Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 1: Location of YF3 in the LnF3 Series According to Its Chemical and Structural Characteristics
Abstract
:1. Introduction
2. Results
2.1. Obtaining YF3 and LnF3 with Ln = Ho-Lu for Structural Studies
2.2. X-ray Diffraction Study of β-YF3 and Five β-LnF3 (Ln = Ho-Lu)
3. Discussion
3.1. Determination of “Pseudo ZY” According to Literature Data
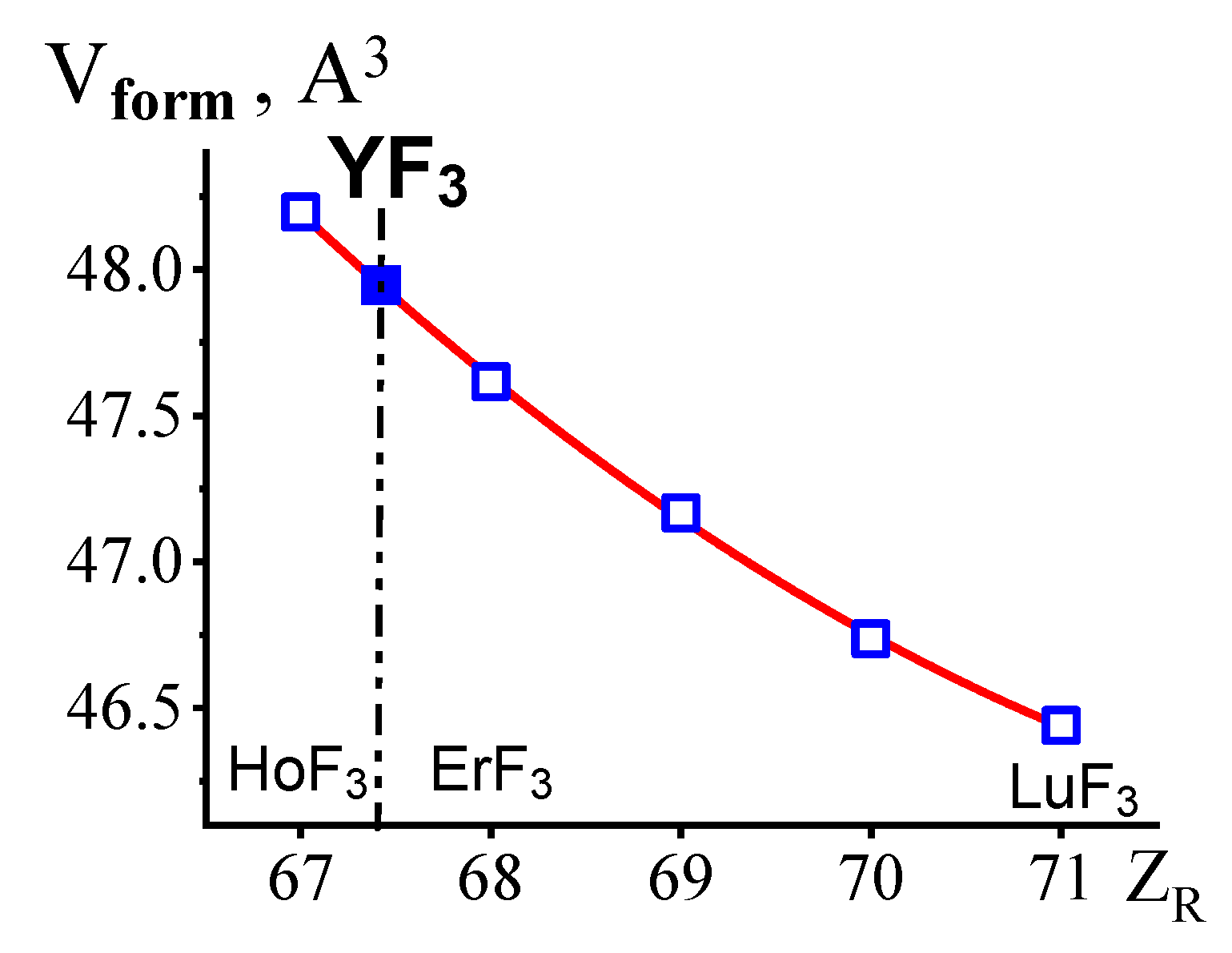
3.2. Location of YF3 in the LnF3 Series in Terms of the Structural Types and Unit Cell Parameters of 16 RF3 (without ScF3) at Pst and Tst
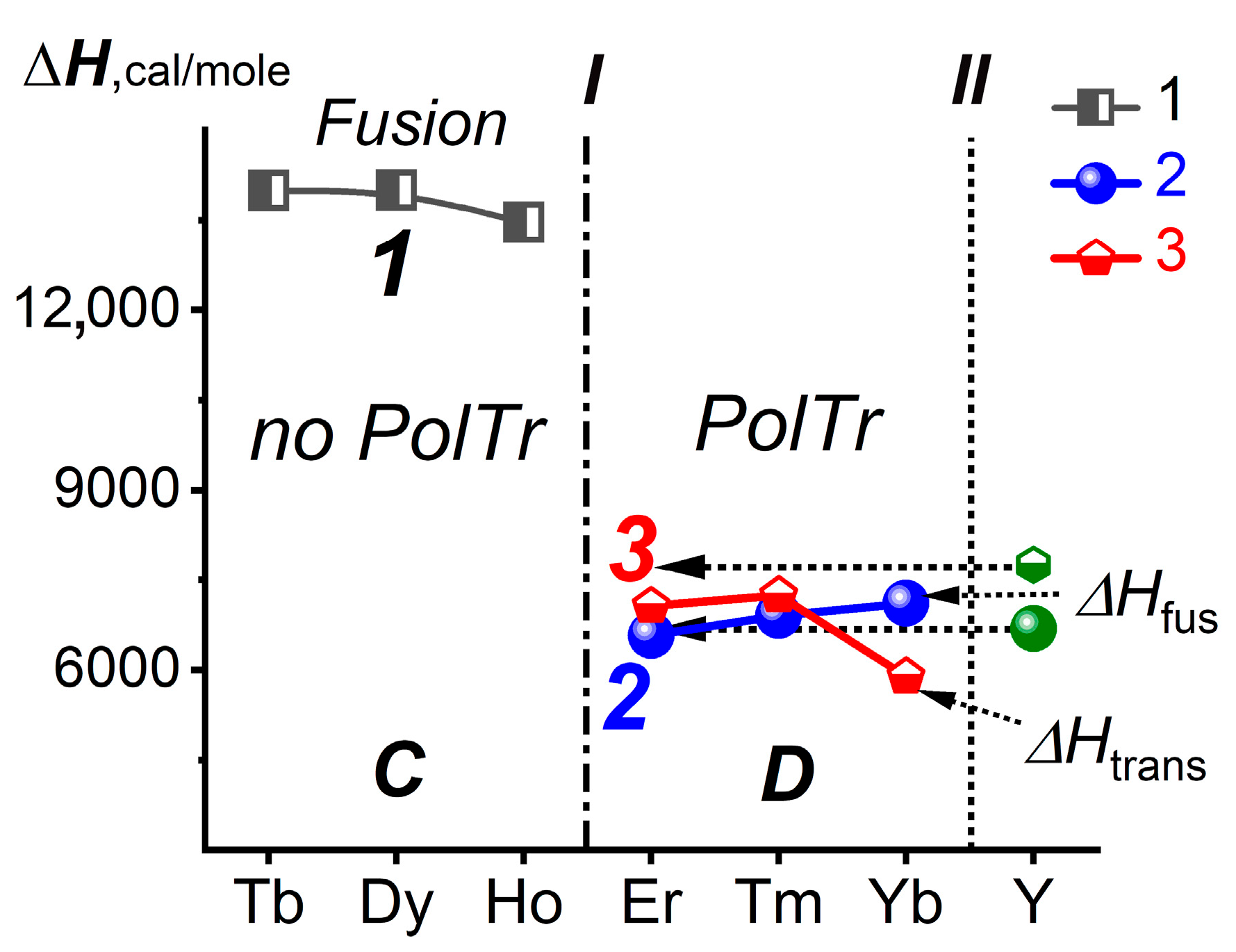
3.3. Location of YF3 in the LnF3 Series in Terms of Tfus and ΔHtrans
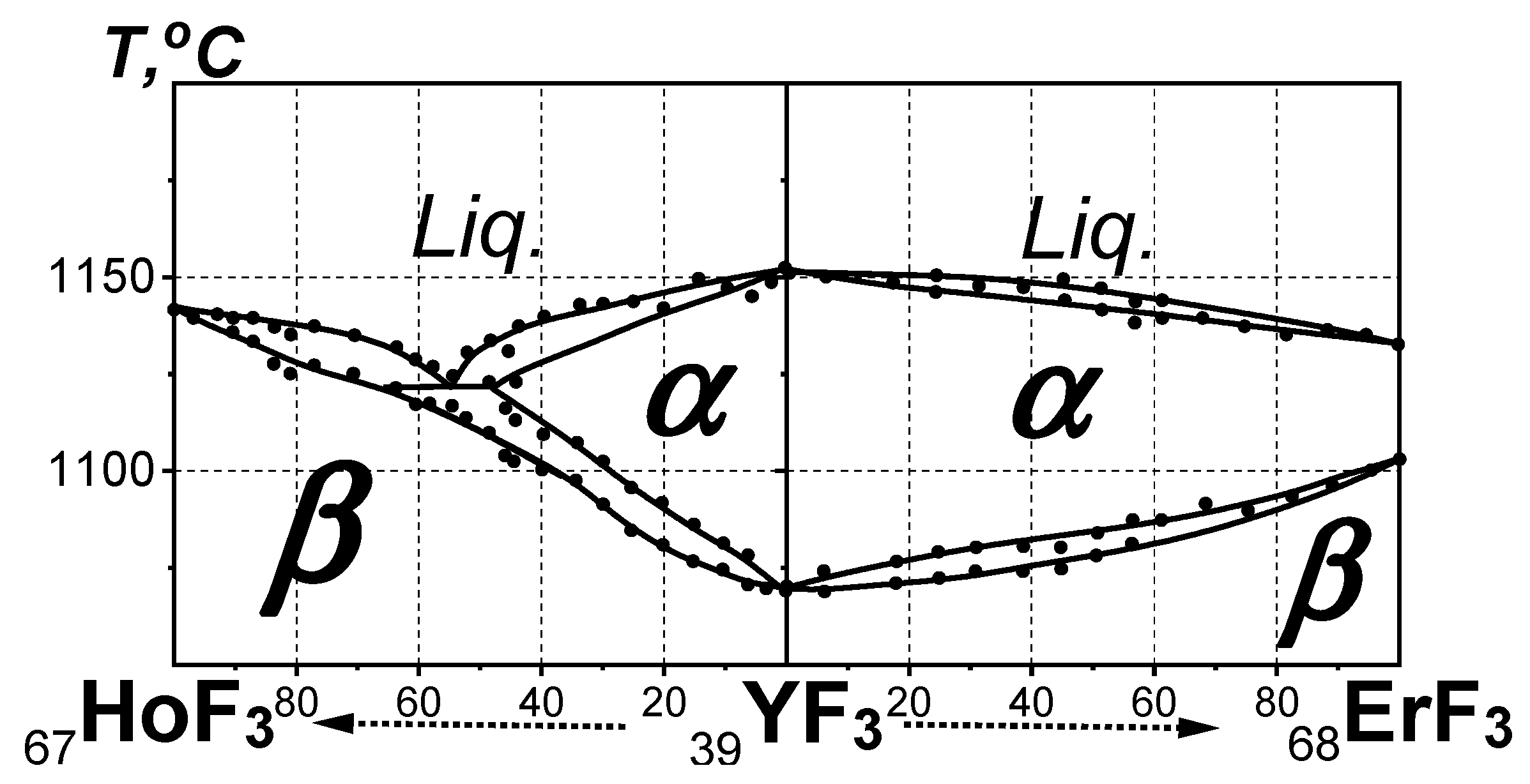
3.4. Location of YF3 Based on the YF3-LnF3 Phase Diagrams at P = Pst, T > Tst
3.5. Location of YF3 Based on the Phase Diagrams of the YF3-LnF3 Systems in the Short Composite “ErF3-YF3-TmF3” QS at P = Pst, T > Tst
- (1)
- Structural data are not available for the entire (R,Ln)F3 series. These data are not sufficient for precise analysis of LC and determination of “pseudo ZY” of YF3.
