Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata
Abstract
:1. Introduction
2. Result
2.1. Systemic Identification of PGs in the A. trifoliata Genome
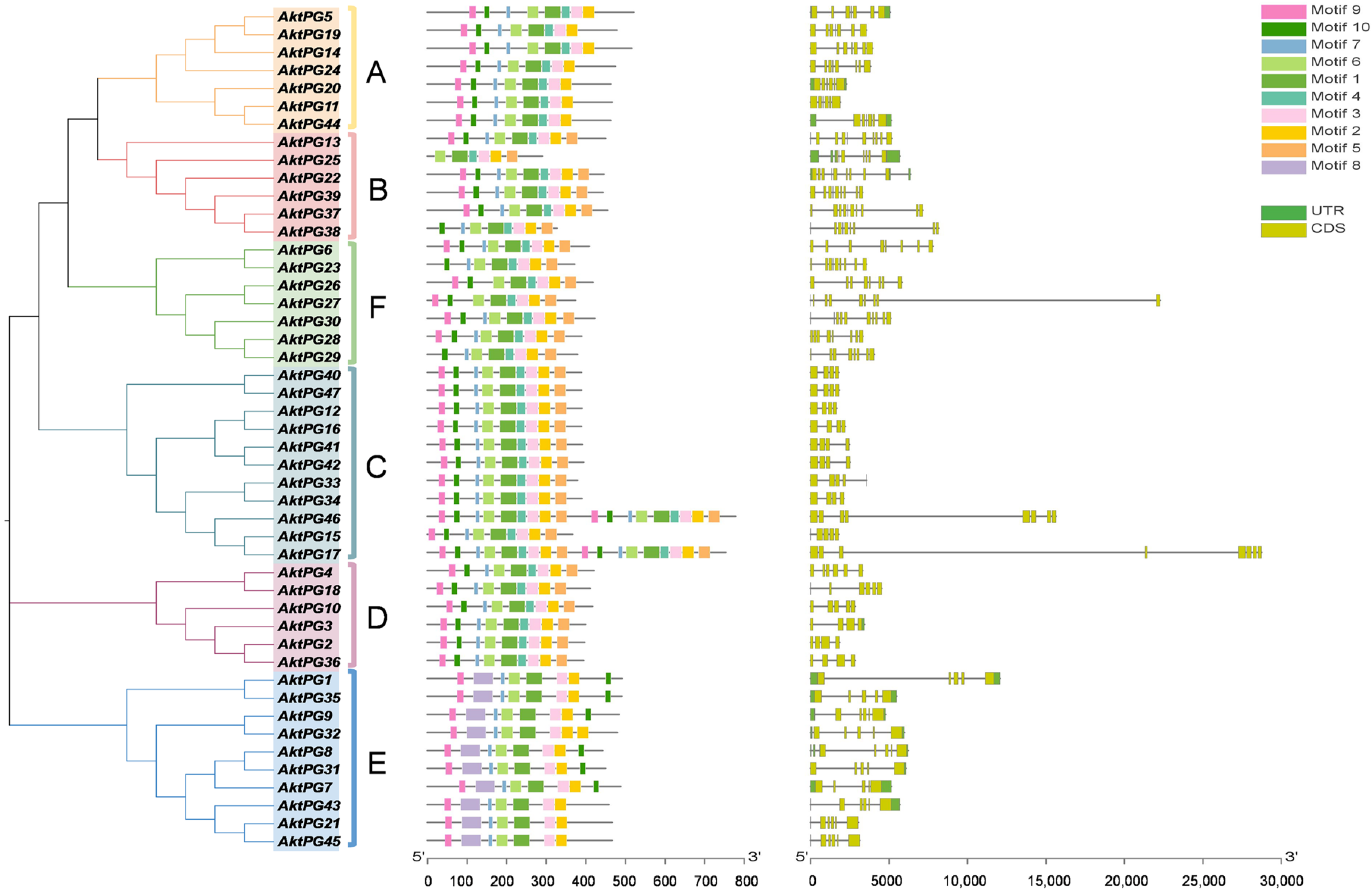
2.2. Phylogenetic Tree of AktPGs
2.3. Interspecific Collinearity
2.4. Conserved Structures and Motifs of Putative AktPGs
2.5. Ka/Ks Value of Homologous AktPG Pairs
2.6. Cis-Acting Elements of the AktPGs
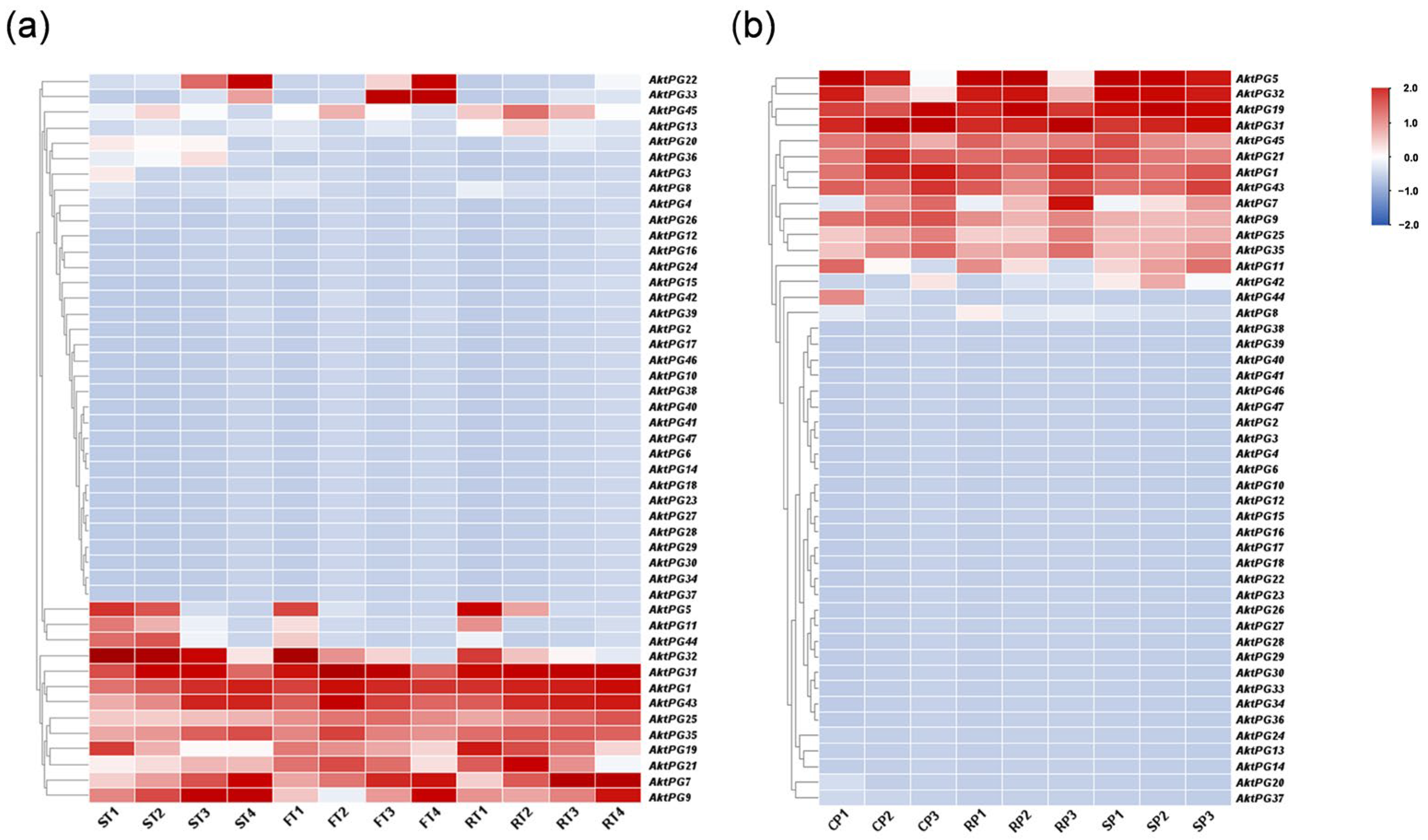
2.7. Expression Profiles of AktPGs in Two Independent Transcriptome Datasets
3. Discussion
3.1. Putative Evolutionary Events Experienced by AktPGs
3.2. A Histidine Residue (H) Substitute Could Influence the Structural Characteristics of AktPGs
3.3. Several Putative Candidate AktPGs Possibly Associated with Fruit Cracking in A. trifoliata
4. Materials and Methods
4.1. Identification and Characterization Analysis of AktPGs
4.2. Phylogenetic Analysis, Selective Pressure, and Collinearity of AktPGs
4.3. Gene Structure and Conserved Motif Analysis
4.4. Analysis of the Cis-Acting Elements of AktPGs
4.5. Expression Analysis of the AktPGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Santos, M.; Egea-Cortines, M.; Gonçalves, B.; Matos, M. Molecular mechanisms involved in fruit cracking: A review. Front. Plant Sci. 2023, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Hay, A. Snap, crack and pop of explosive fruit. Curr. Opin. Genet. Dev. 2018, 51, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2015, 37, 1718. [Google Scholar] [CrossRef]
- Christiansen, L.; Dal Degan, F.; Ulvskov, P.; Borkhardt, B. Examination of the dehiscence zone in soybean pods and isolation of a dehiscence-related endopolygalacturonase gene. Plant Cell Environ. 2002, 25, 479–490. [Google Scholar] [CrossRef]
- Esgici, R.; Pekitkan, F.G.; Sessiz, A. Correlation between rice stem cutting resistance and cracking force of rice kernel. Fresenius Environ. Bull. 2019, 28, 3014–3021. [Google Scholar]
- Khurnpoon, L.; Siriphanich, J.; Labavitch, J. Cell wall metabolism during durian fruit dehiscence. Postharvest Biol. Technol. 2008, 48, 391–401. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A. Ethylene-responsive factor 4 is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnol. J. 2020, 18, 1066–1077. [Google Scholar] [CrossRef]
