Abstract
Endothelial dysfunction is one of the major factors in the pathogenesis of metabolic syndrome (MetS), and its molecular mechanisms are not completely understood. The present study aimed to examine the connection between nuclear factor2-related factor2 (Nrf2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), heme oxygenase 1 (HO-1), and plasma asymmetric dimethylarginine (ADMA) and malondialdehyde (MDA) in people with MetS. Participants in the study were as follows: with MetS (n = 30) and without MetS (Control) (n = 14). Expression of Nrf2, NF-kB, and HO-1 was measured in peripheral blood mononuclear cells (PBMCs). Plasma ADMA was determined using the ELISA technique and MDA via the thiobarbituric acid method. Our study showed that mRNA of NF-kB, Nrf2, and HO-1 levels in PBMCs in the MetS group were significantly higher than in the controls by 53%, 130%, and 185% (p < 0.05), respectively. Similarly, elevated levels of MDA (by 78%, p < 0.001) and ADMA (by 18.7%, p < 0.001) were established in the MetS group. Our findings show the importance of transcription factor Nrf2, playing an integral role in the protection of the endothelium, and of NF-κB, a transcription factor mediating the inflammatory response in MetS. Knowledge of complex cellular–molecular mechanisms would allow the use of biomarkers such as Nrf2, NF-kB, HO-1, and ADMA for the assessment of endothelial dysfunction in clinical practice.
1. Introduction
Endothelial dysfunction (ED) is characterized by decreased bioavailability of nitric oxide (NO) and impaired endothelial-dependent vasodilation, one of the major risk factors of metabolic syndrome (MetS) [1,2]. Endothelial dysfunction is considered to be a key factor in the development of vascular diseases [3]. Asymmetric dimethylarginine (ADMA) is an endogenous competitive inhibitor of nitric oxide synthase [4,5]. Increased ADMA has been also linked to the presence of hypertension, type 2 diabetes, and obesity as components of MetS [6,7,8,9]. The ED’s molecular mechanisms in MetS are not completely understood, but free radicals and inflammatory mediators are thought to be involved. Recent evidence suggests that oxidative stress and inflammation induced by high low density lipoproteins (LDL) or by TNF-α (Tumor necrosis factor alpha) increase ADMA levels, and, respectively, decrease NO bioavailability [6]. Reactive oxygen species (ROS) enhance NO degradation and uncouple endothelial nitric oxide synthase (eNOS) to redirect the enzyme to generate ROS [10].
Heme oxygenase 1 (HO-1) is an enzyme induced by nuclear factor2-related factor2 (Nrf2) and plays a critical role in antioxidant defense in response to various inflammatory and oxidative stimuli [1]. HO-1 expression significantly suppresses the production of proinflammatory mediators and reverses impaired eNOS expression in response to oxidised LDL and TNF-α [11].
Although HO-1 is expressed at low levels in endothelial cells and white blood cells under physiological conditions, it is highly inducible in response to various pathophysiological stresses/stimuli [12,13]. Heme-oxygenase-1 catalyzes the degradation of heme to carbon monoxide and bilirubin, which also have cytoprotective effects for endothelial dysfunction against various stresses [12,14,15]. The beneficial effect of HO-1 expression in the attenuation of metabolic syndrome, obesity, cardiovascular disease, and diabetic cardiomyopathy has been reported [16,17].
Nrf2, a redox-sensitive transcription factor, transcribes many antioxidant genes. Nfr2 is important in the transcriptional activation of HO-1 and several other genes that contain specific sequences called Antioxidant Response Elements (AREs) [18]. There is insufficient data on the role of HO-1 in the pathogenesis of endothelial dysfunction in MetS. It has been reported that HO-1 protects endothelial cells against oxidative stress in aging, hypertension, diabetes, and atherosclerosis [19,20]. Nrf2 decreases oxidative stress by regulating the expression of antioxidant genes while ADMA enhances oxidative stress by inhibiting the nitric oxide pathway via competitive inhibition of the nitric oxide synthase pathway [21]. Nrf2/ARE signaling has recently been shown to play an important role in endothelial protection under oxidative stress [22].
Inflammation plays an important role in ED in MetS and the inhibition of endothelial nitric oxide synthesis by ADMA and may be related to increased concentrations of inflammatory mediators [23]. Previous studies demonstrated that plasma ADMA could concentration-dependently increase ROS production, and that these ROS consequently act as second messengers and stimulate the activation of uclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) in endothelial cells [24]. NF-κB is a key transcription factor in the expression of proinflammatory markers such as TNF-α, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) released by white blood cells [25,26]. Data on investigations of Nrf2, NF-KB transcription factors in humans, and their role in the pathogenesis of ED in MetS are very scarce.
