A Transcriptome Array-Based Approach to Link SGLT-2 and Intrarenal Complement C5 Synthesis in Diabetic Nephropathy
Abstract
:1. Introduction
2. Results
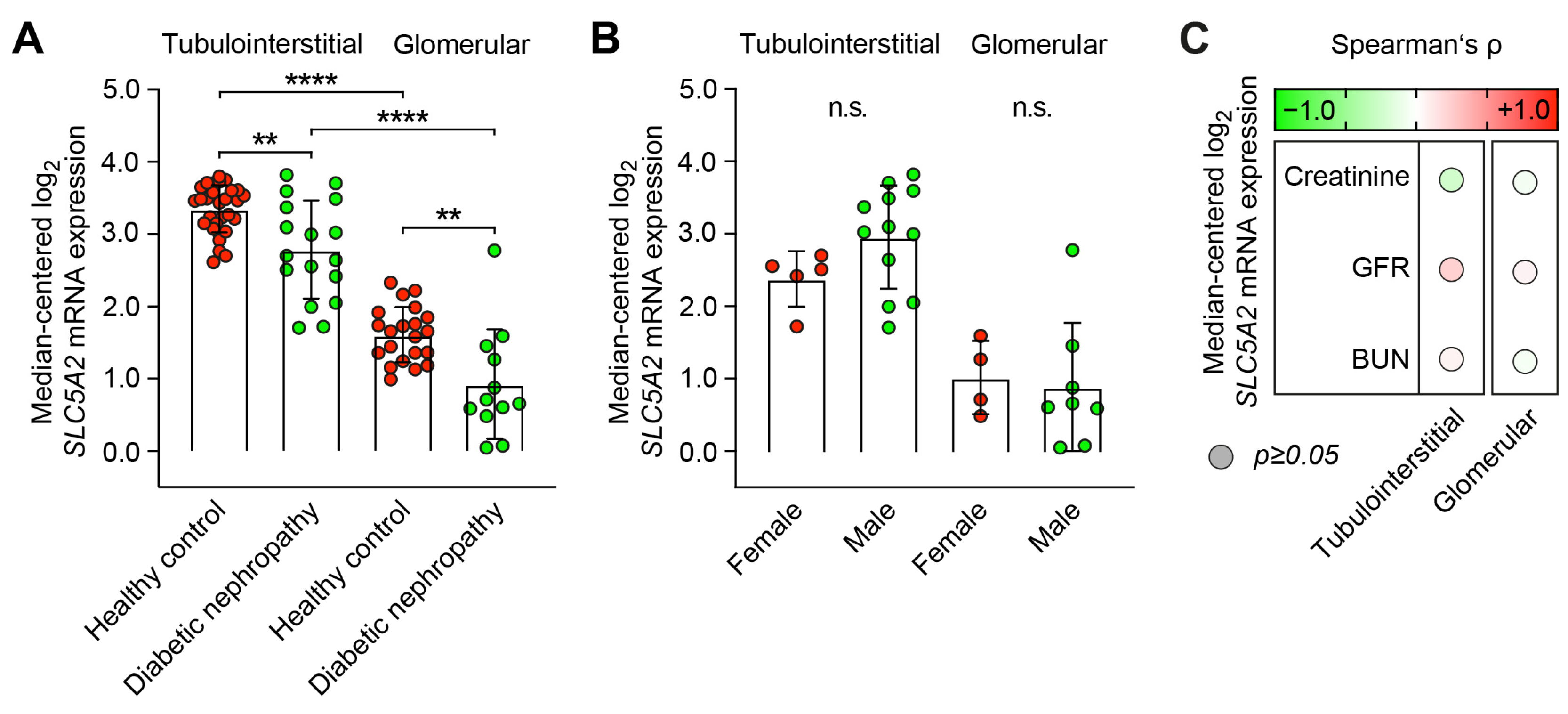
2.1. Predominant Tubulointerstitial SLC5A2 Expression in Healthy Controls and Diabetic Nephropathy
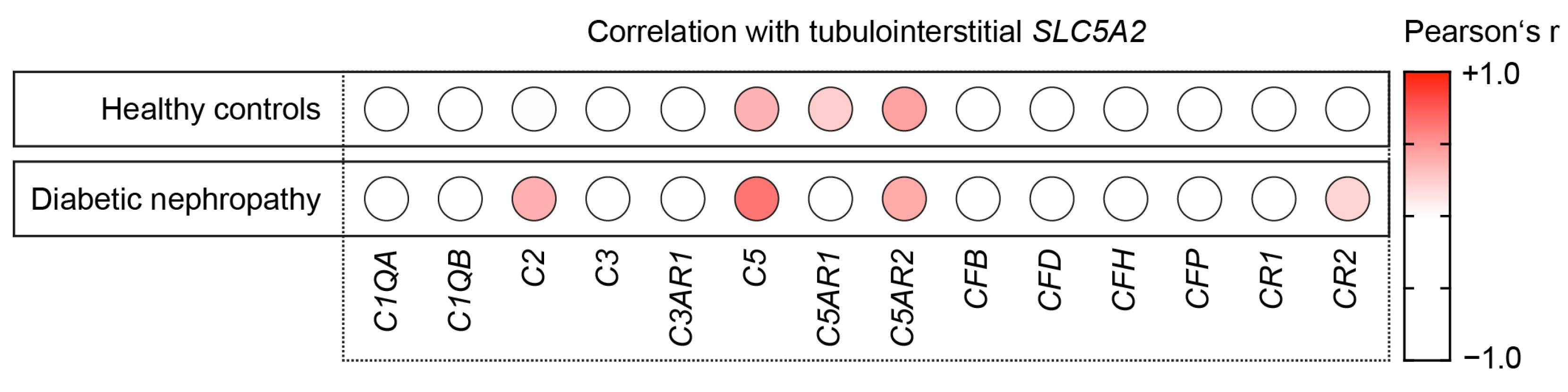
2.2. Intrarenal SLC5A2 Expression Is Associated with Tubulointerstitial Synthesis of Distinct Complement Components
2.3. Intrarenal SLC5A2 Expression Is Associated with Tubulointerstitial Synthesis of Complement C5 in Diabetic Nephropathy
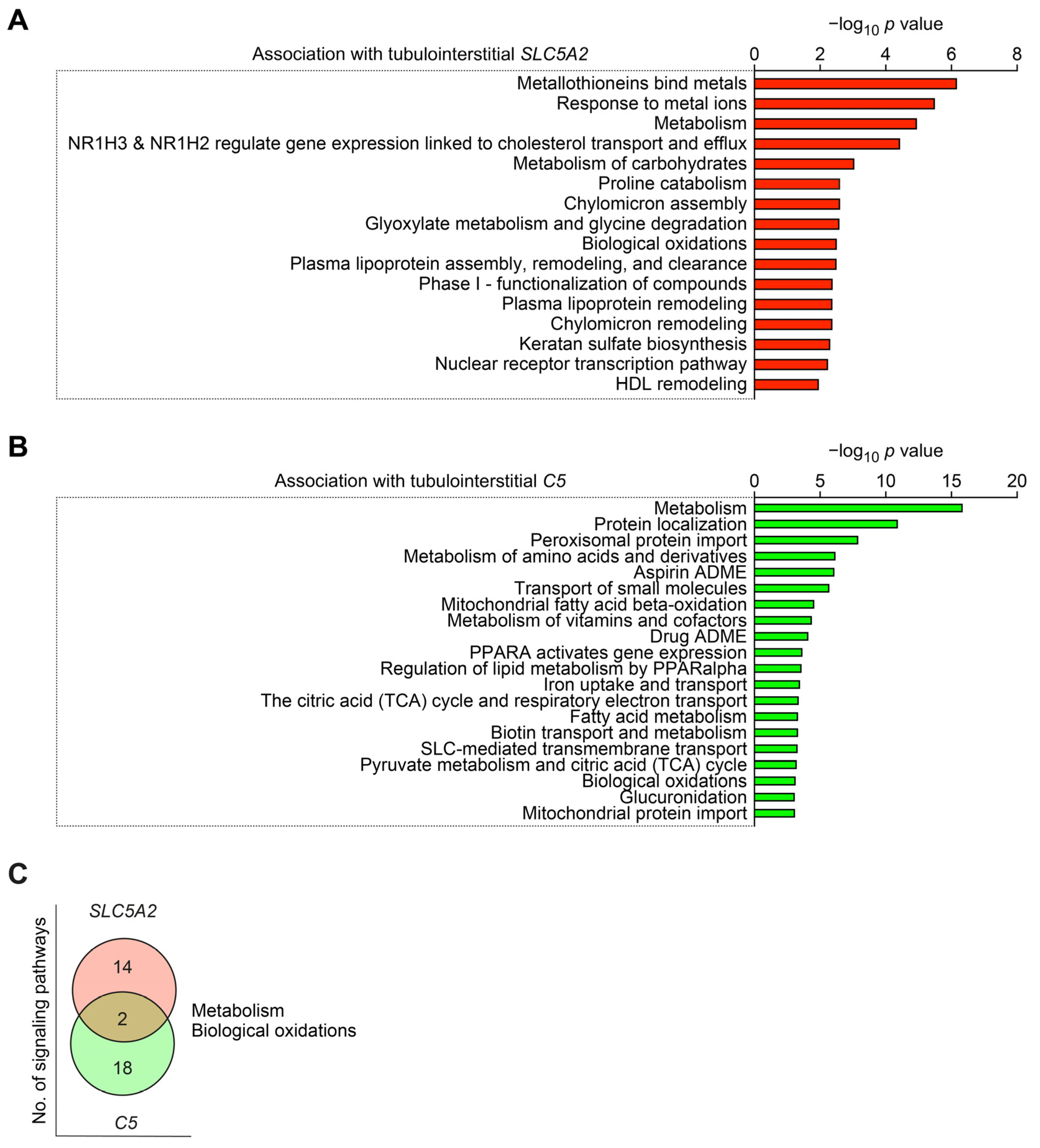
2.4. Intrarenal SLC5A2 Expression and Complement C5 Synthesis Associated with Distinct Molecular Signatures in Diabetic Nephropathy
3. Discussion
4. Materials and Methods
4.1. Analyses of Publicly Available Array Datasets
4.2. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budge, K.; Dellepiane, S.; Yu, S.M.; Cravedi, P. Complement, a Therapeutic Target in Diabetic Kidney Disease. Front. Med. 2020, 7, 599236. [Google Scholar] [CrossRef]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; de Boer, I.H. Clinical Manifestations of Kidney Disease among US Adults with Diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, A.A.; Shapiro, J.P.; Bott, C.N.; Song, H.; Nadasdy, G.M.; Brodsky, S.V.; Hebert, L.A.; Birmingham, D.J.; Nadasdy, T.; Freitas, M.A.; et al. Characterization of Glomerular Diseases Using Proteomic Analysis of Laser Capture Microdissected Glomeruli. Mod. Pathol. 2012, 25, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.M.; Ren, X.G.; Jiang, Z.H.; Chen, D.J.; Zhao, W.J.; Li, L.J. Lectin-Induced Renal Local Complement Activation Is Involved In Tubular Interstitial Injury In Diabetic Nephropathy. Clin. Chim. Acta 2018, 482, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Goldfine, A.; Krumrei, N.; Grubissich, L.; Acosta, J.; Chorev, M.; Hays, A.P.; Halperin, J.A. Glycation Inactivation of The Complement Regulatory Protein Cd59: A Possible Role in the Pathogenesis of the Vascular Complications of Human Diabetes. Diabetes 2004, 53, 2653–2661. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, D.; Korsten, P.; Strobel, P.; Tampe, B. Complement Components C3 and C4 Indicate Vasculitis Manifestations to Distinct Renal Compartments in Anca-Associated Glomerulonephritis. Int. J. Mol. Sci. 2021, 22, 6588. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, D.; Baier, E.; Kluge, I.A.; Strobel, P.; Tampe, B. Intrarenal Synthesis Of Complement C3 Localized to Distinct Vascular Compartments in Anca-Associated Renal Vasculitis. J. Autoimmun. 2022, 133, 102924. [Google Scholar] [CrossRef]
- Hakroush, S.; Kluge, I.A.; Baier, E.; Tampe, D.; Tampe, B. Relevance of Complement C4 Deposits Localized to Distinct Vascular Compartments in Anca-Associated Renal Vasculitis. Int. J. Mol. Sci. 2022, 23, 14325. [Google Scholar] [CrossRef]
- Baier, E.; Tampe, D.; Kluge, I.A.; Hakroush, S.; Tampe, B. Implication of Platelets and Complement C3 as Link between Innate Immunity and Tubulointerstitial Injury in Renal Vasculitis with Mpo-Anca Seropositivity. Front. Immunol. 2022, 13, 1054457. [Google Scholar] [CrossRef]
- Korsten, P.; Baier, E.; Hakroush, S.; Tampe, B. C-Reactive Protein Levels Are Associated with Complement C4 Deposits and Interstitial Arteritis in ANCA-Associated Renal Vasculitis. Int. J. Mol. Sci. 2023, 24, 3075. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.A.; Cosio, F.G.; Neff, J.C. Diagnostic Significance of Hypocomplementemia. Kidney Int. 1991, 39, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Park, A.S.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome Analysis Of Human Diabetic Kidney Disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Nair, V.; Smith, S.; Zhu, L.; Shedden, K.; Song, P.X.K.; Mariani, L.H.; Eichinger, F.H.; Berthier, C.C.; Randolph, A.; et al. Tissue Transcriptome-Driven Identification of Epidermal Growth Factor as a Chronic Kidney Disease Biomarker. Sci. Transl. Med. 2015, 7, 316ra193. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.C.; Satou, R.; Miyata, K.; Katsurada, A.; Dugas, C.M.; Klingenberg, N.C.; Fonseca, V.A.; Navar, L.G. Canagliflozin Prevents Intrarenal Angiotensinogen Augmentation and Mitigates Kidney Injury and Hypertension in Mouse Model of Type 2 Diabetes Mellitus. Am. J. Nephrol. 2019, 49, 331–342. [Google Scholar] [CrossRef]