- (2)
- Structural changes in large arrays of REE compounds of different homologous series with different chemical bonds exhibit uncontrolled shifts in the areas of change in the type of structure along the Z-axis. These data are unsuitable for precise studies of LC.
- (3)
- The thermal and thermochemical properties and phase diagrams are related to the common property—Ttrans. This common property determines the location of YF3 between ErF3 and TmF3, which is shifted by Z = 1 compared to the location based on Vform. The reason for this shift remains unknown.
- (4)
- The phase diagrams of the short composite “HoF3-YF3-ErF3” QS and the particular HoF3-ErF3 system do not confirm the location of YF3 between HoF3 and ErF3.
- (5)
- In the short composite “ErF3-YF3-TmF3” QS, YF3 is located between ErF3 and TmF3.
- (6)
- To analyze the subtle features of the LC evolution in the LnF3 series and obtain Spec-zd Emp SIR for (R,Ln)F3, the structural properties of all REE trifluorides obtained under the same technological conditions are required.
3.6. Definition of “Pseudo ZY” Using X-ray Diffraction Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tantardini, C.; Oganov, A.R. Thermochemical electronegativities of the elements. Nat. Comm. 2021, 12, 20872087. [Google Scholar] [CrossRef]
- Greis, O.; Haschke, J.M. Rare Earth Fluorides. In Handbook on the Physics and Chemistry of Rare Earths; Gscheidner, K.A., Eyring, L.R., Eds.; Elsevier (North Holland Publishing Co.): Amsterdam, The Netherlands, 1982; Volume 5, pp. 387–460. [Google Scholar]
- Greis, O. Uber neue Verbindungen im System SmF2–SmF3. J. Solid State Chem. 1978, 24, 227–232. [Google Scholar] [CrossRef]
- Greis, O. Uber neue Verbindungen in den Systemen EuF2–EuF3 und SrF2–EuF3. Z. Anorgan. Allgem. Chem. 1978, 441, 39–46. [Google Scholar] [CrossRef]
- Karapetian, F.S.; Adamyan, V.E.; Arutunian, M.A.; Seiranian, K.B.; Grigorian, P.A.; Butaev, A.G. Temperature dependence of magnetic susceptibility and X-ray phase analysis of the system SrF2–EuF3. Izv. Akad. Nauk. SSSR Seriya Fiz. 1974, 9, 338–341. (In Russian) [Google Scholar]
- Greis, O. Phasenuntersuchungen am System YbF2–YbF3. Z. Anorgan. Allgem. Chem. 1977, 430, 175–198. [Google Scholar] [CrossRef]
- Petzel, T.; Greis, O. The evaporation behaviour of ytterbium (III) fluoride and ytterbium (II) fluoride. J. Less Common Metals. 1976, 46, 197–207. [Google Scholar] [CrossRef]
- Goldschmidt, V.M.; Barth, T.; Lunde, G.; Zachariasen, W. Geochemische Verteilungsgesetze der Elemente. Part V. Isomorphie und Polymorphie der Sesquioxyde. Die Lanthaniden-Kontraktion und Ihre Konsequenzen; Jacob Dybwad; Kristiania: Oslo, Norway, 1926; Volume 7, pp. 1–117. [Google Scholar]
- Takenaka, K. Progress of Research in Negative Thermal Expansion Materials: Paradigm Shift in the Control of Thermal Expansion. Front. Chem. 2018, 6, 267. [Google Scholar] [CrossRef]