The present study aimed to examine the connection between Nrf2 as a critical regulator of defense against oxidative stress, NF-κB, a transcription factor mediating the inflammatory response, HO-1, endothelial protector and antioxidant, plasma ADMA, and MDA in people with MetS.
2. Results
The basic demographic characteristics of the participants are presented in Table 1.

Table 1.
Baseline characteristics of the study groups.
Thirty subjects (83.5%) met NCEP-ATPIII criteria for metabolic syndrome diagnosis. These subjects showed higher levels of WC, BMI, TG, HDL, and systolic and diastolic pressures.
Our study showed that NF-KB mRNA (over 53%), Nrf2 mRNA (over 130%), and HO-1 mRNA expression (over 185%) in peripheral blood mononuclear cells (PBMCs) in the MetS group were elevated compared to control subjects (Figure 1, Figure 2 and Figure 3).
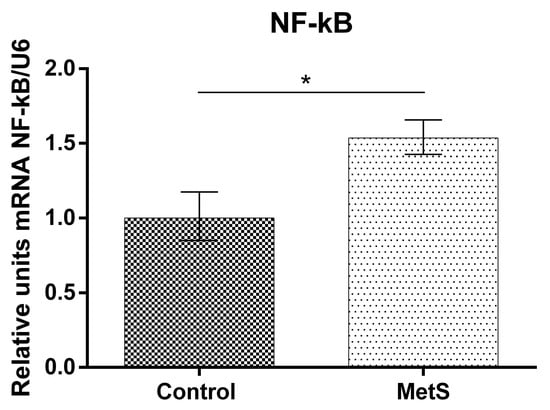
Figure 1.
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) gene expression in Peripheral Blood Mononuclear Cells (PBMCs) of subjects with metabolic syndrome (MetS) and control subjects (Control). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), * p < 0.05.
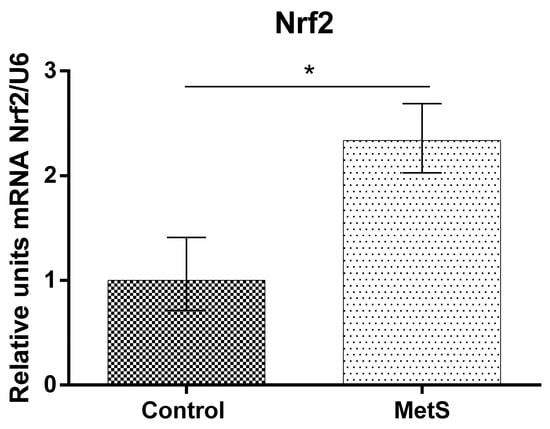
Figure 2.
Nuclear factor2-related factor2 (Nrf2) gene expression in PBMCs of subjects with MetS and control subjects (Control). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), * p < 0.05.
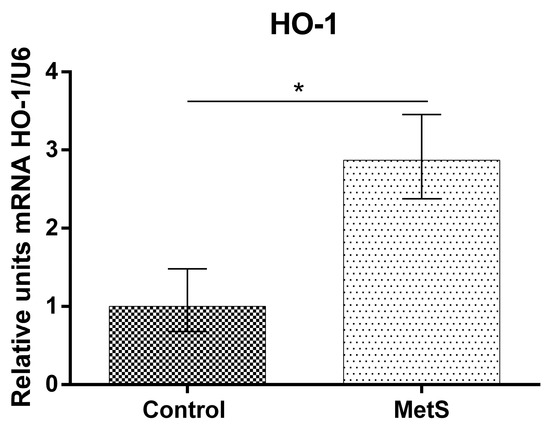
Figure 3.
Heme oxygenase 1 (HO-1) gene expression in PBMCs of subjects with MetS and control subjects (Control). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05) *, p < 0.05.
Plasma MDA was increased significantly in the metabolic group vs. the control by 78% (1.05 ± 0.13 to 1.87 ± 0.11) (Figure 4). Plasma ADMA was also increased by 18.7% (0.45 ± 0.02 to 0.54 ± 0.01) (Figure 5).
