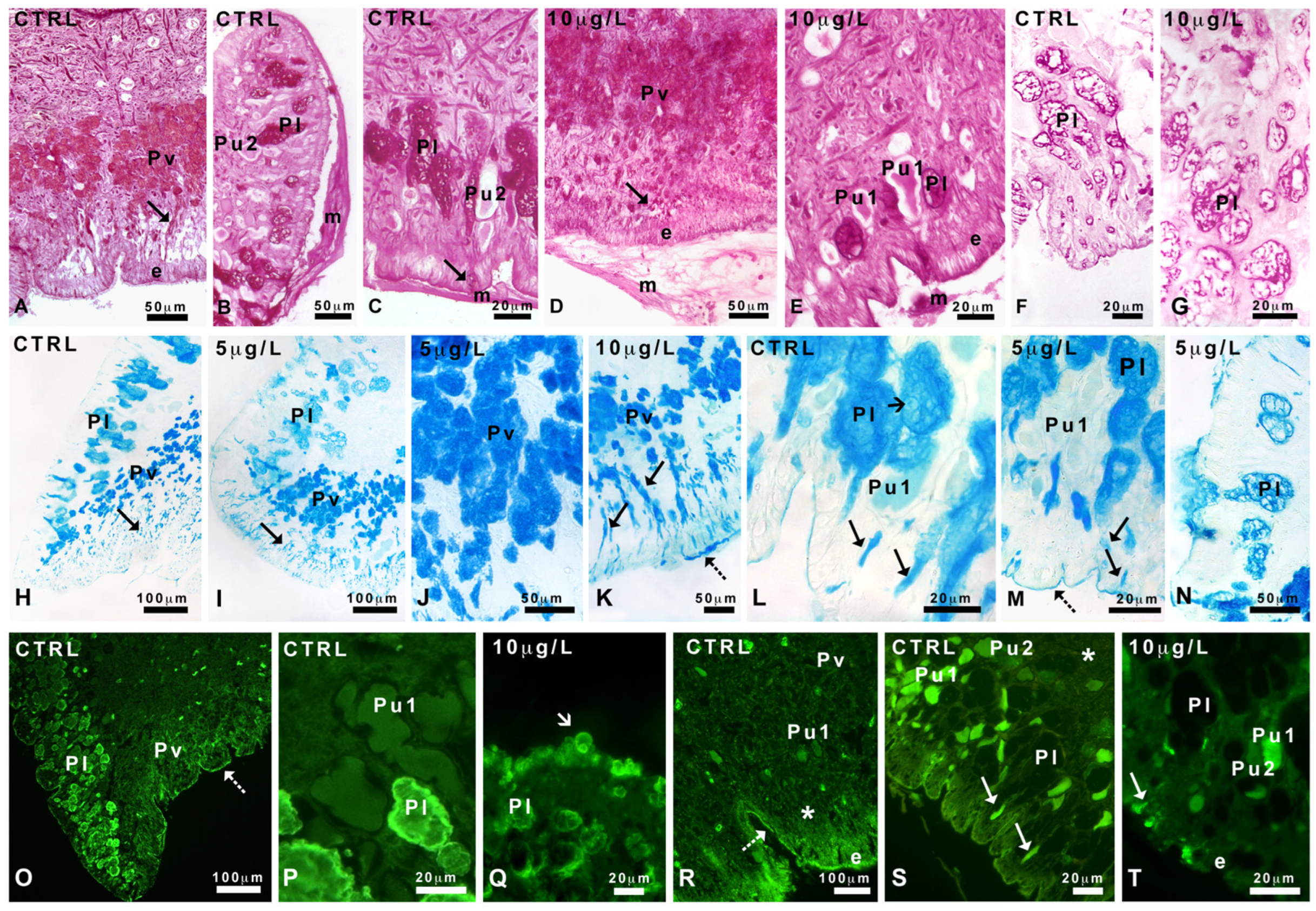
2.3. Effects of Delorazepam on Mucus Texture
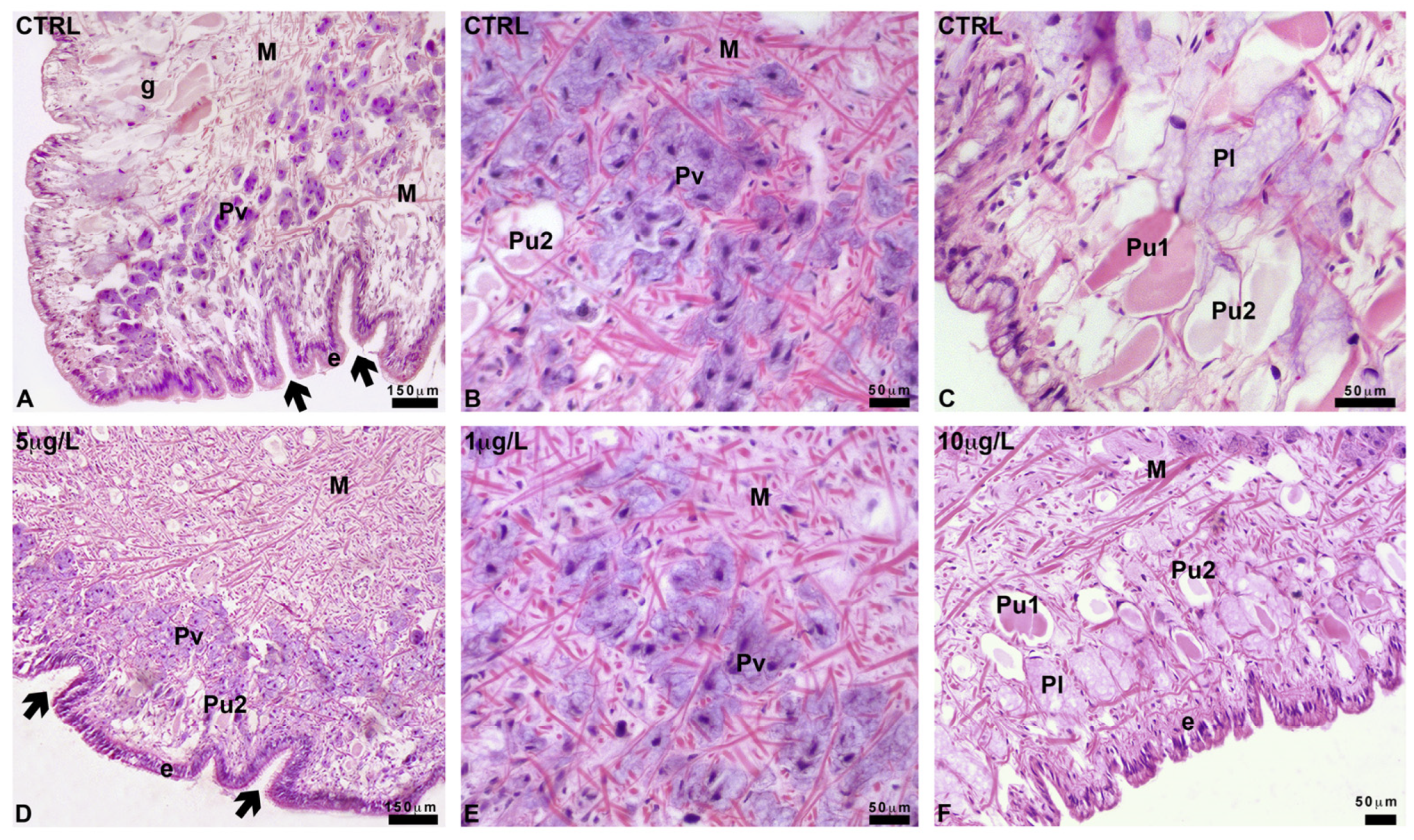
2.4. Pedal Glands and Role in Mucus Release
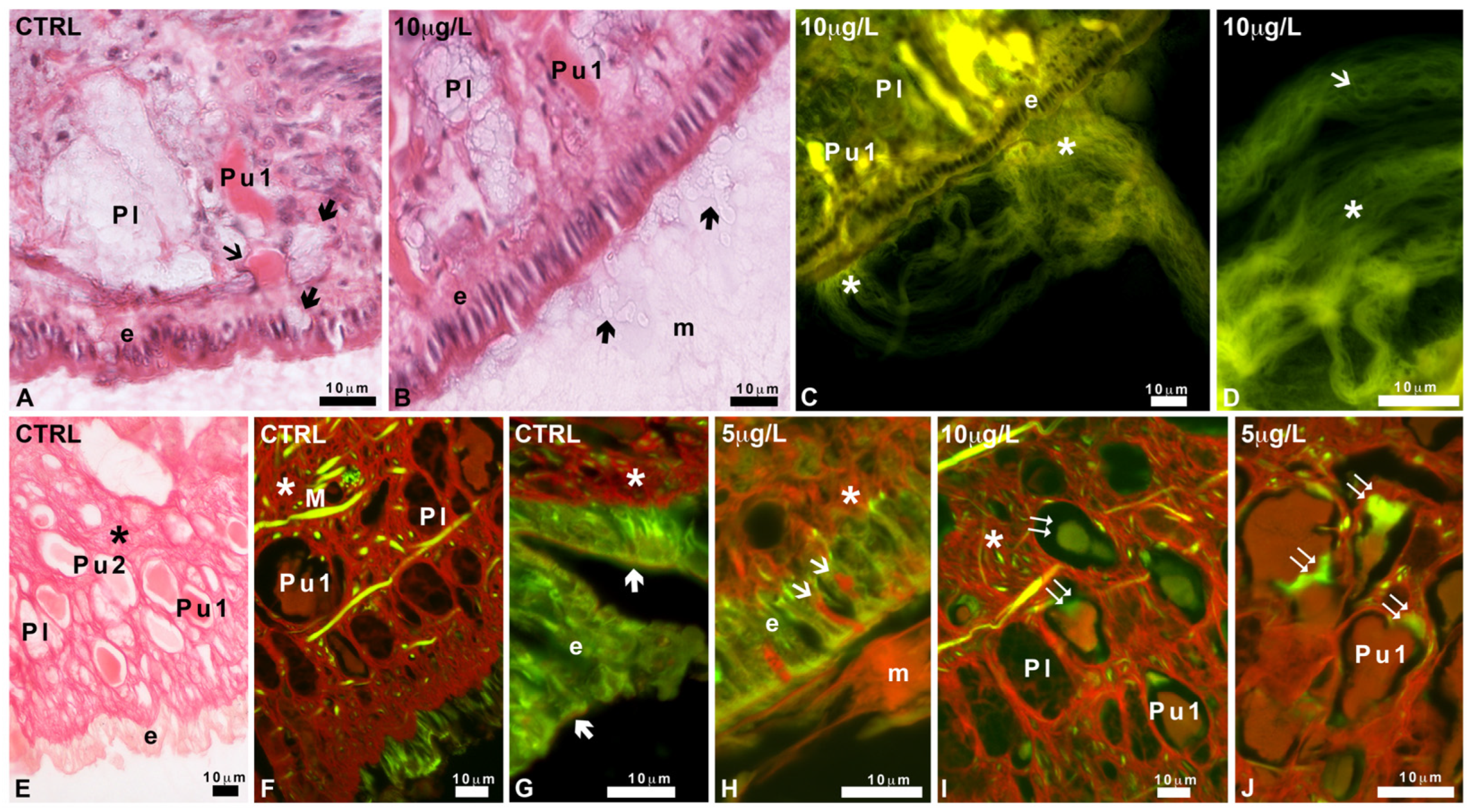
2.5. Characterization of Mucus Glycoconjugates
2.5.1. Pas and Alcian Staining
2.5.2. Staining with FITC-Lectins
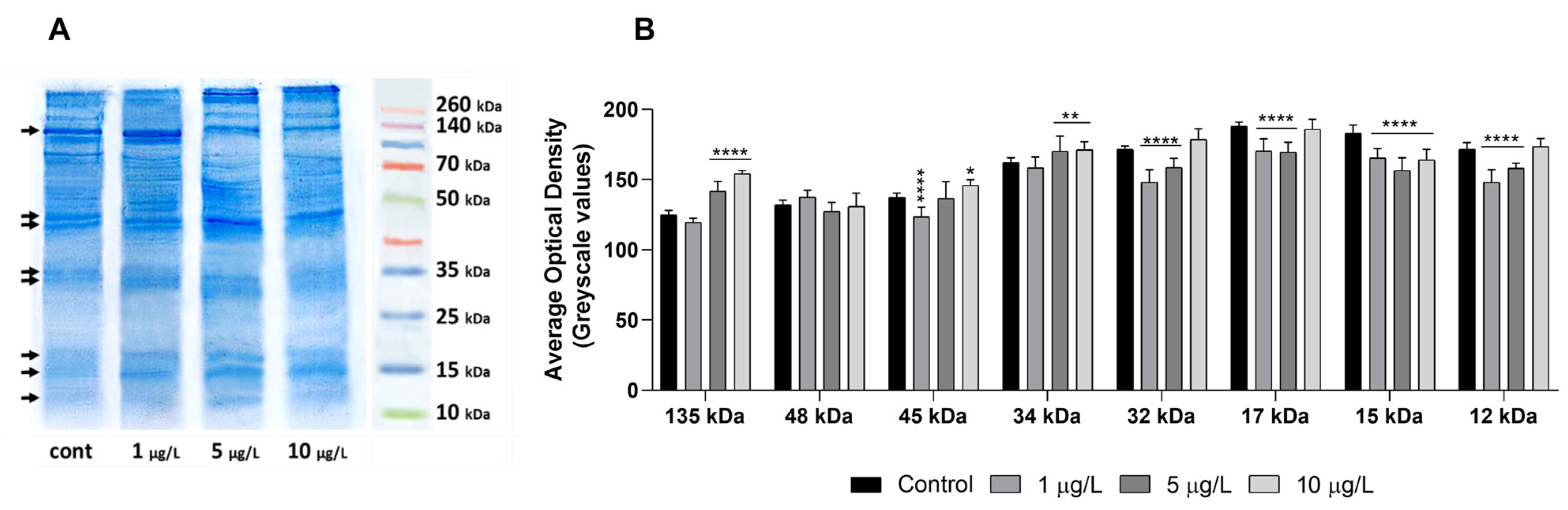
2.6. Effects of DLZ on Foot Protein Pattern
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance and Treatment
4.2. Thigmotactic Response (TR)
4.3. Food Search Test
4.4. Video Analysis and Speed Determination
4.5. Mucus Collection
4.6. Histological Analysis
4.7. Protein Analysis by SDS-PAGE
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, R.T.; Tyrer, P. Benzodiazepine dependence. A review of the evidence. Drugs 1983, 25, 385–398. [Google Scholar] [CrossRef]
- The Medicines Utilisation Monitoring Centre. National Report on Medicines use in Italy. Year 2018; Italian Medicines Agency: Rome, Italy, 2019. Available online: https://www.aifa.gov.it/documents/20142/o/Rapporto_OsMed_2018.pdf (accessed on 1 November 2023).