- Sen, T.; Heerspink, H.J.L. A Kidney Perspective on the Mechanism of Action of Sodium Glucose Co-Transporter 2 Inhibitors. Cell Metab. 2021, 33, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Saemann, M.; Kronbichler, A. Call for action in ANCA-Associated Vasculitis and Lupus Nephritis: Promises and Challenges of Sglt-2 Inhibitors. Ann. Rheum. Dis. 2022, 81, 614–617. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, D.; Kluge, I.A.; Baier, E.; Korsten, P.; Tampe, B. Comparative Analysis of Sglt-2 Expression in Renal Vasculitis and Lupus Nephritis. Ann. Rheum. Dis. 2022, 81, 1048–1050. [Google Scholar] [CrossRef]
- Saemann, M.; Kronbichler, A. Response to: Correspondence on ‘Call for Action in ANCA-Associated Vasculitis and Lupus Nephritis: Promises and Challenges of SGLT-2 Inhibitors’ by Saemann and Kronbichler. Ann. Rheum. Dis. 2022, 8. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, S.S.; He, Y.X.; Yan, L.J.; Lv, F.; Liang, Q.M.; Gan, Y.H.; Han, L.P.; Xu, H.D.; Li, Y.C.; et al. SGLT2 Inhibitors Alleviated Podocyte Damage in Lupus Nephritis by Decreasing Inflammation and Enhancing Autophagy. Ann. Rheum. Dis. 2023, 82, 1328–1340. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. Sglt2 Inhibitor Dapagliflozin Limits Podocyte Damage in Proteinuric Nondiabetic Nephropathy. JCI Insight 2018, 3, e98720. [Google Scholar] [CrossRef]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: Sglt2 Protein Inhibition Decreases Renal Lipid Accumulation, Inflammation, and the Development of Nephropathy in Diabetic Mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.E.; Mullins, R.H.; Morin, L.G. Non-Enzymic Glycation of Individual Plasma Proteins in Normoglycemic and Hyperglycemic Patients. Clin. Chem. 1987, 33, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Peake, P.W.; Charlesworth, J.A.; Timmermans, V.; Gavrilovic, L.; Pussell, B. Does Non-Enzymatic Glycosylation Affect Complement Function In Diabetes? Diabetes Res. 1989, 11, 109–114. [Google Scholar] [PubMed]
- Li, L.; Chen, L.; Zang, J.; Tang, X.; Liu, Y.; Zhang, J.; Bai, L.; Yin, Q.; Lu, Y.; Cheng, J.; et al. C3a and C5a Receptor Antagonists Ameliorate Endothelial-Myofibroblast Transition Via the Wnt/Beta-Catenin Signaling Pathway In Diabetic Kidney Disease. Metabolism 2015, 64, 597–610. [Google Scholar] [CrossRef]