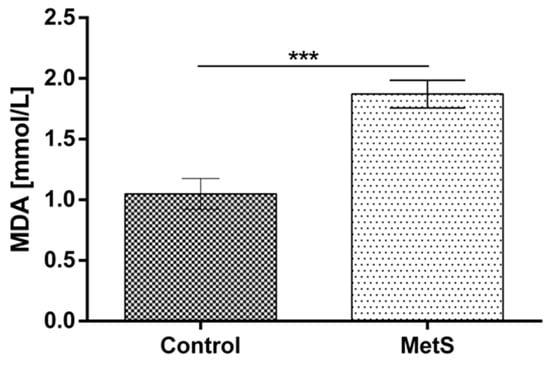
Figure 4.
Plasma malondialdehyde (MDA) levels in subjects with MetS and control subjects (Control). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), *** p < 0.001.
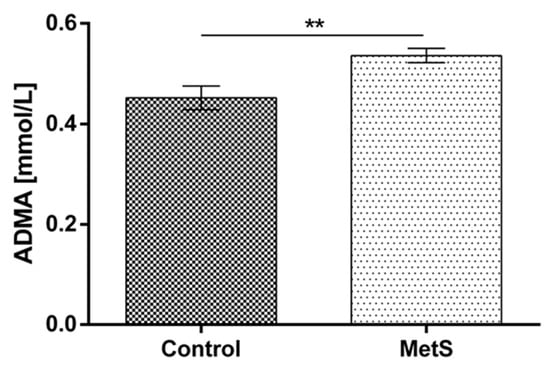
Figure 5.
Plasma asymmetric dimethylarginine (ADMA) levels in subjects with MetS and control subjects (Control). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), ** p < 0.01.
We subdivided MetS individuals into two groups according their plasma ADMA levels: ADMA ≤ median (=0.524 mmol/L) and ADMA > median of the group. The expression levels of Nrf2 and HO-1 in PBMCs were more than 320% (p < 0.001) and 214% (p < 0.01), respectively, higher in the MetS subgroup with ADMA > median. Significantly higher levels (192%, p < 0.01) of NF-kB mRNA were also detected (Figure 6). The same subgrouping according to plasma MDA levels revealed a lower relative expression of Nrf2 (by 61%, p < 0.01) and HO-1 (by 56%, p < 0.05) in the subgroup with plasma MDA higher than the median (=1.930 mmol/L). NF-kB was not found to be differently expressed, depending on plasma MDA levels in the MetS group (Figure 7).
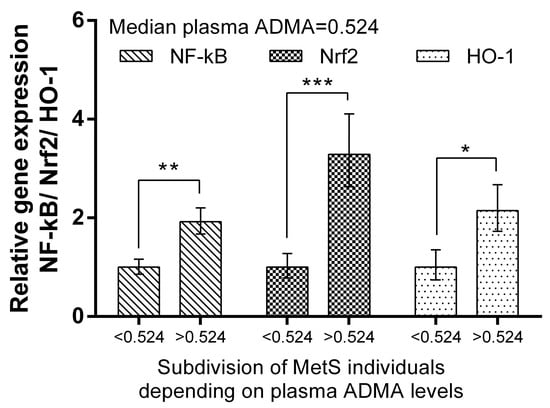
Figure 6.
Differential expression of NF-kB, Nrf2, and HO-1 in MetS subgroups, depending on plasma ADMA levels. Subdivision was as follows: subgroup with ADMA plasma levels ≤ 0.524 mmol/L (median value). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), * p < 0.05; ** p < 0.01; *** p < 0.001.
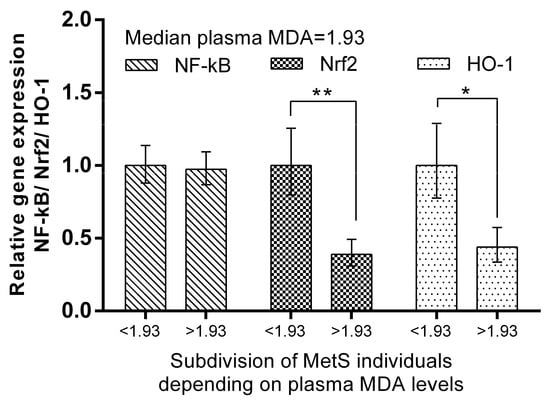
Figure 7.
Differential expression of NF-kB, Nrf2, and HO-1 in MetS subgroups, depending on plasma MDA levels. Subdivision was as follows: subgroup with MDA plasma levels ≤ 1.93 mmol/L (median value). Data are presented as mean ± SEM (statistical significance was indicated at p < 0.05), * p < 0.05; ** p < 0.01.
3. Discussion
In the present study, we investigated the expression of NF-κB, a transcription factor mediating the inflammatory response, Nrf2 as a critical regulator of defense against oxidative stress, HO-1, an endothelial protector and antioxidant, and the relationship between them and plasma MDA and ADMA in people with MetS.