- Bandurkin, G.A.; Dzhurinskii, B.F.; Tananaev, I.V. Features of Crystal Chemistry of REE Compounds; Nauka: Moscow, Russia, 1984; 230p. (In Russian) [Google Scholar]
- Spedding, F.H.; Henderson, D.C. High-temperature heat contents and related thermodynamic functions of seven trifluorides of the rare earths: Y, La, Pr, Nd, Gd, Ho, and Lu. J. Chem. Phys. 1971, 54, 2476–2483. [Google Scholar] [CrossRef]
- Sobolev, B.P. Full quasi-system “from LaF3 to LuF3” as a combination of 14 binary systems of lanthanide trifluorides with maximal chemical proximity. J. Solid State Chem. 2022, 312, 123164. [Google Scholar] [CrossRef]
- Glavin, G.G.; Karpov, Y.A.; Olzhataev, B.A. Oxygen determination in Y, Sr, Ca and Li fluorides by isotope dilution method. Zavod. Lab. Diagn. Mater. 1969, 35, 172–175. (In Russian) [Google Scholar]
- Glavin, G.G.; Karpov, Y.A. Oxygen determination in metallic RE and their fluorides. Zavod. Lab. Diagn. Mater. 1964, 30, 306–308. (In Russian) [Google Scholar]
- Sobolev, B.P.; Fedorov, P.P.; Steinberg, D.V.; Sinitsyn, B.V.; Shakhalamian, G.S. On the problem of polymorphism and fusion of lanthanide trifluorides. I. The influence of oxygen on phase transition temperatures. J. Solid State Chem. 1976, 17, 191–199. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Alexandrov, V.B.; Bondareva, O.S.; Buchinskaya, I.I.; Val’kovskii, M.D.; Sobolev, B.P. Concentration dependence of cell parameters of Na0.5-xR0.5+xF2+2x fluorite phases. Crystallogr. Rep. 2001, 46, 280–286. (In Russian) [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system Jana2006: General features. Z. Kristallogr. 2014, 229, 345. [Google Scholar] [CrossRef]
- Becker, P.J.; Coppens, P. Extinction within the limit of validity of the Darwin transfer equations. I. General formalism for primary and secondary extinction and their applications to spherical crystals. Acta Cryst. A 1974, 30, 129–147. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. The Analytical Expression of the Results of the Theory of Space-Groups; Carnegie Institution of Washington: Washington, DA, USA, 1922; 180p. [Google Scholar]
- Greis, O.; Petzel, T. Ein Beitrag zur Strukturchemie eder Seltenerd-Trifluoride. Z. Anorgan. Allgem. Chem. 1974, 403, 1–22. [Google Scholar] [CrossRef]
- Kent, R.A.; Zmbov, K.E.; Kanan, A.S.; Besenbruch, G.; McDonald, J.D.; Margrave, J.L. Mass spectrometric studies at high temperatures. The sublimation pressures of scandium (III), yttrium (III) and lanthanum (III), trifluorides. J. Inorg. Nucl. Chem. 1966, 28, 1419–1427. [Google Scholar] [CrossRef]
- Rinehart, G.H.; Behrens, R.G. Vapour pressure and vaporization thermodynamics of ScF3. J. Less Common Metals. 1980, 75, 65–78. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Kovacs, A. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Oxford, UK; Amsterdam, The Netherlands; New York, NY, USA, 2003; Volume 33, 147p. [Google Scholar]
