Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents—In Vitro Evaluation against Breast Cancer Cells
Abstract
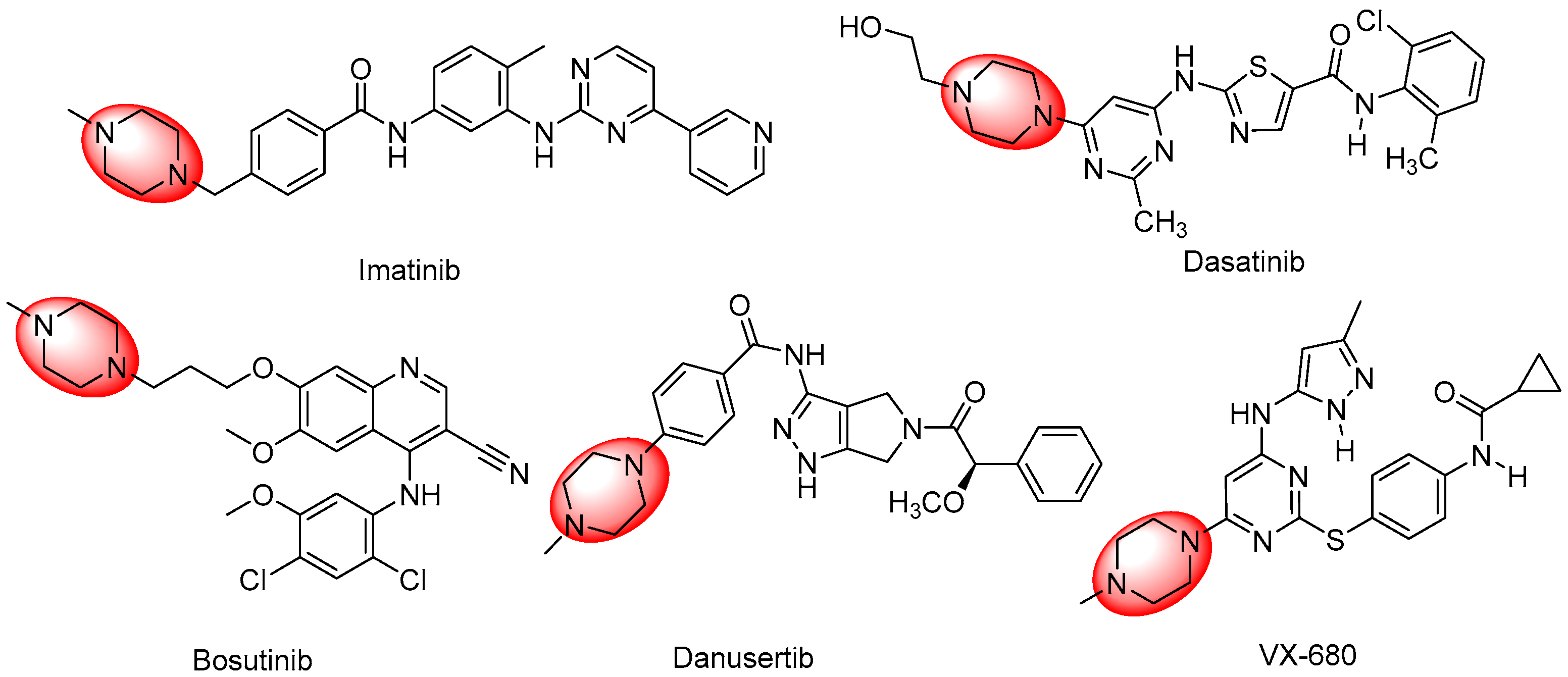
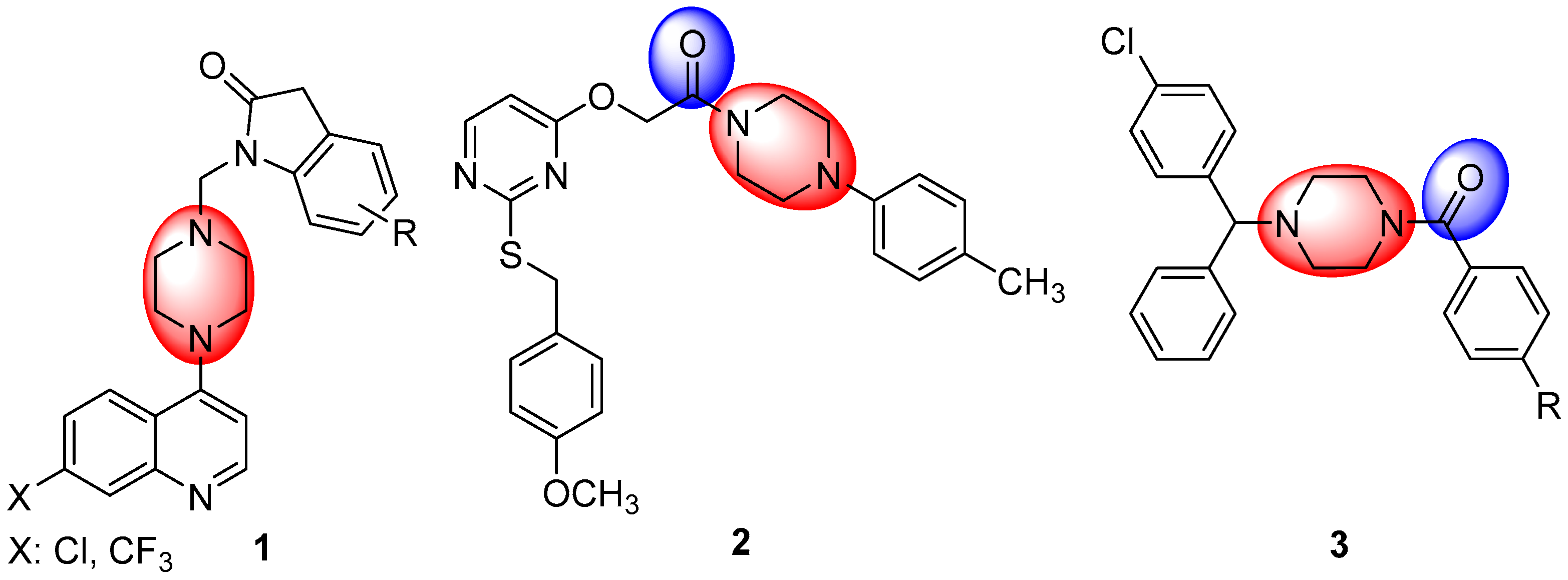
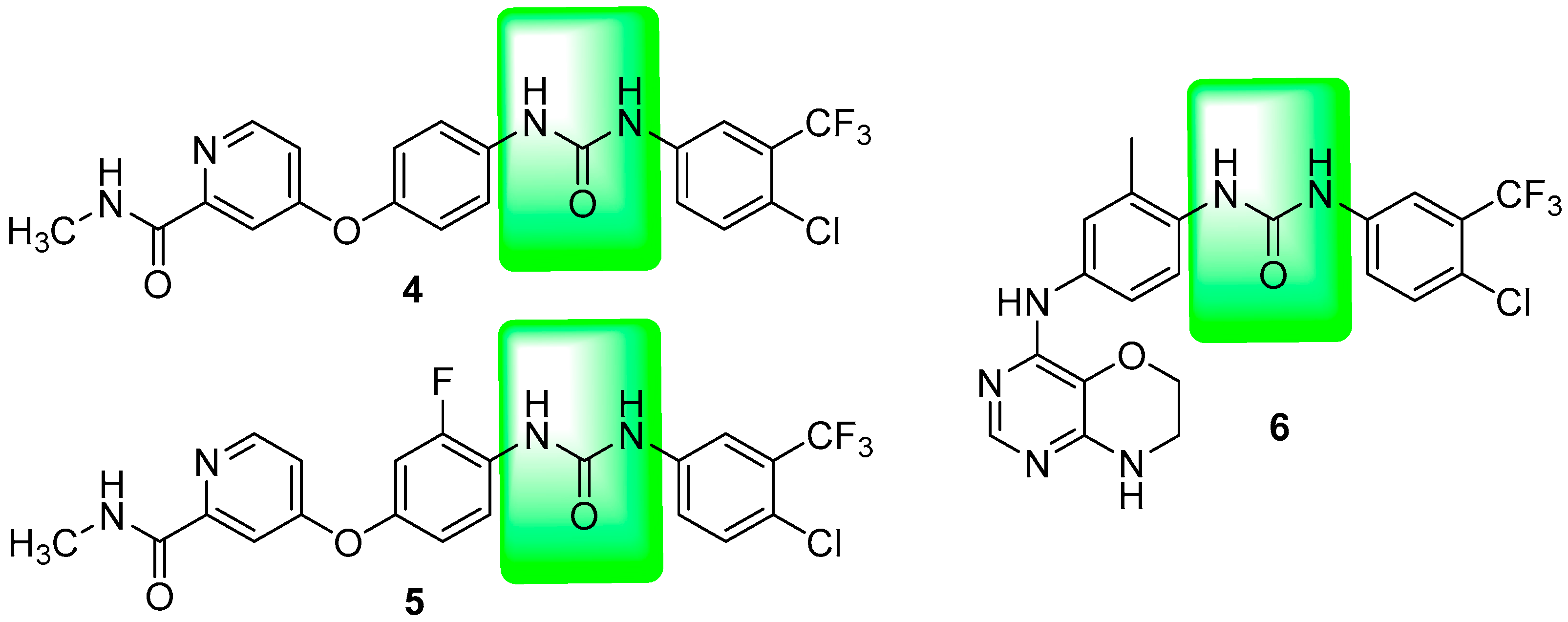
1. Introduction
2. Results and Discussion
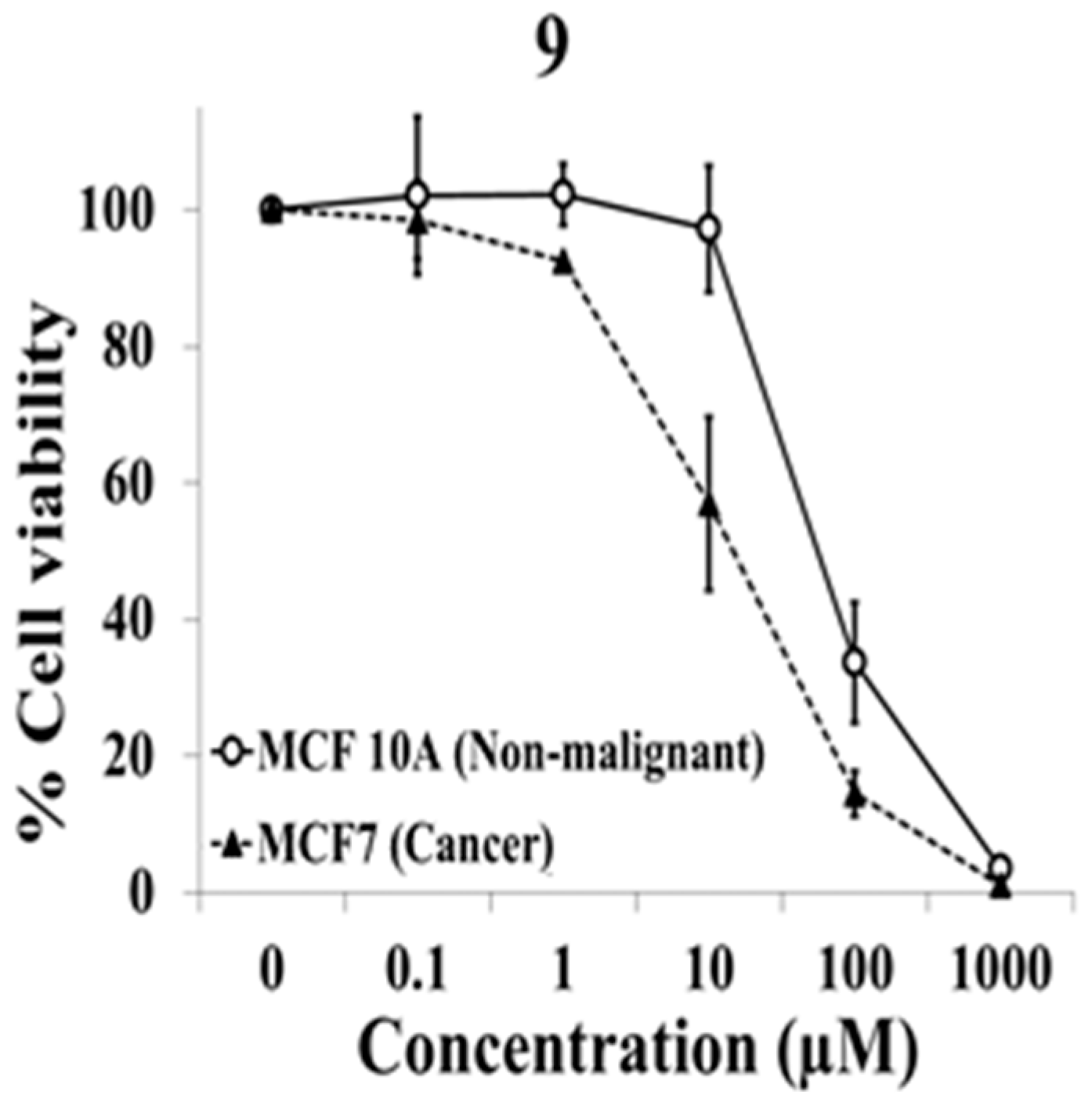
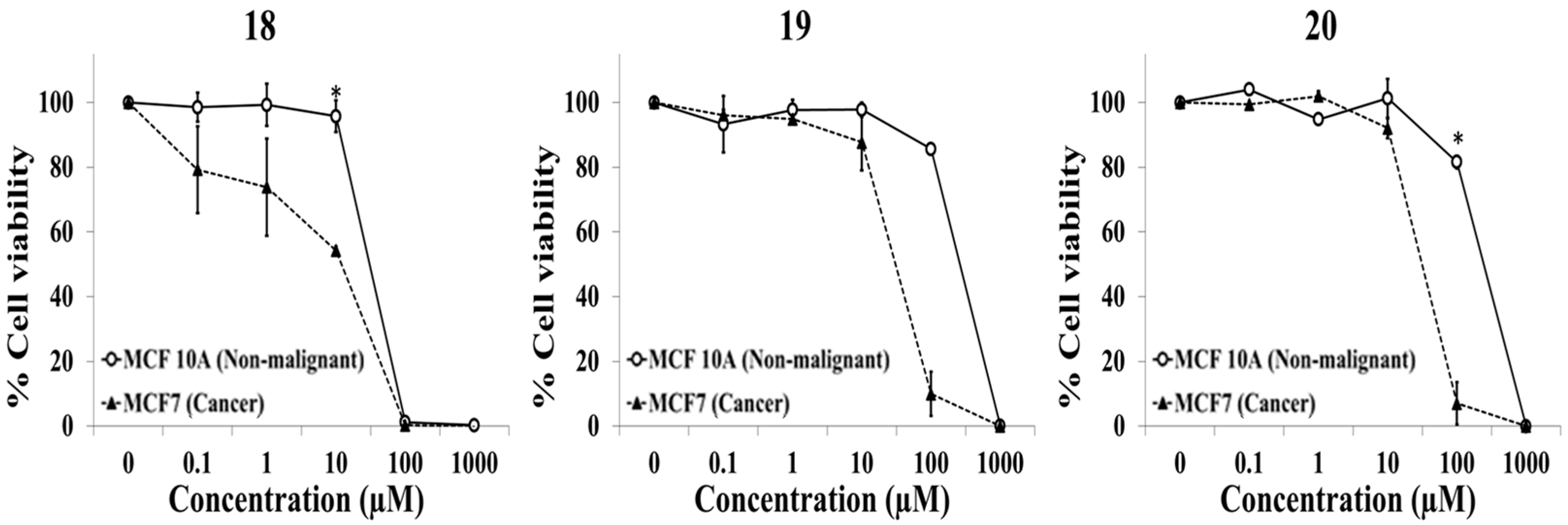
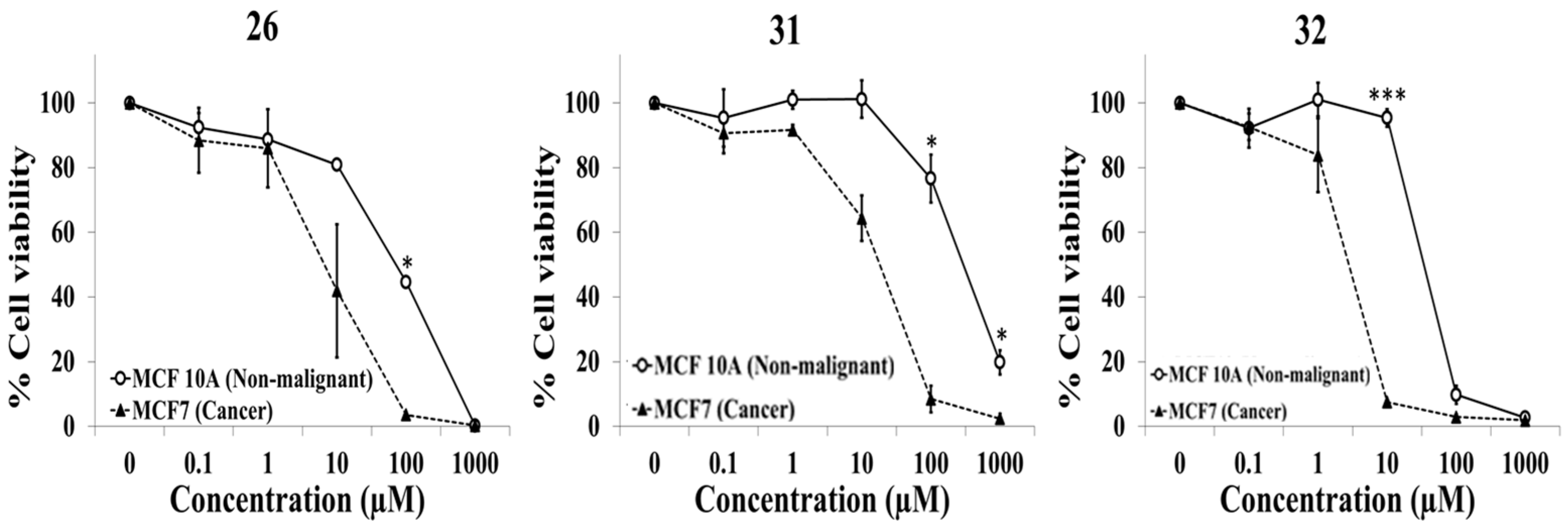
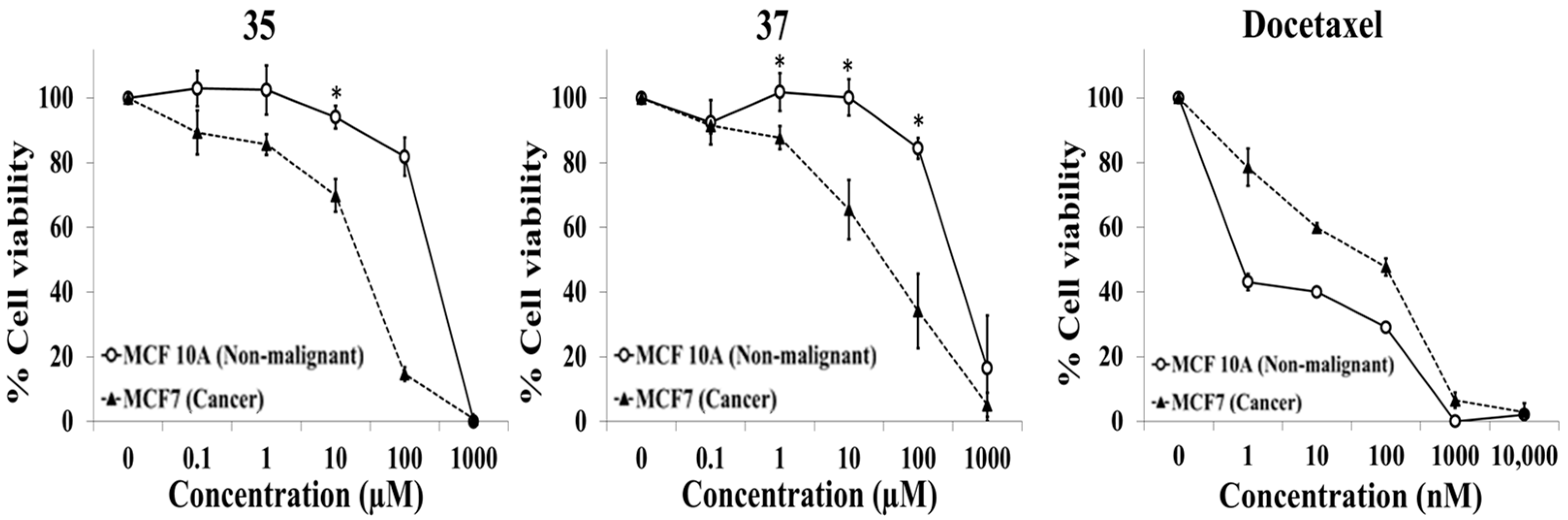
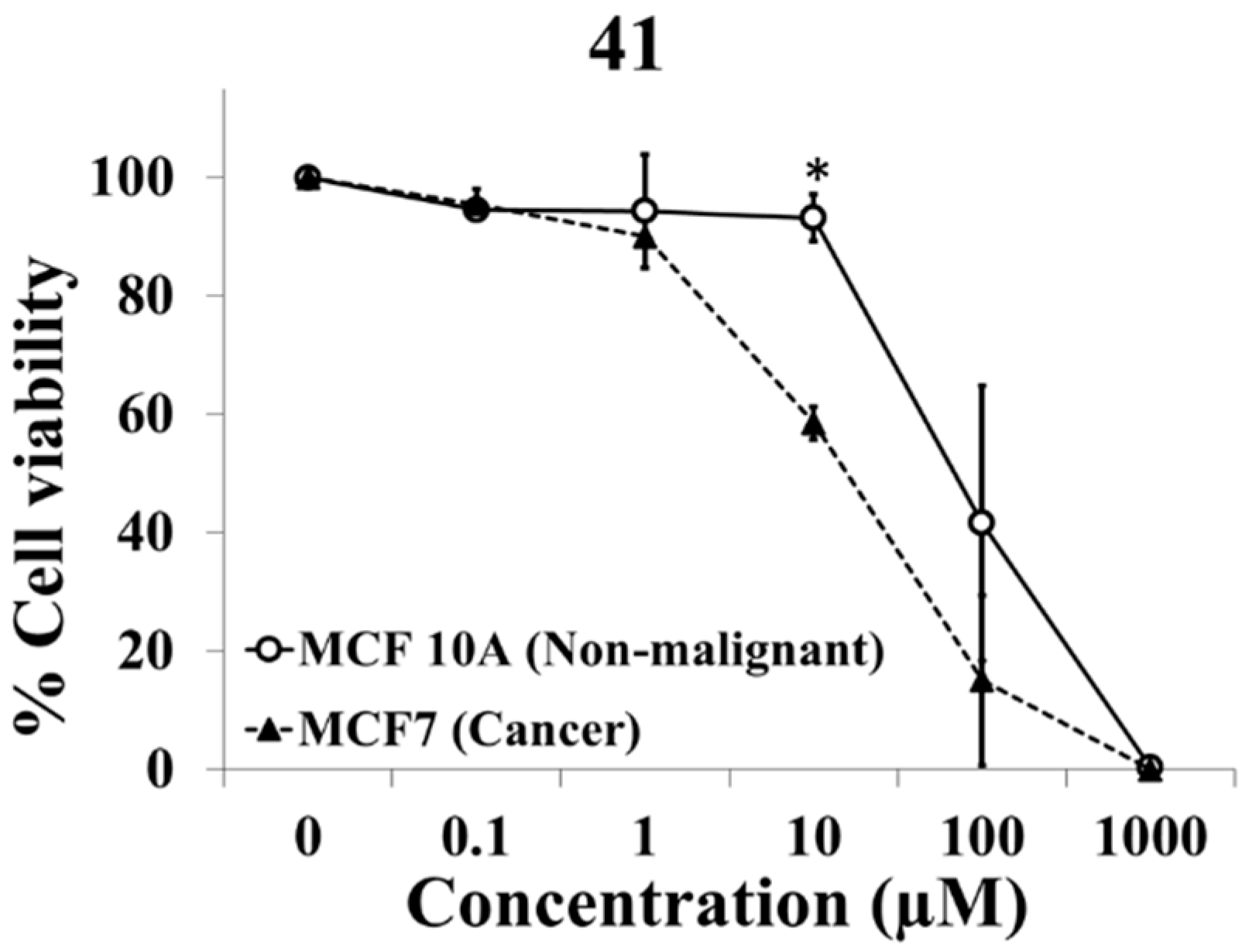
2.1. Evaluation of Selective Cytotoxic Activity of 4-Acyl-2-substituted Piperazine Urea Derivatives against MCF7 Breast Cancer Cells and MCF 10A Normal Breast Cells
2.2. Structure Activity Relationship
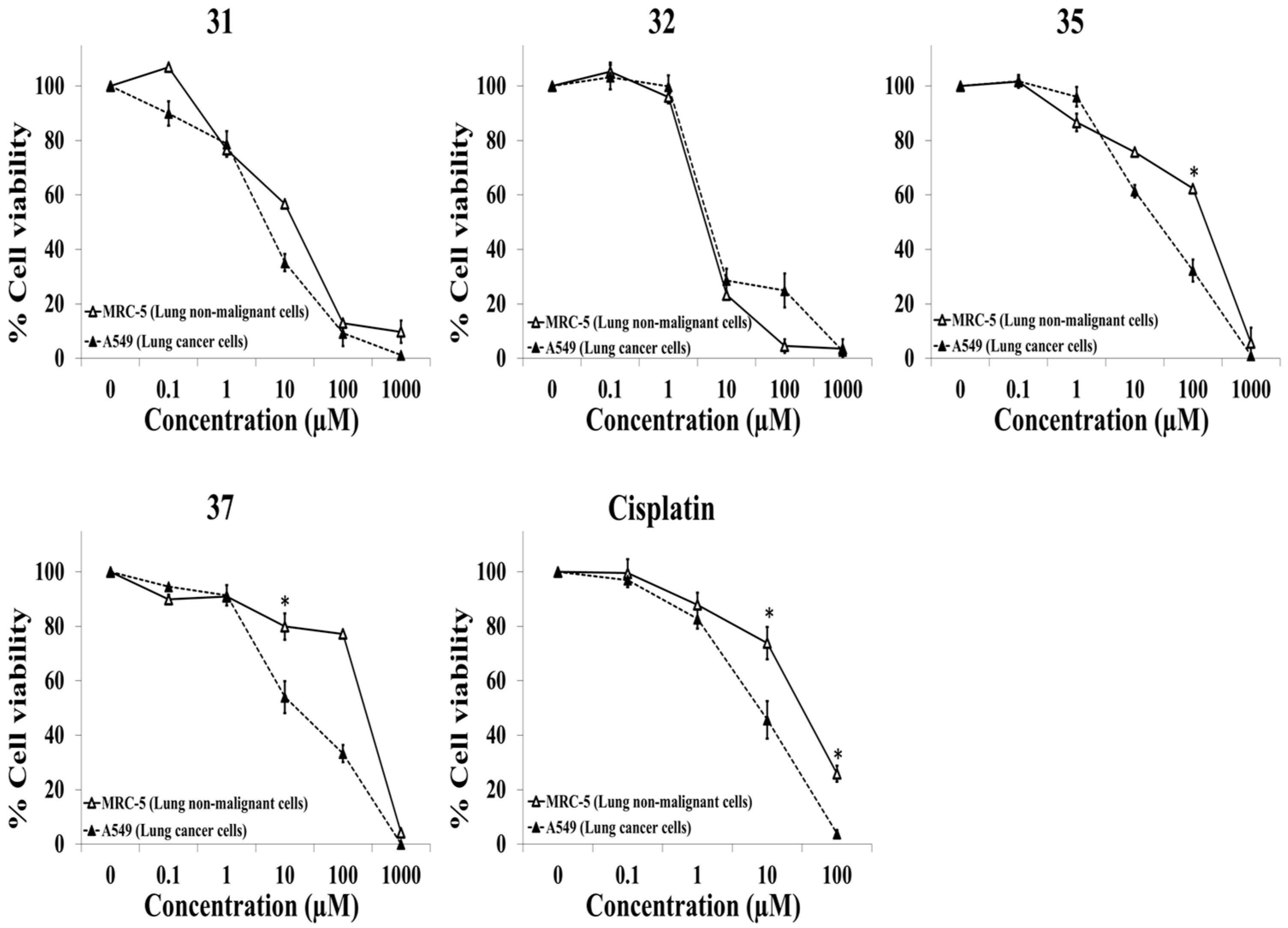
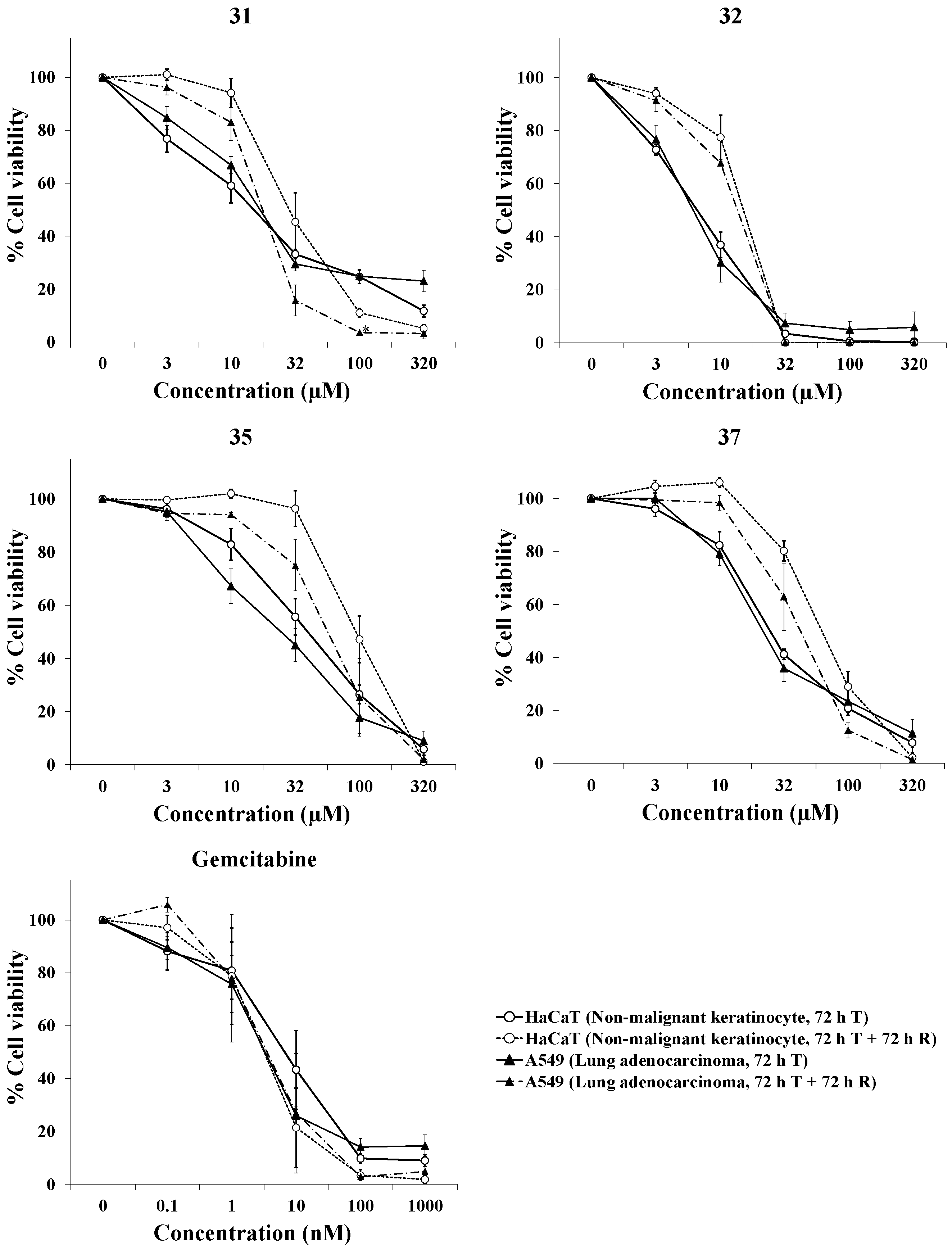
2.3. Evaluation of the Selective Cytotoxic Activity of 4-N-acyl-1-phenylamino(thio)carbonyl-2-substituted Piperazine Derivatives against Nonmalignant Cells and Lung Cancer Cells
2.4. In Silico Evaluation of Physicochemical and Pharmacokinetic Properties of Selected Compounds
3. Materials and Methods
3.1. Biology
3.1.1. Drugs and Reagents
3.1.2. Cell Lines
3.1.3. Cell Viability Assays
3.2. Chemistry
3.2.1. General Methods
3.2.2. Chemoselective N-Acylation Reaction
3.2.3. Synthesis of 4-(Benzofuran-2-carbonyl)-2-methyl-1-[(1-adamantyl)aminocarbonyl]piperazine (41)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E. Current, and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Cancer.Net® Breast Cancer—Metastatic: Statistics. Available online: https://www.cancer.net/cancer-types/breast-cancer-metastatic/statistics#:~:text=Metastatic%20breast%20cancer%20causes%20the,metastatic%20breast%20cancer%20is%2029%25 (accessed on 29 December 2022).
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Gualtieri, G.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Manfreda, L.; Bai, R.; et al. Identification of pyrrolo[3′,4’:3,4]cyclohepta[1,2-d][1,2]oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023, 254, 115372. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Chen, G.; Ji, Y.; Ma, Q.; Qiao, X.; Wu, S.; Zhou, L.; Bu, J.; Zhu, X.; et al. Targeting SOST using a small-molecule compound retards breast cancer bone metastasis. Mol. Cancer 2022, 21, 228. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Raza, A.; Encío, I.; Sharma, A.K.; Plano, D.; Sanmartín, C. Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity. Antioxidants 2023, 12, 1331. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Y.; Lv, L.; Chen, J.; Jiang, W.G.; Li, J.; Lin, Q.; Fang, X.; Gao, J.; Liu, Y.; et al. Small Molecular Inhibitors Reverse Cancer Metastasis by Blockading Oncogenic PITPNM3. Adv. Sci. 2022, 9, 2204649. [Google Scholar] [CrossRef]
- Hatnapure, G.D.; Keche, A.P. Synthesis and biological evaluation of novel piperazine derivatives of flavone as potent anti-inflammatory and antimicrobial agent. Bioorg. Med. Chem. Lett. 2012, 22, 6385–6390. [Google Scholar] [CrossRef]
- Singh, V.; Pacitto, A. Synthesis and Structure-Activity relationship of 1-(5-isoquinolinesulfonyl)piperazine analogues as inhibitors of Mycobacterium tuberculosis IMPDH. Eur. J. Med. Chem. 2019, 174, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Mahmood, T. Design, synthesis and anti-bacterial studies of piperazine derivatives against drug resistant bacteria. Eur. J. Med. Chem. 2019, 166, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cyriac, G. Structure-Activity Relationships for a Novel Series of Citalopram (1-(3-(Dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile). Analogues at Monoamine Transporters. J. Med. Chem. 2010, 53, 6112–6121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzotta, S.; Cebrero-Cangueiro, T.L.; Vega-Holm, M.; Carretero-Ledesma, M.; Sánchez-Céspedes, J.; Cappello, A.R.; Aiello, F.; Pachón, J.; Vega-Pérez, J.M.; Iglesias-Guerra, F.; et al. Exploration of piperazine-derived thioureas as antibacterial and anti-inflammatory agents. In vitro evaluation against clinical isolates of colistin-resistant Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2020, 30, 127411. [Google Scholar]
- Thakur, A.; Khan, S.I. Synthesis of piperazine tethered 4-aminoquinoline-pyrimidine hybrids as potent antimalarial agents. RSC Adv. 2014, 4, 20729–20736. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Z. Ferulic acid derivatives with piperazine moiety as potential antiviral agents. Pest. Manag. Sci. 2022, 78, 1749–1758. [Google Scholar] [CrossRef]