- Palapol, Y.; Kunyamee, S.; Thongkhum, M.; Ketsa, S.; Ferguson, I.B.; van Doorn, W.G. Expression of expansin genes in the pulp and the dehiscence zone of ripening durian (Durio zibethinus) fruit. J. Plant Physiol. 2015, 182, 33–39. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Fu, P.; Zhong, S.; Guan, J.; Luo, P. Developmental stages of Akebia trifoliata fruit based on volume. Hortic. Sci. Technol. 2021, 39, 823–831. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in mechanisms and omics pertaining to fruit cracking in horticultural plants. Agronomy 2021, 11, 1045. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Knoche, M. Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 2017, 245, 765–777. [Google Scholar] [CrossRef]
- Coen, E.; Cosgrove, D.J. The mechanics of plant morphogenesis. Science 2023, 379, e8055. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.; Briggs, S.; Knox, J. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, K.A.; Bennett, A.B. Polygalacturonases: Many genes in search of a function. Plant Physiol. 1998, 117, 337–343. [Google Scholar] [CrossRef]
- Markovič, O.; Janeček, Š. Pectin degrading glycoside hydrolases of family 28: Sequence-structural features, specificities and evolution. Protein Eng. 2001, 14, 615–631. [Google Scholar] [CrossRef]
- Fischer, R.L.; Bennett, A.B. Role of cell wall hydrolases in fruit ripening. Annu. Rev. Plant Biol. 1991, 42, 675–703. [Google Scholar] [CrossRef]
- Lu, L.; Hou, Q.; Wang, L.; Zhang, T.; Zhao, W.; Yan, T.; Zhao, L.; Li, J.; Wan, X. Genome-Wide Identification and Characterization of Polygalacturonase Gene Family in Maize (Zea mays L.). Int. J. Mol. Sci. 2021, 22, 10722. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, Y.; Lv, M.; Wu, J.; Lu, G.; Cao, J. Genome-wide identification and characterization of polygalacturonase genes in Cucumis sativus and Citrullus lanatus. Plant Physiol. Biochem. 2014, 74, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587–598. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Zhao, T.; Han, F.; Zhang, Q.; Liu, X.; Jiang, C.; Zhong, C. Genome-wide identification and expression analysis of polygalacturonase gene family in Kiwifruit (Actinidia chinensis) during fruit softening. Plants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-wide identification and expression profiling of the polygalacturonase (PG) and pectin methylesterase (PME) genes in grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Fernández, R.; Matilla, A.; Rodríguez-Gacio, M.; Fernández-Otero, C.; de La Torre, F. The polygalacturonase gene PdPG1 is developmentally regulated in reproductive organs of Prunus domestica L. subsp. insititia. Plant Sci. 2007, 172, 763–772. [Google Scholar] [CrossRef]
- Peng, G.; Wu, J.; Lu, W.; Li, J. A polygalacturonase gene clustered into clade E involved in lychee fruitlet abscission. Sci. Hortic. 2013, 150, 244–250. [Google Scholar] [CrossRef]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. Arabidopsis dehiscence zone polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef]
- Chen, D.; Wei, X.; Xu, L.; Xu, J.; Zeng, L. Genome-wide identification and expression analysis of PG gene family in wax apple [Syzygium samarangense (Bl.) Merr. et Perry]. J. Fruit Sci. 2022, 39, 548–563. [Google Scholar]
- Li, L.; Yao, X.; Zhong, C.; Chen, X.; Huang, H. Akebia: A potential new fruit crop in China. HortScience 2010, 45, 4–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Jie, B.; Wu, G.; Cao, Y.; Wu, W.; Meng, H. Research progress of Akebia trifoliata (Thunb.) Koidz. Agric. Biotechnol. 2017, 6, 1–8. [Google Scholar]
- Zou, S.; Gao, P.; Jia, T.; Huang, H. Physicochemical characteristics and nutritional composition during fruit ripening of Akebia trifoliata (Lardizabalaceae). Horticulturae 2022, 8, 326. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, D.; Zhu, J.; Yu, H.; Li, G. Study on the respiration physiology of Akebia trifoliate fruit and the suitable storage conditions. J. Fruit Sci. 2003, 20, 512–514. [Google Scholar]
- Niu, J.; Shi, Y.; Huang, K.; Zhong, Y.; Chen, J.; Sun, Z.; Luan, M.; Chen, J. Integrative transcriptome and proteome analyses provide new insights into different stages of Akebia trifoliata fruit cracking during ripening. Biotechnol. Biofuels 2020, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Exploring the mechanism of Akebia trifoliata fruit cracking based on cell-wall metabolism. Food Res. Int. 2022, 157, 111219. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Combination of chitosan coating and heat shock treatments to maintain postharvest quality and alleviate cracking of Akebia trifoliate fruit during cold storage. Food Chem. 2022, 394, 133330. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Kwon, S.J.; Kim, N.S. Intron loss mediated structural dynamics and functional differentiation of the polygalacturonase gene family in land plants. Genes Genom. 2010, 32, 570–577. [Google Scholar] [CrossRef]
- Mahmood, U.; Fan, Y.; Wei, S.; Niu, Y.; Li, Y.; Huang, H.; Chen, Y.; Tang, Z.; Liu, L.; Qu, C. Comprehensive analysis of polygalacturonase genes offers new insights into their origin and functional evolution in land plants. Genomics 2021, 113, 1096–1108. [Google Scholar] [CrossRef]
- Yang, Z.L.; Liu, H.J.; Wang, X.R.; Zeng, Q.Y. Molecular evolution and expression divergence of the Populus polygalacturonase supergene family shed light on the evolution of increasingly complex organs in plants. New Phytol. 2013, 197, 1353–1365. [Google Scholar] [CrossRef]
- Sun, G.; Dilcher, D.L.; Wang, H.; Chen, Z. A eudicot from the Early Cretaceous of China. Nature 2011, 471, 625–628. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Zhang, N.; Shan, H.; Su, K.; Zhang, J.; Meng, Z.; Kong, H.; Chen, Z. Interactions among proteins of floral MADS-box genes in basal eudicots: Implications for evolution of the regulatory network for flower development. Mol. Biol. Evol. 2010, 27, 1598–1611. [Google Scholar] [CrossRef]
- Zhong, S.; Li, B.; Chen, W.; Wang, L.; Guan, J.; Wang, Q.; Yang, Z.; Yang, H.; Wang, X.; Yu, X. The chromosome-level genome of Akebia trifoliata as an important resource to study plant evolution and environmental adaptation in the Cretaceous. Plant J. 2022, 112, 1316–1330. [Google Scholar] [CrossRef]