- Spedding, F.H.; Beaudry, B.J.; Henderson, D.C.; Moorman, J. High temperature enthalpies and related thermodynamic functions of the trifluorides of Sc, Ce, Sm, Eu, Gd, Tb, Dy, Er, Tm, and Yb. J. Chem. Phys. 1974, 60, 1578–1588. [Google Scholar] [CrossRef]
- Sobolev, B.P. The Rare Earth Trifluorides, Part 1: The High Temperature Chemistry of the Rare Earth Trifluorides; Institute of Crystallography: Moscow, Russia; Institut d’Estudis Catalans: Barcelona, Spain, 2000; 520p. [Google Scholar]
- Zalkin, A.; Templeton, D.H. The crystal structures of YF3 and related compounds. J. Amer. Chem. Soc. 1953, 75, 2453–2458. [Google Scholar] [CrossRef]
- Greis, O.; Cader, M.S.R. Polymorphism of high purity rare earth trifluorides. Thermochim. Acta 1985, 87, 145–150. [Google Scholar] [CrossRef]


| Nd | <0.0005 | Dy | 0.0002 | Si | <0.001 | Tm | 0.0005 |
| Sm | 0.0002 | Ho | <0.001 | Mn | <0.00005 | Fe | 0.0005 |
| Gd | 0.0005 | Cu | <0.0005 | Co | <0.00005 | Ti | <0.00005 |
| Tb | 0.0001 | O | 270 ppmw | Ni | 0.00005 | Cr | 0.00005 |
| RF3 | β-YF3 | β-HoF3 | β-ErF3 | β-TmF3 | β-YbF3 | β-LuF3 |
|---|---|---|---|---|---|---|
| ICSD ID | 2254323 | 2254302 | 2254320 | 2254321 | 2254415 | 2254322 |
| Crystal system | Orthorhombic | |||||
| Sp.gr., Z | Pnma, 6 | |||||
| a (Å) | 6.3666 (2) | 6.4055 (2) | 6.3500 (5) | 6.2792 (3) | 6.2168 (3) | 6.1437 (4) |
| b (Å) | 6.8579 (3) | 6.8739 (3) | 6.8435 (7) | 6.8141 (3) | 6.7852 (3) | 6.7606 (3) |
| c (Å) | 4.3927 (1) | 4.3784 (2) | 4.3829 (4) | 4.4095 (3) | 4.4318 (2) | 4.4724 (3) |
| V (Å 3) | 191.79 (1) | 192.78 (1) | 190.46 (3) | 188.67 (2) | 186.94 (2) | 185.76 (2) |
| Vform | 47.948 | 48.195 | 47.615 | 47.168 | 46.735 | 46.440 |
| Dx (g·cm−3) | 5.0528 | 7.6464 | 7.8205 | 7.9538 | 8.1732 | 8.2942 |
| μ (mm−1) | 16.441 | 21.751 | 23.486 | 24.981 | 26.71 | 28.387 |
| Tmin, Tmax | 0.0891, 0.1878 | 0.0594, 0.1564 | 0.0391, 0.1312 | 0.0628, 0.1605 | 0.0222, 0.1051 | 0.0211, 01031 |
| Shape, color | colorless | light yellow | light rose | colorless | colorless | colorless |
| Diameter (mm) | 0.20 | |||||
| Wavelength (Å) | 0.56087 | |||||
| Θ range (deg) | 4.35–72.65 | 4.36–72.85 | 4.36–72.78 | 4.34–73.03 | 4.33–72.82 | 4.31–72.87 |
| Refl. collected | 19,376 | 20,349 | 20,522 | 19,403 | 19,993 | 19,644 |
| Refl. unique/Rint | 3573/4.55 | 3800/5.14 | 3342/5.00 | 3301/5.06 | 3333/4.65 | 2895/5.15 |
| Refin. method | Full matrix least squares on F | |||||
| Param/Restrains | 23 | 38/0 | 38/0 | 38/0 | 38/0 | 66/0 |
| R/wR, % | 2.26/3.70 | 2.38/3.93 | 2.05/3.10 | 2.60/4.01 | 2.80/4.11 | 2.51/3.39 |
| Δρmin/Δρmax, Å−3 | −2.70/1.48 | −4.48/4.39 | −4.29/3.64 | −4.54/2.84 | −4.60/3.64 | −4.26/3.87 |
| GOF | 1.36 | 1.47 | 1.43 | 1.40 | 1.62 | 1.39 |
| RF3 | Ion | W.p. | s.o.f. | x/a | y/b | z/c | Ueq |
|---|---|---|---|---|---|---|---|
| YF3 | Y | 4c | 1 | 0.367845(12) | ¼ | 0.05981(2) | 0.005423(11) |
| F(1) | 4c | 1 | 0.52302(15) | ¼ | 0.5901(2) | 0.00976(12) | |
| F(2) | 8b | 1 | 0.16454(10) | 0.06335(8) | 0.37686(16) | 0.00856(7) | |