ADMA, as a marker of endothelial dysfunction, and MDA, as a marker of oxidative stress and lipid peroxidation, are increased in MetS. Some studies report a significant association between increased ADMA levels, hypercholesterolemia, and endothelial dysfunction in people with MetS [1,27,28]. It is well known that hypercholesterolemia and increased ADMA and MDA levels lead to endothelial dysfunction, which is considered a key factor in the development of proatherogenic changes in endothelial cell and vascular events, including stroke and myocardial infarction. Hypercholesterolemia may disturb the function or regulation of dimethylarginine dimethylaminohydrolase (DDAH), thereby leading to intracellular accumulation of ADMA and increased oxidative stress [29].
In our study, we found a positive association between plasma MDA, a marker of oxidative stress, and plasma ADMA, a marker of endothelial dysfunction in individuals with MetS. It is known that ADMA is metabolized by DDAH to dimethylamine and citrulline [30,31]. Oxidative stress, TNF-α, homocysteine, and nitrosative stress have been shown to reduce DDAH activity. Increased plasma concentrations of ADMA were found to cause an increase in superoxide (O2−) production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, thereby leading to oxidative stress and decreased NO bioavailability [32]. ROS and products of lipid peroxidation are crucial factors for the activation of transcriptional factors Nrf2 and NF-kB [33,34]. As nuclear factor Nrf2r is located in the cytoplasm, its translocation to the nucleus is triggered by different stress stimuli which have the potential to disturb cellular redox balance [18,35,36]. Therefore, in stressful milieu such as oxidative/inflammatory insults, Nrf2 moves to the nucleus and binds to its ARE sites to cause the upregulation of enzymes to counteract the effect of increased ROS.
Nrf2 may activate DDAH-1, which metabolizes ADMA, the endogenous inhibitor of nitric oxide synthase eNOS [31]. Increased DDAH-1 is accompanied by decreased levels of ADMA in endothelium and plasma. This would support endothelial function through adequate NO production and the homeostasis of endothelial function [21]. Therefore, Nrf2 can enhance endothelial NO generation and protect endothelial function by regulating the DDAH/ADMA/eNOS pathway (Figure 8).
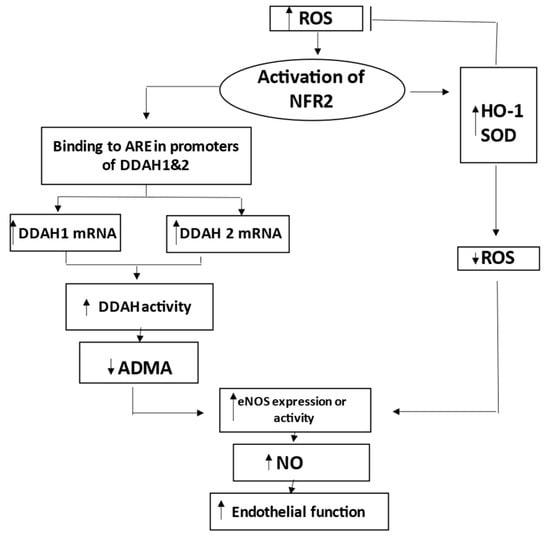
Figure 8.
Schematic representation of the role of nuclear factor erythroid 2-related factor 2 (Nrf2) in preserving endothelial function during activation by reactive oxygen species (ROS). Nrf2 increases expression of HO-1 and other genes to decrease ROS. Nrf2 also increases DDAH-1 and DDAH-2, leading to degradation and decrease of ADMA, which is inhibitor of eNOS. Decreased levels of ADMA result in improved NO production and related endothelial function. Thus, Nrf2 coordinates HO-1/DDAH/eNOS pathways that enhance endothelial NO generation and endothelial function. ADMA indicates asymmetric dimethylarginine; ARE, antioxidant response element; DDAH, dimethylarginine dimethylaminohydrolase; eNOS, endothelial nitric oxide synthase; HO-1, hemoxygenase-1; and SOD, superoxide dismutase.