- Calisto, V.; Domingues, M.R.; Esteves, V.I. Photodegradation of psychiatric pharmaceuticals in aquatic environments—Kinetics and photodegradation products. Water Res. 2011, 45, 6097–6106. [Google Scholar] [CrossRef]
- Fick, J.; Brodin, T.; Heynen, M.; Klaminder, J.; Jonsson, M.; Grabicova, K.; Randak, T.; Grabic, R.; Kodes, V.; Slobodnik, J.; et al. Screening of benzodiazepines in thirty European rivers. Chemosphere 2017, 176, 324–332. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef]
- Kosjek, T.; Perko, S.; Zupanc, M.; Zanoški Hren, M.; Landeka Dragičević, T.; Zigon, D.; Kompare, B.; Heath, E. Environmental occurrence, fate and transformation of benzodiazepines in water treatment. Water Res. 2012, 46, 355–368. [Google Scholar] [CrossRef]
- Mendoza, A.; Rodríguez-Gil, J.L.; González-Alonso, S.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Valcárcel, Y. Drugs of abuse and benzodiazepines in the Madrid Region (Central Spain): Seasonal variation in river waters, occurrence in tap water and potential environmental and human risk. Environ. Int. 2014, 70, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, J.; Rodríguez-Gil, J.L.; Postigo, C.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Valcárcel, Y. Psychoactive pharmaceuticals and illicit drugs in coastal waters of North-Western Spain: Environmental exposure and risk assessment. Chemosphere 2019, 224, 379–389. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; de Araujo, F.G.; Marques, M. Psychoactive drugs: Occurrence in aquatic environment, analytical methods, and ecotoxicity—A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 24076–24091. [Google Scholar] [CrossRef] [PubMed]
- Calisto, V.; Esteves, V.I. Psychiatric pharmaceuticals in the environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef]
- Gomez, E.; Bachelot, M.; Boillot, C.; Munaron, D.; Chiron, S.; Casellas, C.; Fenet, H. Bioconcentration of two pharmaceuticals (benzodiazepines) and two personal care products (UV filters) in marine mussels (Mytilus galloprovincialis) under controlled laboratory conditions. Environ. Sci. Pollut. Res. Int. 2011, 19, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Klaminder, J.; Brodin, T.; Sundelin, A.; Anderson, N.J.; Fahlman, J.; Jonsson, M.; Fick, J. Long-Term Persistence of an Anxiolytic Drug (Oxazepam) in a Large Freshwater Lake. Environ. Sci. Technol. 2015, 49, 10406–10412. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Castiglioni, S.; Fanelli, R.; Reitano, G.; Bagnati, R.; Chiabrando, C.; Pomati, F.; Rossetti, C.; Calamari, D. Pharmaceuticals in the environment in Italy: Causes, occurrence, effects and control. Environ. Sci. Pollut. Res. Int. 2006, 13, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Darlison, M.G.; Pahal, I.; Thode, C. Consequences of the evolution of the GABA(A) receptor gene family. Cell. Mol. Neurobiol. 2005, 25, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Verginica, S.; Mihaela, I.I.; Pavalanche, G. Effects of diazepam on biotester organisms. Turk. J. Health Sci. Life 2018, 1, 1–6. [Google Scholar]
- Lebreton, M.; Sire, S.; Carayon, J.L.; Malgouyres, J.M.; Vignet, C.; Géret, F.; Bonnafé, E. Low concentrations of oxazepam induce feeding and molecular changes in Radix balthica juveniles. Aquat. Toxicol. 2021, 230, 105694. [Google Scholar] [CrossRef]
- Genario, R.; Giacomini, A.C.V.V.; de Abreu, M.S.; Marcon, L.; Demin, K.A.; Kalueff, A.V. Sex differences in adult zebrafish anxiolytic-like responses to diazepam and melatonin. Neurosci. Lett. 2020, 714, 134548. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental concentrations of a delorazepam-based drug impact on embryonic development of non-target Xenopus laevis. Aquat. Toxicol. 2022, 250, 106244. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Nunes, B. Effects of paracetamol on the polychaete Hediste diversicolor: Occurrence of oxidative stress, cyclooxygenase inhibition and behavioural alterations. Environ. Sci. Pollut. Res. Int. 2021, 28, 26772–26783. [Google Scholar] [CrossRef]