- Ou, S.; Chen, J.; Jiang, N. Assessing genome assembly quality using the LTR Assembly Index (LAI). Nucleic Acids Res. 2018, 46, e126. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhong, S.; Yang, H.; Chen, C.; Chen, W.; Yang, H.; Guan, J.; Fu, P.; Tan, F.; Ren, T. Identification and characterization of NBS resistance genes in Akebia trifoliata. Front. Plant Sci. 2021, 12, 758559. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yang, H.; Guan, J.; Shen, J.; Ren, T.; Li, Z.; Tan, F.; Li, Q.; Luo, P. Characterization of the MADS-Box Gene Family in Akebia trifoliata and Their Evolutionary Events in Angiosperms. Genes 2022, 13, 1777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhong, S.; Guan, J.; Chen, W.; Yang, H.; Yang, H.; Chen, C.; Tan, F.; Ren, T.; Li, Z. Genome-Wide Identification and Expression Analysis of WRKY Transcription Factors in Akebia trifoliata: A Bioinformatics Study. Genes 2022, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Wu, X.; Li, J.; Xu, C.; Huang, M.; Wang, H.; Liu, H.; Zhao, Z. Identification of the NAC Transcription Factor Family during Early Seed Development in Akebia trifoliata (Thunb.) Koidz. Plants 2023, 12, 1518. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhong, S.; Yang, H.; Ren, T.; Chen, C.; Tan, F.; Cao, G.; Liu, J.; Luo, P. Genome-Wide Identification of Superoxide Dismutase and Expression in Response to Fruit Development and Biological Stress in Akebia trifoliata: A Bioinformatics Study. Antioxidants 2023, 12, 726. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, S.; Dong, Q.; Yang, H.; Yang, H.; Tan, F.; Chen, C.; Ren, T.; Shen, J.; Cao, G. Identification of Photoperiod-and Phytohormone-Responsive DNA-Binding One Zinc Finger (Dof) Transcription Factors in Akebia trifoliata via Genome-Wide Expression Analysis. Int. J. Mol. Sci. 2023, 24, 4973. [Google Scholar] [CrossRef]
- Lyu, M.; Iftikhar, J.; Guo, R.; Wu, B.; Cao, J. Patterns of expansion and expression divergence of the polygalacturonase gene family in Brassica oleracea. Int. J. Mol. Sci. 2020, 21, 5706. [Google Scholar] [CrossRef]
- Tebbutt, S.J.; Rogers, H.J.; Lonsdale, D.M. Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Mol. Biol. 1994, 25, 283–297. [Google Scholar] [CrossRef]
- Rexová-Benková, L. Evidence for the role of carboxyl groups in activity of endopolygalacturonase of Aspergillus niger. Chemical modification by carbodiimide reagent. Collect. Czechoslov. Chem. Commun. 1990, 55, 1389–1395. [Google Scholar] [CrossRef]