- Tan, S.M.; Ziemann, M.; Thallas-Bonke, V.; Snelson, M.; Kumar, V.; Laskowski, A.; Nguyen, T.V.; Huynh, K.; Clarke, M.V.; Libianto, R.; et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes 2020, 69, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, T.; Liu, S.; Wang, C.; Zhao, M.; Feng, Y.; Ma, L.; Lu, Y.; Fu, P.; Liu, J. Complement C5 Activation Promotes Type 2 Diabetic Kidney Disease Via Activating Stat3 Pathway And Disrupting The Gut-Kidney Axis. J. Cell Mol. Med. 2021, 25, 960–974. [Google Scholar] [CrossRef]
- Zahedi, R.; Braun, M.; Wetsel, R.A.; Ault, B.H.; Khan, A.; Welch, T.R.; Frenzke, M.; Davis, A.E. The C5a Receptor is Expressed by Human Renal Proximal Tubular Epithelial Cells. Clin. Exp. Immunol. 2000, 121, 226–233. [Google Scholar] [CrossRef]
- Weiss, S.; Rosendahl, A.; Czesla, D.; Meyer-Schwesinger, C.; Stahl, R.A.; Ehmke, H.; Kurts, C.; Zipfel, P.F.; Kohl, J.; Wenzel, U.O. The Complement Receptor C5ar1 Contributes to Renal Damage But Protects the Heart in Angiotensin Ii-Induced Hypertension. Am. J. Physiol. Renal Physiol. 2016, 310, F1356–F1365. [Google Scholar] [CrossRef]
- Mathern, D.R.; Heeger, P.S. Molecules Great and Small: The Complement System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1636–1650. [Google Scholar] [CrossRef]
- Abe, K.; Miyazaki, M.; Koji, T.; Furusu, A.; Nakamura-Kurashige, T.; Nishino, T.; Ozono, Y.; Harada, T.; Sakai, H.; Kohno, S. Enhanced Expression of Complement C5a Receptor Mrna in Human Diseased Kidney Assessed by In Situ Hybridization. Kidney Int. 2001, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular Pathways that Drive Diabetic Kidney Disease. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Zhang, L.; Fu, J.; Verghese, D.A.; Chauhan, K.; Nadkarni, G.N.; Li, Z.; Ju, W.; Kretzler, M.; Cai, G.Y.; et al. LRG1 Promotes Diabetic Kidney Disease Progression by Enhancing TGF-beta-Induced Angiogenesis. J. Am. Soc. Nephrol. 2019, 30, 546–562. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2022, 102, S1–S127. [Google Scholar] [CrossRef] [PubMed]
- Ring, T.; Pedersen, B.B.; Salkus, G.; Goodship, T.H. Use of Eculizumab in Crescentic IgA Nephropathy: Proof of Principle and Conundrum? Clin. Kidney J. 2015, 8, 489–491. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Duggan, S. Risankizumab: First Global Approval. Drugs 2019, 79, 893–900. [Google Scholar] [CrossRef]