- Belal, I.E.H.; Assem, H. Pharmacological Mechanisms of Diazepam in Fish: Effect on Growth. J. Environ. Sci. Eng. 2011, 5, 1363–1369. [Google Scholar]
- Fogliano, C.; Carotenuto, R.; Panzuto, R.; Spennato, V.; De Bonis, S.; Simoniello, P.; Raggio, A.; Avallone, B.; Agnisola, C.; Motta, C.M. Behavioral alterations and gills damage in Mytilus galloprovincialis exposed to an environmental concentration of delorazepam. Environ. Toxicol. Pharmacol. 2022, 97, 104030. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Casellas, P.; Galiegue, S.; Basile, A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002, 40, 475–486. [Google Scholar] [CrossRef]
- Lang, S. The role of peripheral benzodiazepine receptors (PBRs) in CNS pathophysiology. Curr. Med. Chem. 2002, 9, 1411–1415. [Google Scholar] [CrossRef]
- Rivadeneira, P.R.; Agrelo, M.; Otero, S.; Kristoff, G. Different effects of subchronic exposure to low concentrations of the organophosphate insecticide chlorpyrifos in a freshwater gastropod. Ecotoxicol. Environ. Saf. 2013, 90, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Agrelo, M.; Rivadeneira, P.R.; Cossi, P.F.; Cacciatore, L.C.; Kristoff, G. Azinphos-methyl causes in Planorbarius corneus toxic effects on reproduction, offspring survival and B-esterases depending on the exposure time. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2019, 217, 114–121. [Google Scholar] [CrossRef]
- Laimou-Geraniou, M.; Heath, D.; Heath, E. Analytical methods for the determination of antidepressants, antipsychotics, benzodiazepines and their metabolites through wastewater-based epidemiology. Trends Environ. Anal. Chem. 2023, 37, e00192. [Google Scholar] [CrossRef]
- Gent, L.; Paul, R. The detection of new psychoactive substances in wastewater. A comprehensive review of analytical approaches and global trends. Sci. Total Environ. 2021, 776, 146028. [Google Scholar] [CrossRef]
- Sarangi, A.; McMahon, T.; Gude, J. Benzodiazepine Misuse: An Epidemic Within a Pandemic. Cureus 2021, 13, e15816. [Google Scholar] [CrossRef]
- de Dios, C.; Fernandes, B.S.; Whalen, K.; Bandewar, S.; Suchting, R.; Weaver, M.F.; Selvaraj, S. Prescription fill patterns for benzodiazepine and opioid drugs during the COVID-19 pandemic in the United States. Drug Alcohol Depend. 2021, 229 Pt A, 109176. [Google Scholar] [CrossRef]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Martin, J.R. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J. Neurobiol. 2005, 62, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.A.; Parker, M.O.; Ford, A.T. Shape and size of the arenas affect amphipod behaviours: Implications for ecotoxicology. PeerJ 2018, 6, e5271. [Google Scholar] [CrossRef]
- Laurent Salazar, M.-O.; Planas-Sitjà, I.; Sempo, G.; Deneubourg, J.-L. Individual Thigmotactic Preference Affects the Fleeing Behavior of the American Cockroach (Blattodea: Blattidae). J. Insect Sci. 2018, 18, 9. [Google Scholar] [CrossRef]
- Wright, P.A.; Turko, A.J. Amphibious fishes: Evolution and phenotypic plasticity. J. Exp. Biol. 2016, 219 Pt 15, 2245–2259. [Google Scholar] [CrossRef]
- Treit, D.; Fundytus, M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 1988, 31, 959–962. [Google Scholar] [CrossRef]
- Jethva, S.; Liversage, K.; Kundu, R. Does topography of rocky intertidal habitat affect aggregation of cerithiid gastropods and co-occurring macroinvertebrates? Oceanologia 2022, 64, 387–395. [Google Scholar] [CrossRef]
- Moisez, E.; Seuront, L. Deciphering the known unknowns in the behavioural ecology of the intertidal gastropod Littorina littorea. J. Exp. Mar. Biol. Ecol. 2020, 524, 151313. [Google Scholar] [CrossRef]