HO-1 is an Nrf2-dependent gene that exerts beneficial effects through protection against oxidative damage [15]. HO-1 is important in attenuating the overall production of reactive oxygen species (ROS) through its ability to degrade heme and produce carbon monoxide and biliverdin/bilirubin, and release free iron [37]. In clinical cases, human HO-1 deficiency causes oxidative stress and endothelial cell injury [14]. In contrast, the up-regulation of HO-1 protein may represent an attempt to minimize cellular injury [38]. Biliverdin and CO are gaining particular interest because these two have been found to mediate most of the anti-inflammatory, antioxidant, and anti-apoptotic effects of HO-1. The present study showed increased HO-1 expression in PBMCs of MetS subjects. Elevated plasma HO-1 levels have been reported in diabetes [39] as well as in other chronic diseases [40,41,42]. HO-1 is an Nrf2-dependent gene that exerts beneficial effects through endothelial protection against oxidative damage [37].
The activation of Nrf2 promoting HO-1 expression can inhibit oxidative stress and increase eNOS activity and NO production [43]. Furthermore, HO-1 induction inhibits ROS production in the aorta isolated from animals with fructose-induced MetS [44]. HO-1, by modulating the activation of Nrf2, sets an adaptive reprogramming that enhances antioxidant defenses [45]. It has been reported that the increased HO-1 expression and heme degradation products can improve vascular function, at least in part, by compensating for the loss of NO bioavailability [43].
Nrf2 increases the expression of HO-1 and other genes to decrease ROS, as well as increases DDAH-1 and DDAH-2 to decrease ADMA and to increase the eNOS-mediated bioavailability of NO [31]. Therefore, Nrf2 coordinates HO-1/DDAH/eNOS pathways that enhance endothelial NO generation and endothelial function (Figure 8).
Previous studies demonstrated that ADMA could concentration-dependently increase the production of ROS, which act as second messengers and activate NF-kB in endothelial cells [46]. The data from the present study showed an increased NF-κB expression in PBMCs, which corresponds to higher plasma MDA and ADMA levels in MetS. ADMA may activate NF-κB, the expression of cytokines, soluble ICAM-1 and VCAM-1 expression, and monocyte adhesiveness, and may mediate their proinflammatory actions in the endothelium [23]. It has been proven that the reduced eNOS/NO activity activates NF-κB responsible for the expression of endothelin-1 and other proinflammatory mediators including TNF-α, interleukin 6 (IL-6), free radicals, chemokines, and adhesives molecules (ICAM-1, endothelial leucocyte adhesion molecule-1 (ELAM-1)), as well as inducible NO synthase (iNOS) contributing to endothelial dysfunction.
ADMA is produced in many tissues and is known to induce the expression of Nrf2, which in response stimulates DDAH [47]. In this study, we subdivided MetS individuals in two groups according to their plasma ADMA levels: ADMA ≤ median (=0.524 mmol/L) and ADMA > median of the group. Expression levels of Nrf2 in PBMCs were more than 320% (p < 0.0.001) higher in the MetS subgroup with ADMA > median. Similarly, HO-1, which is a target of Nrf2, was, correspondingly, 214% (p < 0.01) higher in the MetS subgroup with high ADMA plasma levels. Significantly higher levels (192%, p < 0.01) of mRNA were also detected for NF-kB in the same subgroup (Figure 6). Data suggest that the gene expression of respective genes in PBMCs may reflect plasma ADMA levels in metabolic syndrome. In support of this hypothesis is the observation that, under ADMA treatment, cells, including endothelial, produce ROS and upregulate NF-kB [25,48].
The same subgrouping according to plasma MDA levels revealed lower relative expression of Nrf2 (by 61%, p < 0.01) and HO-1 (by 56%, p < 0.05) in the subgroup with plasma MDA higher than the median (=1.930 mmol/L). At the same time, NF-kB, which is related to inflammation pathways [49], was not found to be differently expressed depending on plasma MDA levels in the MetS group (Figure 7). This observation suggests other mechanisms by which the expression of respective genes in PBMCs is dependent on MDA plasma levels in metabolic syndrome. In pathological conditions, cells may have decreased HO-1 expression. For example, this enzyme is downregulated in PBMCs in multiple sclerosis [50]. Further studies would reveal whether impaired balance of the protective mechanisms in PBMCs in MetS could be a reason for this observation.
4. Materials and Methods
4.1. Participants
This study was approved by the local Ethical Committee at the Medical University of Varna (Protocol № 86/26.09.2019). Written informed consent was obtained from all participants in the study. A total of 44 people (39 females, 5 males) were included in the study. The participants of the study were divided into two groups: those newly diagnosed with metabolic syndrome (MetS) (n = 30), and nonmetabolic, clinically healthy individuals (Control) (n = 14). Individuals in the MetS group were selected according to CEP-ATP III criteria [51] (the presence of three or more of the following: waist circumference ≥102 cm (men), ≥88 cm (women); blood pressure ≥130/85 mm Hg or treatment for hypertension; triglycerides ≥ 1.7 mmol/L (≥150 mg/dL); HDL-cholesterol < 1.03 mmol/L (<40 mg/dL) for men, <1.29 mmol/L (<50 mg/dL) for women). The participants were newly diagnosed and indicated as MetS based on the abovementioned criteria and were not under treatment for metabolic syndrome.