- Sánchez-Céspedes, J.; Martínez-Aguado, P.; Vega-Holm, M.; Serna-Gallego, A.; Candela, J.I.; Marrugal-Lorenzo, J.A.; Pachón, J.; Iglesias-Guerra, F.; Vega-Pérez, J.M. New 4-Acyl-1-Phenylaminocarbonyl-2-Phenylpiperazine Derivatives as PotentialInhibitors of Adenovirus Infection. Synthesis, Biological Evaluation, and Structure-Activity Relationships. J. Med. Chem. 2016, 59, 5432–5448. [Google Scholar] [PubMed]
- Mazzotta, S.; Marrugal-Lorenzo, J.A.; Vega-Holm, M.; Serna-Gallego, A.; Alvarez-Vidal, J.; Berastegui-Cabrera, J.; Pérez del Palacio, J.; Díaz, C.; Aiello, F.; Pachón, J.; et al. Optimization of piperazine-derived ureas privileged structures for effective antiadenovirus agents. Eur. J. Med. Chem. 2020, 185, 111840. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, M.K.; Revanasiddappa, H.D. Synthesis, characterization, antidepressant, and antioxidant activity of novel piperamides bearing piperidine and piperazine analogues. Bioorg. Med. Chem. Lett. 2012, 22, 7065–7070. [Google Scholar] [CrossRef]
- Xie, S.; Lia, X. Design, synthesis and biological evaluation of isochroman-4-one hybrids bearing piperazine moiety as antihypertensive agent candidates. Bioorg. Med. Chem. 2019, 27, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, S.; Venkataramaiah, C. Design, synthesis, in vitro and in silico bioactivity profiles of new urea/thiourea derivatives of 2-pyridyl piperazine as potent antioxidant and antimicrobial agents: Chemo-bio-computational approach. J. Biomol. Struct. Dyn. 2022, 41, 4786–4797. [Google Scholar] [CrossRef]
- Buchdunger, E.; O’Reilly, T.; Wood, J. Pharmacology of imatinib (STI571). Eur. J. Cancer 2002, 38, S28–S36. [Google Scholar] [CrossRef]
- Johnson, F.M.; Saigal, B.; Talpaz, M.; Donato, N.J. Dasatinib (BMS-354825) Tyrosine Kinase Inhibitor Suppresses Invasion and Induces Cell Cycle Arrest and Apoptosis of Head and Neck Squamous Cell Carcinoma and Non Small Cell Lung Cancer Cells. Clin. Cancer Res. 2005, 11, 6924–6932. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.M.; Brümmendorf, T.H.; Kim, D.W.; Turkina, A.G.; Shen, Z.X.; Pasquini, R.; Khoury, H.J.; Arkin, S.; Volkert, A.; et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphiachromosome–positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011, 118, 4567–4576. [Google Scholar] [CrossRef]