- Denny, M. The role of gastropod pedal mucus in locomotion. Nature 1980, 285, 160–161. [Google Scholar] [CrossRef]
- Davies, M.S.; Hawkins, S.J. Mucus from Marine Molluscs. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1–71. [Google Scholar]
- Iwamoto, M.; Ueyama, D.; Kobayashi, R. The advantage of mucus for adhesive locomotion in gastropods. J. Theor. Biol. 2014, 353, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, V.; Lengerer, B.; Wattiez, R.; Flammang, P. Molecular insights into the powerful mucus-based adhesion of limpets (Patella vulgata L.). Open Biol. 2020, 10, 200019. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, A.R.; McDermott, M.B.; Pepi, L.E.; Liu, Z.L.; Barry, D.; Zhang, S.; Yang, X.; Chen, X.; Azadi, P.; Holford, M.; et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum Secretions. Nat. Commun. 2023, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. J. Morphol. Sci. 2017, 22, 97–104. [Google Scholar]
- Dalesman, S.; Lukowiak, K. Effect of acute exposure to low environmental calcium on respiration and locomotion in Lymnaea stagnalis (L.). J. Exp. Biol. 2010, 213 Pt 9, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Bury, T.B.; Dworkin-Brodsky, A.D.; Jasion, C.M.; Kell, R.C. The antidepressants venlafaxine (“Effexor”) and fluoxetine (“Prozac”) produce different effects on locomotion in two species of marine snail, the oyster drill (Urosalpinx cinerea) and the starsnail (Lithopoma americanum). Mar. Environ. Res. 2015, 103, 89–94. [Google Scholar] [CrossRef]
- Domenici, P.; Torres, R.; Manríquez, P.H. Effects of elevated carbon dioxide and temperature on locomotion and the repeatability of lateralization in a keystone marine mollusc. J. Exp. Biol. 2017, 220 Pt 4, 667–676. [Google Scholar] [CrossRef]
- Ziegler, M.; Eckstein, H.; Köhler, H.-R.; Tisler, S.; Zwiener, C.; Triebskorn, R. Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus). Water 2021, 13, 1722. [Google Scholar] [CrossRef]
- Bose, A.P.; Brodin, T.; Cerveny, D.; McCallum, E.S. Uptake, depuration, and behavioural effects of oxazepam on activity and foraging in a tropical snail (Melanoides tuberculata). Environ. Adv. 2022, 8, 100187. [Google Scholar] [CrossRef]
- Argyropoulos, S.V.; Nutt, D.J. The use of benzodiazepines in anxiety and other disorders. Eur. Neuropsychopharmacol. 1999, 9, S407–S412. [Google Scholar] [CrossRef]
- Stewart, P.; Williams, E.A.; Stewart, M.J.; Soonklang, N.; Degnan, S.M.; Cummins, S.F.; Hanna, P.J.; Sobhon, P. Characterization of a GABAA receptor β subunit in the abalone Haliotis asinina that is upregulated during larval development. J. Exp. Mar. Biol. Ecol. 2011, 410, 53–60. [Google Scholar] [CrossRef]
- Lambshead, P.J.D.; Schalk, P.H. Invertebrates, marine, overview. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 543–559. [Google Scholar]
- Romanova, E.; Rubakhin, S.; Rozsa, K. Behavioural changes induced by GABA-receptor agonists in Lymnaea stagnalis L. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Orlovsky, G.N. Control of locomotion in the freshwater snail Planorbis corneus ii. differential control of various zones of the ciliated epithelium. J. Exp. Biol. 1990, 152, 405–423. [Google Scholar] [CrossRef]
- Arshavsky, Y.I.; Deliagina, T.G.; Meizerov, E.S.; Orlovsky, G.N. Control of feeding movements in the freshwater snail Planorbis corneus. I. Rhythmical neurons of buccal ganglia. Exp. Brain Res. 1988, 70, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Paton, C. Benzodiazepines and disinhibition: A review. Psychiatr. Bull. 2002, 26, 460–462. [Google Scholar] [CrossRef]
- Jones, K.A.; Nielsen, S.; Bruno, R.; Frei, M.; Lubman, D.I. Benzodiazepines—Their role in aggression and why GPs should prescribe with caution. Aust. Fam. Physician 2011, 40, 862–865. [Google Scholar]
- van der Bijl, P.; Roelofse, J.A. Disinhibitory reactions to benzodiazepines: A review. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1991, 49, 519–523. [Google Scholar] [CrossRef]