For biochemical analyses, venous fasting blood was collected in vacutainers (EDTA). Plasma was separated by centrifugation at 1500 rpm for 15 min, aliquoted, and stored at −20 °C. Subsequent analyses were performed immediately after the thawing of the samples. Plasma lipid parameters (total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides) were measured using routine methods.
4.2. ADMA and MDA Measurement
Plasma ADMA concentrations were determined using the ELISA assay kit (DLD Diagnostica GMBH, Germany) after it was validated locally. The method was validated in compliance with the international standard of quality and competence of medical laboratories (BDS/EN/ISO 15189). Measurements were performed in duplicate. Data are presented in mM/L.
Lipid peroxidation was assayed via MDA, measured according to its thiobarbituric acid (TBA) reactivity in plasma using the method of Porter et al. [52]. Data are presented in mmol/L.
4.3. Peripheral Blood Mononuclear Cells (PBMCs) Collection
Whole blood samples were used for peripheral blood mononuclear cells collection. Blood (6 mL) was collected in lithium heparin vacutainer tubes. The separation of PBMCs was carried out using a Ficoll separation medium with a density of 1.077 g/L and 50 mL LeucoSep™ centrifuge separation tubes (GreinerBioOne, Kremsmünster, Austria) containing a porous barrier, which enables cell separation by means of density gradient centrifugation and purification following the manufacturer’s instructions.
4.4. Gene Expression Analysis
RNA was extracted from collected PBMCs with Tri reagent (Ambion®, Life Technologies, Waltham, USA) using the manufacturer’s protocol. First-strand cDNA synthesis was performed with 0.1 µg of total RNA using a RevertAid First Strand cDNA Synthesis Kit (ThermoScientific, Waltham, MA, USA),a nd using (dT)18 primer and following the steps in the manufacturer’s instructions. Quantitative gene expression analysis was performed using two-step real-time qPCR with SYBR Green qPCR 1 × Master Mix with ROX (KAPA SYBR FAST qPCR Kit, KAPA BIOSYSTEMS, USA). The primer sequences used were as follows: U6 Forward GCTTCGGCAGCACATATACTAAAAT; Reverse CGCTTCACGAATTTGCGTGTCAT; HMOX1 Forward TCAGGCAGAGGGTGATAGAAG; Reverse TTGGTGTCATGGGTCAGC; NRF2 Forward TCCAGTCAGAAACCAGTGGAT; Reverse GAATGTCTGCGCCAAAAGCTG; NF-kB Forward AGAGGCTTCCGATTTCGATATGG; Reverse GGATAGGTCTTTCGGCCCTTC. Analysis was performed on an Applied Biosystems® 7500 Real-Time qPCR instrument (Waltham, USA). The amount of mRNA of each gene of interest was normalized according to the amount of mRNA encoding U6 as an internal control. Gene expression levels were calculated using the 2−ΔΔCt method. Gene expression levels of respective genes were presented in relative units as compared to the control group, where expression was considered to be equal to 1 as described by Livak and Schmittgen [53].
4.5. Statistical Analysis
Data were presented as mean ± SEM or percentage (%), as appropriate. Data analysis was performed on GraphPad Prism v. 8.3. and SPSS v. 23. Standard statistical methods such as descriptive statistics, unpaired Student’s t-tests for normally distributed parameters, and one-way ANOVA with Bonferroni correction were used.
5. Conclusions
In conclusion, our findings show the importance of transcription factor Nrf2, playing an integral role in the protection of the endothelium and of NF-κB, the transcription factor mediating the inflammatory response in in MetS. Investigating the relationship between MDA, ADMA, HO-1, and the transcription factors Nrf2 and NF-kB would contribute to elucidating the complex pathophysiological mechanisms of cellular damage in MetS, and this is important for the prevention, early diagnosis, and treatment strategy of cardiovascular diseases and MetS. The knowledge of complex cellular–molecular mechanisms in PBMCs would allow the use of biomarkers such as HO-1, ADMA, Nrf2, and NF- kB for the assessment of imbalance in PBMCs in MetS.