- Fraedrich, K.; Schrader, J.; Ittrich, H.; Keller, G.; Gontarewicz, A.; Matzat, V.; Kromminga, A.; Pace, A.; Moll, J.; Bläker, M.; et al. Targeting Aurora Kinases with Danusertib (PHA-739358) Inhibits Growth of Liver Metastases from Gastroenteropancreatic Neuroendocrine Tumors in an Orthotopic Xenograft Model. Clin. Cancer Res. 2012, 18, 4621–4632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tyler, R.K.; Shpiro, N.; Marquez, R.; Eyers, P.A. VX-680 Inhibits Aurora A and Aurora B Kinase Activity in Human Cells. Cell Cycle 2007, 6, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Hu, C.; Lee, H. Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: A hybrid pharmacophore approach. Bioorg. Med. Chem. 2010, 18, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Deveshegowda, S.N.; Metri, P.K.; Shivakumar, R.; Yang, J.-R.; Rangappa, S.; Swamynayaka, A.; Shanmugam, M.K.; Nagaraja, O.; Madegowda, M.; Shubha, P.B.; et al. Development of 1-(4-(Substituted)piperazin-1-yl)-2-((2-((4-methoxybenzyl)thio)pyrimidin-4-yl)oxy)ethanones That Target Poly (ADP-Ribose) Polymerase in Human Breast Cancer Cells. Molecules 2022, 27, 2848. [Google Scholar] [CrossRef] [PubMed]
- Yarim, M.; Koksal, M.; Durmaz, I.; Atalay, R. Cancer cell cytotoxicities of 1-(4-substitutedbenzoyl)-4-(4-chlorobenzhydryl)piperazine derivatives. Int. J. Mol. Sci. 2012, 13, 8071–8085. [Google Scholar] [CrossRef] [PubMed]
- Elmeligie, S.; Aboul-Magd, A.M. Design and synthesis of phthalazine-based compounds as potent anticancer agents with potential antiangiogenic activity via VEGFR-2 inhibition. J. Enzym. Inhib. Med. Chem. 2019, 34, 1347–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, K.; Zhang, G.; Yang, Q.-Y.; Li, Y.-S.; Huang, S.-Z.; Wang, Y.-L.; Yang, W.; Jiang, X.-J.; Yan, H.-X.; et al. Structural optimization and structure-activity relationship studies of N-phenyl-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-amine derivatives as a new class of inhibitors of RET and its drug resistance mutants. Eur. J. Med. Chem. 2018, 143, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.M. Drug repurposing as a current strategy in medicine discovery. Semergen 2022, 48, 101790. [Google Scholar] [CrossRef]