- Benjamin, P.R. Distributed network organization underlying feeding behavior in the mollusk Lymnaea. Neural Syst. Circuits 2012, 2, 4. [Google Scholar] [CrossRef]
- Cropper, E.C.; Evans, C.G.; Hurwitz, I.; Jing, J.; Proekt, A.; Romero, A.; Rosen, S.C. Feeding neural networks in the mollusc Aplysia. Neurosignals 2004, 13, 70–86. [Google Scholar] [CrossRef]
- Arshavsky, Y.I.; Deliagina, T.G.; Gamkrelidze, G.N.; Orlovsky, G.N.; Panchin, Y.V.; Popova, L.B.; Shupliakov, O.V. Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusk Clione limacina. I. Effects of GABA. J. Neurophysiol. 1993, 69, 512–521. [Google Scholar] [CrossRef]
- Arshavsky, Y.L.; Gamkrelidze, G.N.; Orlovsky, G.N.; Panchin, Y.V.; Popova, L.B. Gamma-aminobutyric acid induces feeding behaviour in the marine mollusk, Clione limacina. Neuroreport 1991, 2, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, N.L.; Zhukov, V.V. Multiplicity of chemical mechanisms of regulation of muscle contractions in Lymnaea stagnalis L. J. Evol. Biochem. Phys. 2005, 41, 54–62. [Google Scholar] [CrossRef]
- Lecci, A.; Santicioli, P.; Maggi, C.A. Pharmacology of transmission to gastrointestinal muscle. Curr. Opin. Pharmacol. 2002, 2, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Trueman, E.R.; Hodgson, A.N. The fine structure and function of the foot of Nassarius kraussianus, a gastropod moving by ciliary locomotion. J. Molluscan Stud. 1990, 56, 221–228. [Google Scholar] [CrossRef]
- Tonar, Z.; Markoš, A. Microscopy and morphometry of integument of the foot of pulmonate gastropods Arion rufus and Helix pomatia. Acta Vet. Brno 2004, 73, 3–8. [Google Scholar] [CrossRef]
- Jung, G.K.; Park, J.J.; Lee, J.W.; Lee, J.S. Microscopic anatomy of foot of the spiny top shell, Batillus cornutus (lightfoot, 1786) (Gastropoda: Turbinidae). J. Shellfish. Res. 2006, 25, 1071–1077. [Google Scholar]
- Pavlova, G.A. Correlation between the sole shape and the locomotor speed in the pulmonate snails Helix lucorum and Helix pomatia. Zh. Evol. Biokhim. Fiziol. 1994, 26, 702–706. [Google Scholar]
- Greistorfer, S.; Klepal, W.; Cyran, N.; Gugumuck, A.; Rudoll, L.; Suppan, J.; von Byern, J. Snail mucus—Glandular origin and composition in Helix pomatia. Zoology 2017, 122, 126–138. [Google Scholar] [CrossRef]
- Motta, C.M.; Califano, E.; Scudiero, R.; Avallone, B.; Fogliano, C.; De Bonis, S.; Raggio, A.; Simoniello, P. Effects of Cadmium Exposure on Gut Villi in Danio rerio. Int. J. Mol. Sci. 2022, 23, 1927. [Google Scholar] [CrossRef]
- Avallone, B.; Agnisola, C.; Cerciello, R.; Panzuto, R.; Simoniello, P.; Cretì, P.; Motta, C.M. Structural and functional changes in the zebrafish (Danio rerio) skeletal muscle after cadmium exposure. Cell Biol. Toxicol. 2015, 31, 273–283. [Google Scholar] [CrossRef]
- Waluga-Kozlowska, E.; Jasik, K.P.; Wcisło-Dziadecka, D.; Pol, P.; Kuźnik-Trocha, K.; Komosińska-Vassev, K.; Olczyk, K.Z.; Waluga, M.; Olczyk, P.; Zimmermann, A. Snail mucus: A natural origin substance with potential use in medicine. Acta Pol. Pharm. 2021, 78, 793–800. [Google Scholar] [CrossRef]
- Zhong, T.; Min, L.; Wang, Z.; Zhang, F.; Zuo, B. Controlled self-assembly of glycoprotein complex in snail mucus from lubricating liquid to elastic fiber. RSC Adv. 2018, 8, 13806–13812. [Google Scholar] [CrossRef]
- Manjunatha, B.S.; Agrawal, A.; Shah, V. Histopathological evaluation of collagen fibers using picrosirius red stain and polarizing microscopy in oral squamous cell carcinoma. J. Cancer Res. Ther. 2015, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Suhre, M.H.; Gertz, M.; Steegborn, C.; Scheibel, T. Structural and functional features of a collagen-binding matrix protein from the mussel byssus. Nat. Commun. 2014, 5, 3392. [Google Scholar] [CrossRef]