- Jayne, D.R.W.; Merkel, P.A.; Schall, T.J.; Bekker, P.; Group, A.S. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2021, 384, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Rung, J.; Brazma, A. Reuse of Public Genome-Wide Gene Expression Data. Nat. Rev. Genet. 2013, 14, 89–99. [Google Scholar] [CrossRef]
- Berthier, C.C.; Bethunaickan, R.; Gonzalez-Rivera, T.; Nair, V.; Ramanujam, M.; Zhang, W.; Bottinger, E.P.; Segerer, S.; Lindenmeyer, M.; Cohen, C.D.; et al. Cross-Species Transcriptional Network Analysis Defines Shared Inflammatory Responses in Murine and Human Lupus Nephritis. J. Immunol. 2012, 189, 988–1001. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome Pathway Analysis: A High-Performance In-Memory Approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
| Variable | ß | p Value |
|---|---|---|
| C2—median-centered log2 mRNA expression | 0.2981 | 0.1633 |
| C5—median-centered log2 mRNA expression | 0.5997 | 0.0109 |
| C5AR2—median-centered log2 mRNA expression | 0.3918 | 0.0572 |
| CR2—median-centered log2 mRNA expression | 0.0976 | 0.6626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsten, P.; Tampe, B. A Transcriptome Array-Based Approach to Link SGLT-2 and Intrarenal Complement C5 Synthesis in Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 17066. https://doi.org/10.3390/ijms242317066
Korsten P, Tampe B. A Transcriptome Array-Based Approach to Link SGLT-2 and Intrarenal Complement C5 Synthesis in Diabetic Nephropathy. International Journal of Molecular Sciences. 2023; 24(23):17066. https://doi.org/10.3390/ijms242317066
Chicago/Turabian StyleKorsten, Peter, and Björn Tampe. 2023. "A Transcriptome Array-Based Approach to Link SGLT-2 and Intrarenal Complement C5 Synthesis in Diabetic Nephropathy" International Journal of Molecular Sciences 24, no. 23: 17066. https://doi.org/10.3390/ijms242317066
APA StyleKorsten, P., & Tampe, B. (2023). A Transcriptome Array-Based Approach to Link SGLT-2 and Intrarenal Complement C5 Synthesis in Diabetic Nephropathy. International Journal of Molecular Sciences, 24(23), 17066. https://doi.org/10.3390/ijms242317066