- Schein, C.H. Repurposing approved drugs on the pathway to novel therapies. Med. Res. Rev. 2020, 40, 586–605. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.D.; Chew, H.; et al. NCCN Guidelines Version 1.2023 Breast Cancer. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (accessed on 27 January 2023).
- Gaoa, H.; Zhang, X.; Pu, X.-J.; Zheng, X.; Liu, B.; Rao, G.-X.; Wan, C.-P.; Mao, Z.-W. 2-Benzoylbenzofuran derivatives possessing piperazine linker as anticancer agents. Bioorg. Med. Chem. Lett. 2019, 29, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, P.; Machowska, M.; Wisniewski, K.; Grynkiewicz, G.; Hrynyk, R.; Rzepecki, R.; Gubernator, J. Novel pegylated liposomal formulation of docetaxel with 3-n-pentadecylphenol derivative for cancer therapy. Eur. J. Pharm. Sci. 2021, 163, 105838. [Google Scholar] [CrossRef] [PubMed]
- Kozubík, A.; Horváth, V.; Svihálková-Sindlerová, L.; Soucek, K.; Hofmanová, J.; Suva, P.; Kroutil, A.; Zák, F.; Mistr, A.; Turánek, J. High effectiveness of platinum(IV) complex with adamantylamine in overcoming resistance to cisplatin and suppressing proliferation of ovarian cancer cells in vitro. Biochem. Pharmacol. 2005, 69, 373–383. [Google Scholar] [CrossRef]
- Herůdková, J.; Paruch, K.; Khirsariya, P.; Souček, K.; Krkoška, M.; Blanářová, O.V.; Sova, P.; Kozubík, A.; Vaculová, A.H. CHK1 inhibitor SCH900776 effectively potentiates the cytotoxic effects of platinum-based chemotherapeutic drugs in human colon cancer cells. Neoplasia 2017, 19, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, V.; Saxena, R.; Pitushkin, D.; Butov, G.M.; Chung, L.-G.; Aggarwal, M. Adamantyl Isothiocyanates as Mutant p53 Rescuing Agents and Their Structure−Activity Relationships. J. Med. Chem. 2021, 64, 6621–6663. [Google Scholar] [CrossRef] [PubMed]
- Zefirov, N.A.; Hoppe, M.; Kuznetsova, I.V.; Chernyshov, N.A.; Grishin, Y.K.; Maloshitskaya, O.A.; Kuznetsov, S.A.; Zefirova, O.N. Homologous series of novel adamantane–colchicine conjugates: Synthesis and cytotoxic effect on human cancer cells. Mendeleev Commun. 2018, 28, 308–310. [Google Scholar] [CrossRef]