- Söderström, K.O. Lectin binding to collagen strands in histologic tissue sections. Histochemistry 1987, 87, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Jürgensen, H.J.; Madsen, D.H.; Ingvarsen, S.; Melander, M.C.; Gårdsvoll, H.; Patthy, L.; Engelholm, L.H.; Behrendt, N. A novel functional role of collagen glycosylation: Interaction with the endocytic collagen receptor uparap/ENDO180. J. Biol. Chem. 2011, 286, 32736–32748. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Bettiol, A.; Crescioli, G.; Ravaldi, C.; Bonaiuti, R.; Venegoni, M.; Vighi, G.D.; Mugelli, A.; Mannaioni, G.; Vannacci, A.; et al. Risk of hospitalisation associated with benzodiazepines and z-drugs in Italy: A nationwide multicentre study in emergency departments. Intern. Emerg. Med. 2020, 15, 1291–1302. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Acloque, H.; Avallone, B.; Carotenuto, R. Water contamination by delorazepam induces epigenetic defects in the embryos of the clawed frog Xenopus laevis. Sci. Total Environ. 2023, 896, 165300. [Google Scholar] [CrossRef]
- Croll, R.P. Gastropod chemoreception. Biol. Rev. 1983, 58, 293–319. [Google Scholar] [CrossRef]
- Motta, C.M.; Frezza, V.; Simoniello, P. Caspase 3 in molluscan tissues: Localization and possible function. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 548–559. [Google Scholar] [CrossRef]
- Motta, C.M.; Tizzano, M.; Tagliafierro, A.M.; Simoniello, P.; Panzuto, R.; Esposito, L.; Migliaccio, V.; Rosati, L.; Avallone, B. Biocide triclosan impairs byssus formation in marine mussels Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.A.; Keikhosravi, A.; Eliceiri, K.W.; Vezina, C.M. Fluorescence of Picrosirius Red Multiplexed With Immunohistochemistry for the Quantitative Assessment of Collagen in Tissue Sections. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2017, 65, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.R.; Simoniello, P.; Giacomelli, S.; Oreste, U.; Motta, C.M. Investigation of immunoglobulins in skin of the Antarctic teleost Trematomus bernacchii. Fish. Shellfish Immunol. 2014, 39, 206–214. [Google Scholar] [CrossRef]
- Vincent, S.G.; Cunningham, P.R.; Stephens, N.L.; Halayko, A.J.; Fisher, J.T. Quantitative densitometry of proteins stained with coomassie blue using a Hewlett Packard scanjet scanner and Scanplot software. Electrophoresis 1997, 18, 67–71. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogliano, C.; Carotenuto, R.; Agnisola, C.; Simoniello, P.; Karam, M.; Manfredonia, C.; Avallone, B.; Motta, C.M. Benzodiazepine Delorazepam Induces Locomotory Hyperactivity and Alterations in Pedal Mucus Texture in the Freshwater Gastropod Planorbarius corneus. Int. J. Mol. Sci. 2023, 24, 17070. https://doi.org/10.3390/ijms242317070
Fogliano C, Carotenuto R, Agnisola C, Simoniello P, Karam M, Manfredonia C, Avallone B, Motta CM. Benzodiazepine Delorazepam Induces Locomotory Hyperactivity and Alterations in Pedal Mucus Texture in the Freshwater Gastropod Planorbarius corneus. International Journal of Molecular Sciences. 2023; 24(23):17070. https://doi.org/10.3390/ijms242317070
Chicago/Turabian StyleFogliano, Chiara, Rosa Carotenuto, Claudio Agnisola, Palma Simoniello, Myriam Karam, Claudia Manfredonia, Bice Avallone, and Chiara Maria Motta. 2023. "Benzodiazepine Delorazepam Induces Locomotory Hyperactivity and Alterations in Pedal Mucus Texture in the Freshwater Gastropod Planorbarius corneus" International Journal of Molecular Sciences 24, no. 23: 17070. https://doi.org/10.3390/ijms242317070
APA StyleFogliano, C., Carotenuto, R., Agnisola, C., Simoniello, P., Karam, M., Manfredonia, C., Avallone, B., & Motta, C. M. (2023). Benzodiazepine Delorazepam Induces Locomotory Hyperactivity and Alterations in Pedal Mucus Texture in the Freshwater Gastropod Planorbarius corneus. International Journal of Molecular Sciences, 24(23), 17070. https://doi.org/10.3390/ijms242317070