| HoF3 | Ho | 4c | 1 | 0.36809(3) | ¼ | 0.06137(4) | 0.00483(5) |
| F(1) | 4c | 1 | 0.5217(2) | ¼ | 0.5870(3) | 0.00925(16) | |
| F(2) | 8b | 1 | 0.16429(16) | 0.06318(16) | 0.3803(2) | 0.00866(10) | |
| ErF3 | Er | 4c | 1 | 0.36802(3) | ¼ | 0.06027(4) | 0.00521(4) |
| F(1) | 4c | 1 | 0.5231(2) | ¼ | 0.5909(3) | 0.00967(14) | |
| F(2) | 8b | 1 | 0.16486(14) | 0.06248(12) | 0.37654(17) | 0.00871(9) | |
| TmF3 | Tm | 4c | 1 | 0.36725(3) | ¼ | 0.05609(4) | 0.00477(4) |
| F(1) | 4c | 1 | 0.5252(2) | ¼ | 0.5971(5) | 0.0103(2) | |
| F(2) | 8b | 1 | 0.16454(16) | 0.06304(15) | 0.3716(3) | 0.00867(12) | |
| YbF3 | Yb | 4c | 1 | 0.36718(3) | ¼ | 0.05395(5) | 0.00577(5) |
| F(1) | 4c | 1 | 0.5264(3) | ¼ | 0.6017(4) | 0.0114(2) | |
| F(2) | 8b | 1 | 0.16510(16) | 0.06231(14) | 0.3681(2) | 0.00897(12) | |
| LuF3 | Lu | 4c | 1 | 0.36689(7) | ¼ | 0.05044(10) | 0.00759(17) |
| F(1) | 4c | 1 | 0.5278(3) | ¼ | 0.6056(4) | 0.0110(2) | |
| F(2) | 8b | 1 | 0.16448(18) | 0.06265(13) | 0.3634(3) | 0.00951(11) |
| SSGrs | RF3 | RF3 Structural Types |
|---|---|---|
| A | LaF3, CeF3, PrF3, and NdF3 monomorphic | t- |
| B | PmF3, SmF3, EuF3, and GdF3 dimorphic | (1) t- high-temperature (2) β- low-temperature |
| C | TbF3, DyF3, and HoF3 monomorphic | β- |
| D | ErF3, TmF3, YbF3, and LuF3 dimorphic | (1) α- high-temperature (2) β- low-temperature |
| Reference | Unit Cell Parameters, Å | Vform (Tst, Pst) | YF3 Location | ||
|---|---|---|---|---|---|
| a | b | c | |||
| 1953 Zalkin [26] | 6.353 | 6.850 | 4.393 | 47.79 | between HoF3 and ErF3 |
| 1971 Spedding [11] | 6.367 | 6.859 | 4.394 | 47.97 | between HoF3 and ErF3 |
| 1974 Greis [20] | 6.4027 | 6.8843 | 4.3980 | 48.46 | between DyF3 and HoF3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, B.P.; Sulyanova, E.A. Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 1: Location of YF3 in the LnF3 Series According to Its Chemical and Structural Characteristics. Int. J. Mol. Sci. 2023, 24, 17013. https://doi.org/10.3390/ijms242317013
Sobolev BP, Sulyanova EA. Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 1: Location of YF3 in the LnF3 Series According to Its Chemical and Structural Characteristics. International Journal of Molecular Sciences. 2023; 24(23):17013. https://doi.org/10.3390/ijms242317013
Chicago/Turabian StyleSobolev, Boris P., and Elena A. Sulyanova. 2023. "Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 1: Location of YF3 in the LnF3 Series According to Its Chemical and Structural Characteristics" International Journal of Molecular Sciences 24, no. 23: 17013. https://doi.org/10.3390/ijms242317013
APA StyleSobolev, B. P., & Sulyanova, E. A. (2023). Lanthanide Contraction in LnF3 (Ln = Ce-Lu) and Its Chemical and Structural Consequences: Part 1: Location of YF3 in the LnF3 Series According to Its Chemical and Structural Characteristics. International Journal of Molecular Sciences, 24(23), 17013. https://doi.org/10.3390/ijms242317013