- Schumacher, T.J.; Sah, N.; Palle, K.; Rumbley, J.; Mereddy, V.R. Synthesis and biological evaluation of benzofuran piperazine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2023, 93, 129425. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Gao, H.; Wan, C.; Rao, G.; Mao, Z. Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety. Molecules 2016, 21, 1684. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, W.; Ding, X.; Zhang, L.; Xia, Q.; Wang, Q.; Chen, Q.; Gao, Q.; Yan, J.; Lesyk, R.; et al. Design, synthesis, and anticancer evaluation of nitrobenzoxadiazolepiperazine hybrids as potent pro-apoptotic agents. Tetrahedron 2023, 138, 133393. [Google Scholar] [CrossRef]
- Eldehnaa, W.M.; Hassanb, G.S.; Al-Rashoodc, S.T.; Al-Warhid, T.; Altyare, A.E.; Alkahtanic, H.M.; Almehiziac, A.A.; Abdel-Azizf, H.A. Synthesis and in vitro anticancer activity of certain novel 1-(2-methyl-6-arylpyridin3-yl)-3-phenylureas as apoptosis-inducing agent. J. Enzym. Inhib. Med. Chem. 2019, 34, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Chen, C.B.; Wu, M.Y.; Wu, J.; Yang, C.H.; Hui, R.C.; Chang, Y.C.; Lu, C.W. Anticancer Drugs Induced Severe Adverse Cutaneous Drug Reactions: An Updated Review on the Risks Associated with Anticancer Targeted Therapy or Immunotherapies. J. Immunol. Res. 2018, 2018, 5376476. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Y.; Li, J.Y.; Hao, G.F.; Yang, G.F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Amin, N.H.; El-Saadi, M.T.; Ibrahim, A.A.; Abdel-Rahman, H.M. Design, synthesis and mechanistic study of new 1,2,4-triazole derivatives as antimicrobial agents. Bioorg. Chem. 2021, 111, 104841. [Google Scholar] [CrossRef]
- Morcoss, M.M.; Abdelhafez, E.S.M.N.; Ibrahem, R.A.; Abdel-Rahman, H.M.; Abdel-Aziz, M.; Abou El-Ella, D.A. Design, synthesis, mechanistic studies and in silico ADME predictions of benzimidazole derivatives as novel antifungal agents. Bioorg. Chem. 2020, 101, 103956. [Google Scholar] [CrossRef]
- Lamie, P.F.; Philoppes, J.N. 2-Thiopyrimidine/chalcone hybrids: Design, synthesis, ADMET prediction, and anticancer evaluation as STAT3/STAT5a inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 864–879. [Google Scholar] [CrossRef]
- PreADMET. Bioinformatics and Molecular Design Research Center. Available online: https://preadmet.bmdrc.kr (accessed on 29 November 2023).