Author Contributions
Conceptualization, G.Y.B. and Y.D.K.-K.; methodology, D.G.V., A.S.S., S.M.S. and D.I.G.; validation, V.H.M. and D.I.G.; formal analysis, D.G.V., A.S.S., D.I.G., S.M.S. and N.A.B.; investigation, N.A.B., D.G.V., A.S.S., S.M.S., D.I.G. and V.H.M.; resources, V.H.M.; data curation, N.A.B., D.G.V., A.S.S., S.M.S. and D.I.G.; writing—original draft preparation, G.Y.B.; writing—review and editing, Y.D.K.-K. and D.G.I.; visualization, Y.D.K.-K.; supervision, Y.D.K.-K.; project administration, Y.D.K.-K.; funding acquisition, D.G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union, NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0009-C02.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Medical University of Varna (Protocol № 86/26.09.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palomo, I.; Contreras, A.; Alarcón, L.M.; Leiva, E.; Guzmán, L.; Mujica, V.; Icaza, G.; Díaz, N.; González, D.R.; Moore-Carrasco, R. Elevated concentration of asymmetric dimethylarginine (ADMA) in individuals with metabolic syndrome. Nitric Oxide 2011, 24, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Ignarro, L.J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009, 32, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. ADMA: Its role in vascular disease. Vasc. Med. 2005, 10 (Suppl. 1), S11–S17. [Google Scholar] [CrossRef]
- Böger, R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef]
- Böger, R.H. Association of asymmetric dimethylarginine and endothelial dysfunction. Clin. Chem. Lab. Med. 2003, 41, 1467–1472. [Google Scholar] [CrossRef]
- Bekyarova, G.; Ivanova, D.G.; Madjova, V. Molecular mechanisms associated oxidative stress and endothelial dysfunction in the development of vascular complications in diabetes mellitus. Folia Med. 2007, 49, 13–19. [Google Scholar]
- Abbasi, F.; Asagmi, T.; Cooke, J.P.; Lamendola, C.; McLaughlin, T.; Reaven, G.M.; Stuehlinger, M.; Tsao, P.S. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2001, 88, 1201–1203. [Google Scholar] [CrossRef]
- Bouras, G.; Deftereos, S.; Tousoulis, D.; Giannopoulos, G.; Chatzis, G.; Tsounis, D.; WCleman, M.; Stefanadis, C. Asymmetric Dimethylarginine (ADMA): A promising biomarker for cardiovascular disease? Curr. Top. Med. Chem. 2013, 13, 180–200. [Google Scholar] [CrossRef]
- Chen, A.F.; Chen, D.-D.; Daiber, A.; Faraci, F.M.; Li, H.; Rembold, C.M.; Laher, I. Free radical biology of the cardiovascular system. Clin. Sci. 2012, 123, 73–91. [Google Scholar] [CrossRef]
- Kushida, T.; Li Volti, G.; Quan, S.; Goodman, A.; Abraham, N.G. Role of human heme oxygenase-1 in attenuating TNF-alpha-mediated inflammation injury in endothelial cells. J. Cell Biochem. 2002, 87, 377–385. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005, 139, 1–25. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Pae, H.-O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.-H.; Choi, Y.K.; Lee, B.-S.; Kim, S.R.; Gong, Y.-Y.; et al. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef]
- Haines, D.D.; Tosaki, A. Role of Heme Oxygenases in Cardiovascular Syndromes and Co-morbidities. Curr. Pharm. Des. 2018, 24, 2322–2325. [Google Scholar] [CrossRef]
- Facchinetti, M.M. Heme-Oxygenase-1. Antioxid. Redox Signal. 2020, 32, 1239–1242. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Yang, Y.C.; Li, C.C.; Liu, K.L.; Lii, C.K.; Chen, H.W. Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem. Pharmacol. 2014, 91, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Gongora, E. Oxidative Stress and Cardiovascular Aging: Interaction Between NRF-2 and ADMA. Curr. Cardiol. Rev. 2017, 13, 183–188. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Jimenez, R.; Losonczy, G.; Zhang, C.; Ballabh, P.; Recchia, F.A.; Wilkerson, D.C.; Sonntag, W.E.; et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1133–H1140. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Tommasi, S.; Sotgia, S.; Zinellu, A.; Paliogiannis, P.; Piga, M.; Cauli, A.; Pintus, G.; Carru, C.; Erre, G.L. Asymmetric Dimethylarginine: A Key Player in the Pathophysiology of Endothelial Dysfunction, Vascular Inflammation and Atherosclerosis in Rheumatoid Arthritis? Curr. Pharm. Des. 2021, 27, 2131–2140. [Google Scholar] [CrossRef]