- Gavilán, E.; Sánchez-Aguayo, I.; Daza, P.; Ruano, D. WorzellGSK-3β signaling determines autophagy activation in the breast tumor cell line MCF7 and inclusion formation in the non-tumor cell line MCF10A in response to proteasome inhibition. Cell Death Dis. 2013, 4, e572. [Google Scholar] [CrossRef][Green Version]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Bael, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 27 January 2023).
- Lopez-Lazaro, M. A Simple and Reliable Approach for Assessing Anticancer Activity In Vitro. Curr. Med. Chem. 2015, 22, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
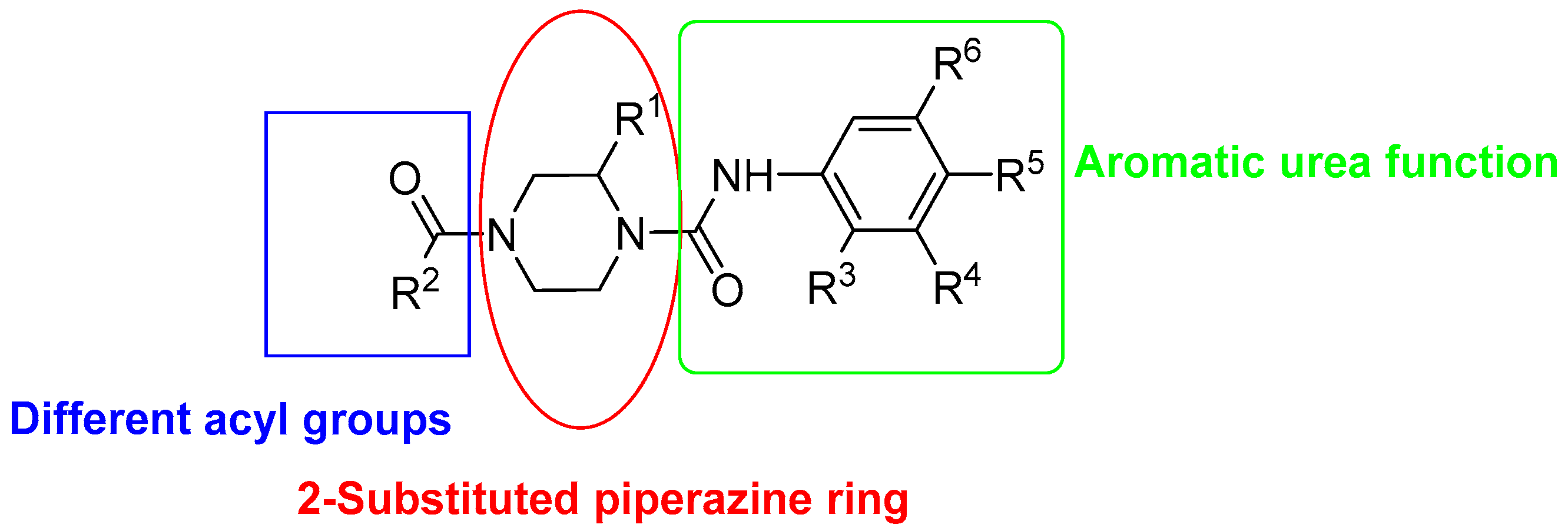

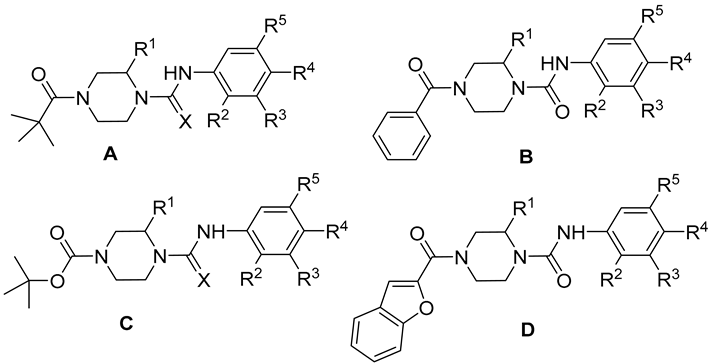
| Compound | Structure | X | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|
| 7 | A | O | CH3 | H | H | NO2 | H |
| 8 | A | O | CH3 | H | H | OCH3 | H |
| 9 | A | S | CH3 | H | H | NO2 | H |
| 10 | B | O | CH3 | H | H | NO2 | H |
| 11 | B | O | CH3 | H | H | OCH3 | H |
| 12 | C | O | CH3 | H | H | NO2 | H |
| 13 | C | O | CH3 | H | H | OCH3 | H |
| 14 | C | S | CH3 | H | H | NO2 | H |
| 15 | C | O | CH3 | H | H | Cl | H |
| 16 | C | O | CH3 | H | H | CF3 | H |
| 17 | C | O | CH3 | H | Cl | CF3 | H |
| 18 | D | O | CH3 | H | H | NO2 | H |
| 19 | D | O | CH3 | H | H | Cl | H |
| 20 | D | O | CH3 | H | H | CF3 | H |
| 21 | D | O | CH3 | H | H | CN | H |
| 22 | D | O | CH3 | NO2 | H | H | H |
| 23 | D | O | CH3 | OCH3 | H | H | H |
| 24 | D | O | CH3 | Br | H | H | H |
| 25 | D | O | CH3 | H | H | H | H |
| 26 | C | O | Ph | H | H | NO2 | H |
| 27 | C | O | Ph | H | H | Cl | H |
| 28 | C | O | Ph | H | H | CN | H |
| 29 | C | O | Ph | H | H | F | H |
| 30 | C | O | Ph | NO2 | H | H | H |
| 31 | C | O | Ph | Cl | H | H | CF3 |
| 32 | C | O | Ph | H | CF3 | Cl | H |
| 33 | C | O | Ph | H | H | OCH3 | H |
| 34 | C | O | Ph | H | H | CH3 | H |
| 35 | D | O | Ph | H | H | NO2 | H |
| 36 | D | O | Ph | H | H | Cl | H |
| 37 | D | O | Ph | H | H | CN | H |
| 38 | D | O | Ph | Cl | H | H | CF3 |
| 39 | D | O | Ph | NO2 | H | H | H |
| IC50 (Mean ± SEM; μM) a | |||
|---|---|---|---|
| Compound | MCF 10A (Normal) | MCF7 (Cancer) | Selectivity Index b |
| 7 | 38.5 ± 4.6 | 60.8 ± 1.2 | 0.6 ± 0.1 |
| 8 | 540.6 ± 259.1 | 117.6 ± 48.7 | 5.2 ± 3.1 |
| 9 | 58.2 ± 18.3 | 15.8 ± 8.2 | 4.2 ± 1.0 |
| 10 | 34.1 ± 1.7 | 26.7 ± 3.3 | 1.3 ± 0.1 |
| 11 | 477.6 ± 35.6 | 358.8 ± 74.7 | 1.4 ± 0.4 |
| 12 | 84.2 ± 12.4 | 34.6 ± 4.8 | 2.4 ± 0.0 |
| 13 | 915.1 ± 226.4 | 250.5 ± 19.4 | 3.6 ± 0.6 |
| 14 | 35.6 ± 4.5 | 34.2 ± 0.1 | 1.0 ± 0.1 |
| 15 | 169.9 ± 61.6 | 43.4 ± 3.7 | 3.8 ± 1.1 |
| 16 | 60.1 ± 19.8 | 31.3 ± 3.6 | 1.9 ± 0.4 |
| 17 | 41.7 ± 4.1 | 22.7 ± 1.6 | 1.9 ± 0.2 |
| 18 | 30.4 ± 1.8 | 12.1 ± 0.6 | 2.5 ± 0.2 |
| 19 | 261.0 ± 2.6 | 30.5 ± 1.1 | 8.6 ± 0.4 |
| 20 | 243.8 ± 8.2 | 31.7 ± 4.1 | 7.8 ± 0.7 |
| 21 | 31.2 ± 2.8 | 19.4 ± 3.8 | 1.6 ± 0.2 |
| 22 | 32.4 ± 1.9 | 28.1 ± 1.2 | 1.2 ± 0.1 |
| 23 | 74.7 ± 30.2 | 31.4 ± 5.5 | 2.3 ± 0.6 |
| 24 | 33.5 ± 3.5 | 25.3 ± 1.6 | 1.3 ± 0.1 |
| 25 | 160.9 ± 64.6 | 48.5 ± 10.1 | 3.2 ± 0.7 |
| 26 | 71.3 ± 3.6 | 9.6 ± 6.7 | 15.2 ± 11.1 |
| 27 | 33.8 ± 6.0 | 16.8 ± 4.0 | 2.2 ± 0.9 |
| 28 | 55.2 ± 24.4 | 19.5 ± 3.5 | 2.7 ± 0.8 |
| 29 | 56.3 ± 16.5 | 24.8 ± 1.7 | 2.2 ± 0.5 |
| 30 | 63.2 ± 10.9 | 24.3 ± 3.4 | 2.6 ± 0.1 |
| 31 | 297.7 ± 56.4 | 18.6 ± 4.2 | 16.6 ± 1.9 |
| 32 | 34.1 ± 2.2 | 2.7 ± 0.6 | 12.6 ± 2.6 |
| 33 | 325.0 ± 27.6 | 130.4 ± 31.4 | 2.6 ± 0.4 |
| 34 | 68.7 ± 2.8 | 24.4 ± 3.1 | 2.9 ± 0.5 |
| 35 | 244.4 ± 24.9 | 23.0 ± 3.8 | 11.1 ± 2.9 |
| 36 | 160.8 ± 16.5 | 41.3 ± 4.2 | 4.0 ± 0.9 |
| 37 | 484.2 ± 233.0 | 57.1 ± 34.7 | 17.9 ± 7.9 |
| 38 | 116.9 ± 21.1 | 91.9 ± 35.7 | 1.4 ± 0.3 |
| 39 | >1000 | 225.9 ± 175.0 | >2.5 |
| Docetaxel (nM) | ˂1 | 76.1 ± 26.2 | ˂1 |
| IC50 (Mean ± SEM; μM) a | |||
|---|---|---|---|
| Compound | MRC-5 (Normal) | A549 (Cancer) | Selectivity Index b |
| 31 | 14.2 ± 0.5 | 4.8 ± 0.8 | 2.1 ± 0.3 |
| 32 | 4.3 ± 0.2 | 5.2 ± 0.4 | 1.0 ± 0.1 |
| 35 | 165.4 ± 4.1 | 25.6 ± 2.9 | 6.5 ± 2.1 |
| 37 | 235.7 ± 5.4 | 18.7 ± 2.7 | 12.8 ± 1.6 |
| Cisplatin | 31.8 ± 5.6 | 8.2 ± 2.2 | 6.3 ± 1.8 |
| 72 h Treatment | 72 h Treatment + 72 h Recovery Period | |||||
|---|---|---|---|---|---|---|
| IC50 (Mean ± SEM; μM) a | IC50 (Mean ± SEM; μM) a | |||||
| Compound | HaCaT (Nonmalignant) | A549 (Cancer) | Selectivity Index b | HaCaT (Nonmalignant) | A549 (Cancer) | Selectivity Index b |
| 31 | 14.5 ± 3.2 | 17.0 ± 1.6 | 0.8 ± 0.2 | 30.9 ± 7.5 | 17.8 ± 2.0 | 1.8 ± 0.5 |
| 32 | 6.7 ± 0.8 | 6.2 ± 0.6 | 1.1 ± 0.1 | 14.9 ± 1.3 | 13.6 ± 0.1 | 1.1 ± 0.1 |
| 35 | 41.6 ± 8.1 | 28.4 ± 7.3 | 1.6 ± 0.2 | 97.5 ± 16.2 | 69.3 ± 20.2 | 2.0 ± 0.8 |
| 37 | 24.8 ± 1.8 | 22.5 ± 2.7 | 1.1 ± 0.1 | 64.6 ± 6.8 | 41.6 ± 8.9 | 1.9 ± 0.6 |
| Gemcitabine (nM) | 13.1 ± 6.2 | 3.5 ± 0.8 | 3.3 ± 1.4 | 8.8 ± 6.5 | 9.3 ± 7.0 | 1.0 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Mancina, E.; García-Lozano, M.d.R.; Burgos-Morón, E.; Mazzotta, S.; Martínez-Aguado, P.; Calderón-Montaño, J.M.; Vega-Pérez, J.M.; López-Lázaro, M.; Iglesias-Guerra, F.; Vega-Holm, M. Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents—In Vitro Evaluation against Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 17041. https://doi.org/10.3390/ijms242317041
Guillén-Mancina E, García-Lozano MdR, Burgos-Morón E, Mazzotta S, Martínez-Aguado P, Calderón-Montaño JM, Vega-Pérez JM, López-Lázaro M, Iglesias-Guerra F, Vega-Holm M. Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents—In Vitro Evaluation against Breast Cancer Cells. International Journal of Molecular Sciences. 2023; 24(23):17041. https://doi.org/10.3390/ijms242317041
Chicago/Turabian StyleGuillén-Mancina, Emilio, María del Rosario García-Lozano, Estefanía Burgos-Morón, Sarah Mazzotta, Pablo Martínez-Aguado, José Manuel Calderón-Montaño, José Manuel Vega-Pérez, Miguel López-Lázaro, Fernando Iglesias-Guerra, and Margarita Vega-Holm. 2023. "Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents—In Vitro Evaluation against Breast Cancer Cells" International Journal of Molecular Sciences 24, no. 23: 17041. https://doi.org/10.3390/ijms242317041
APA StyleGuillén-Mancina, E., García-Lozano, M. d. R., Burgos-Morón, E., Mazzotta, S., Martínez-Aguado, P., Calderón-Montaño, J. M., Vega-Pérez, J. M., López-Lázaro, M., Iglesias-Guerra, F., & Vega-Holm, M. (2023). Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents—In Vitro Evaluation against Breast Cancer Cells. International Journal of Molecular Sciences, 24(23), 17041. https://doi.org/10.3390/ijms242317041