- Hammad, A.S.A.; Ahmed, A.F.; Heeba, G.H.; Taye, A. Heme oxygenase-1 contributes to the protective effect of resveratrol against endothelial dysfunction in STZ-induced diabetes in rats. Life Sci. 2019, 239, 117065. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-κB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar]
- Dayal, S.; Lentz, S.R. ADMA and hyperhomocysteinemia. Vasc. Med. 2005, 10 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Isola, G.; Maggio, S.; Bertuccio, M.P.; Trovato-Salinaro, A.; Matarese, G.; Alibrandi, A.; Caccamo, D.; Ientile, R. Changes in the Biomarkers of Oxidative/Nitrosative Stress and Endothelial Dysfunction Are Associated with Cardiovascular Risk in Periodontitis Patients. Curr. Issues Mol. Biol. 2021, 43, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef]
- Leiper, J.; Nandi, M.; Torondel, B.; Murray-Rust, J.; Malaki, M.; O’Hara, B.; Rossiter, S.; Anthony, S.; Madhani, M.; Selwood, D.; et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat. Med. 2007, 13, 198–203. [Google Scholar] [CrossRef]
- Pope, A.J.; Karuppiah, K.; Cardounel, A.J. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol. Res. 2009, 60, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Munzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Niki, E. 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life 2006, 58, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef]
- Peterson, S.J.; Rubinstein, R.; Faroqui, M.; Raza, A.; Boumaza, I.; Zhang, Y.; Stec, D.; Abraham, N.G. Positive Effects of Heme Oxygenase Upregulation on Adiposity and Vascular Dysfunction: Gene Targeting vs. Pharmacologic Therapy. Int. J. Mol. Sci. 2019, 20, 2514. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004, 37, 1995–2011. [Google Scholar] [CrossRef]
- Bao, W.; Song, F.; Li, X.; Rong, S.; Yang, W.; Zhang, M.; Yao, P.; Hao, L.; Yang, N.; Hu, F.B.; et al. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS ONE 2010, 5, e12371. [Google Scholar] [CrossRef]
- Sato, T.; Takeno, M.; Honma, K.; Yamauchi, H.; Saito, Y.; Sasaki, T.; Morikubo, H.; Nagashima, Y.; Takagi, S.; Yamanaka, K.; et al. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-inducedlung injury. Am. J. Respir. Crit. Care Med. 2006, 174, 906–914. [Google Scholar] [CrossRef]
- Mateo, I.; Infante, J.; Sánchez-Juan, P.; García-Gorostiaga, I.; Rodríguez-Rodríguez, E.; Vázquez-Higuera, J.L.; Berciano, J.; Combarros, O. Serum heme oxygenase-1 levels are increased in Parkinson’s disease but not in Alzheimer’s disease. Acta Neurol. Scand. 2010, 121, 136–138. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kirino, Y.; Takeno, M.; Hama, M.; Ushihama, A.; Watanabe, R.; Takase, K.; Tachibana, T.; Matsumoto, K.; Tanaka, M.; et al. Serum HO-1 is useful to make differential diagnosis of secondary hemophagocytic syndrome from other similar hematological conditions. Int. J. Hematol. 2010, 91, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Son, Y.; Kim, N.H.; Jeong, H.J.; Chang, K.C.; Chung, H.T. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide 2010, 23, 251–257. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Hassan, N.; Zakaria, M.N. Heme oxygenase-1 alleviates vascular complications associated with metabolic syndrome: Effect on endothelial dependent relaxation and NO production. Chem. Biol. Interact. 2014, 223, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.M.A.; Natani, S.; Sruthi, K.K.; Khilar, P.; Ummanni, R. HIF-1α and Nrf2 regulates hypoxia induced overexpres-sion of DDAH1 through promoter activation in prostate cancer. Int J Biochem Cell Biol 2022, 147, 106232. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship be-tween Vascular Dysfunction and Inflammation in Atherosclerosis. J Clin Med 2020, 9, 3026. [Google Scholar] [CrossRef]
- Yadav, U.C.; Ramana, K.V. Regulation of NF-κB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev 2013, 2013, 690545. [Google Scholar] [CrossRef]
- Fagone, P.; Patti, F.; Mangano, K.; Mammana, S.; Coco, M.; Touil-Boukoffa, C.; Chikovani, T.; Di Marco, R.; Nicoletti, F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neu-roimmunol. 2013, 261, 82–86. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Nixon, J.R.; Isaac, R. Cyclic peroxidase and thiobarbituric assay. Biochem Biophys Acta 1976, 441, 506–512. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).