- Rao, M.N.; Kembhavi, A.A.; Pant, A. Implication of tryptophan and histidine in the active site of endo-polygalacturonase from Aspergillus ustus: Elucidation of the reaction mechanism. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1996, 1296, 167–173. [Google Scholar]
- Bussink, H.J.; Buxton, F.P.; Visser, J. Expression and sequence comparison of the Aspergillus niger and Aspergillus tubigensis genes encoding polygalacturonase II. Curr. Genet. 1991, 19, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Son, J.H.; Park, K.C.; Oh, H.Y.; Kim, P.H.; Byeon, W.H.; Kim, N.S. Structural dynamics and divergence of the polygalacturonase gene family in land plants. Nat. Preced. 2008. [Google Scholar] [CrossRef]
- Mahmood, U.; Li, X.; Qian, M.; Fan, Y.; Yu, M.; Li, S.; Shahzad, A.; Qu, C.; Li, J.; Liu, L. Comparative transcriptome and co-expression network analysis revealed the genes associated with senescence and polygalacturonase activity involved in pod shattering of rapeseed. Biotechnol. Biofuels Bioprod. 2023, 16, 20–36. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Li, W.H.; Yang, J.; Gu, X. Expression divergence between duplicate genes. TRENDS Genet. 2005, 21, 602–607. [Google Scholar] [CrossRef]
- Kim, J.; Shiu, S.H.; Thoma, S.; Li, W.H.; Patterson, S.E. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 2006, 7, 1–14. [Google Scholar]
- Shcherbakov, V.P. Biological species is the only possible form of existence for higher organisms: The evolutionary meaning of sexual reproduction. Biol. Direct 2010, 5, 1–22. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, Y.; Cui, J.; Lyu, M.; Xu, L.; Cao, J. A comparative analysis of the evolution, expression, and cis-regulatory element of polygalacturonase genes in grasses and dicots. Funct. Integr. Genom. 2016, 16, 641–656. [Google Scholar] [CrossRef]
- Liu, L. Identification of PG Gene Family in Durian and Pectin Changes during Fruit Dehiscence. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2022. [Google Scholar]
- Russo, C.A.; Selvatti, A.P. Bootstrap and rogue identification tests for phylogenetic analyses. Mol. Biol. Evol. 2018, 35, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
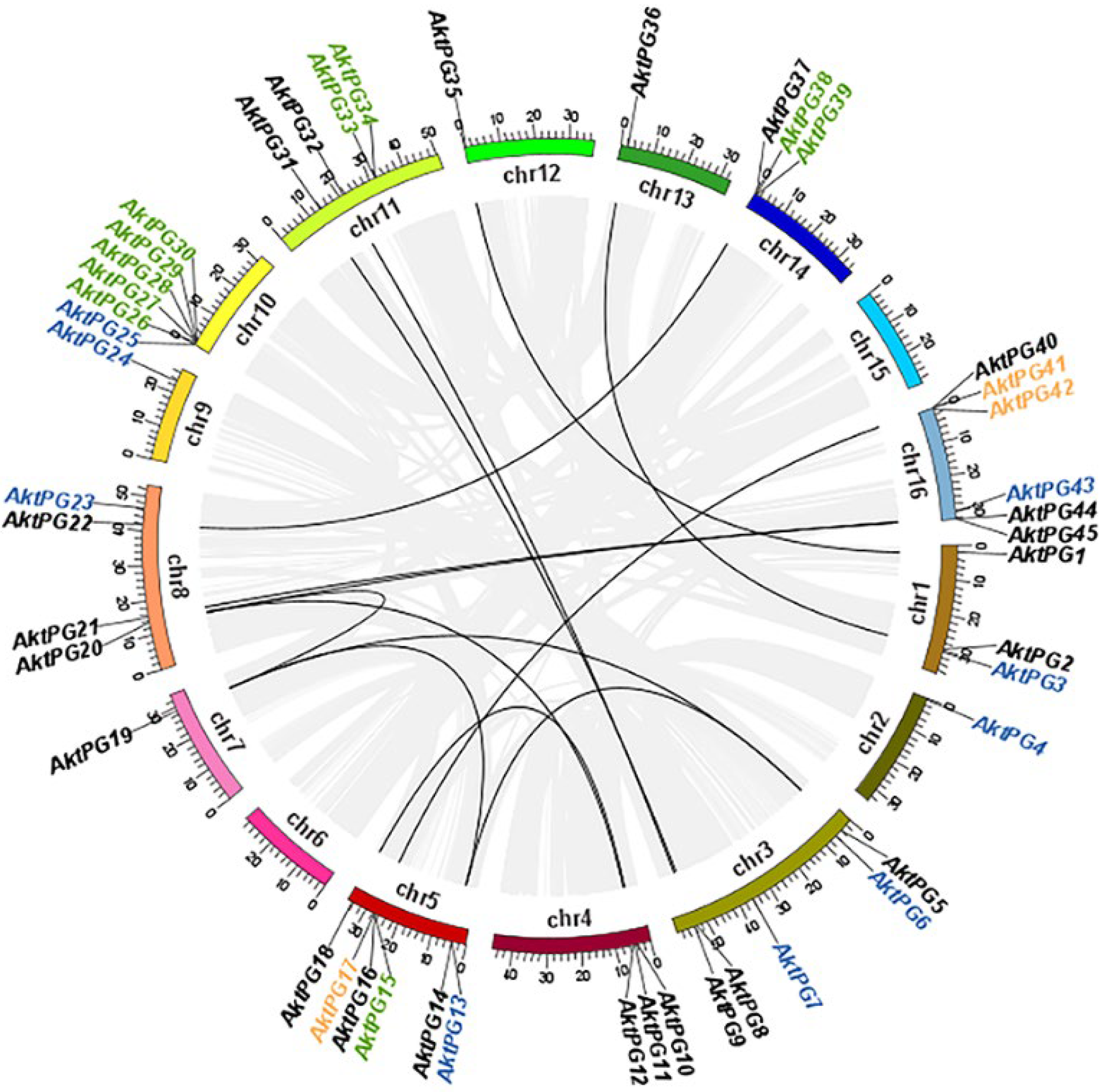
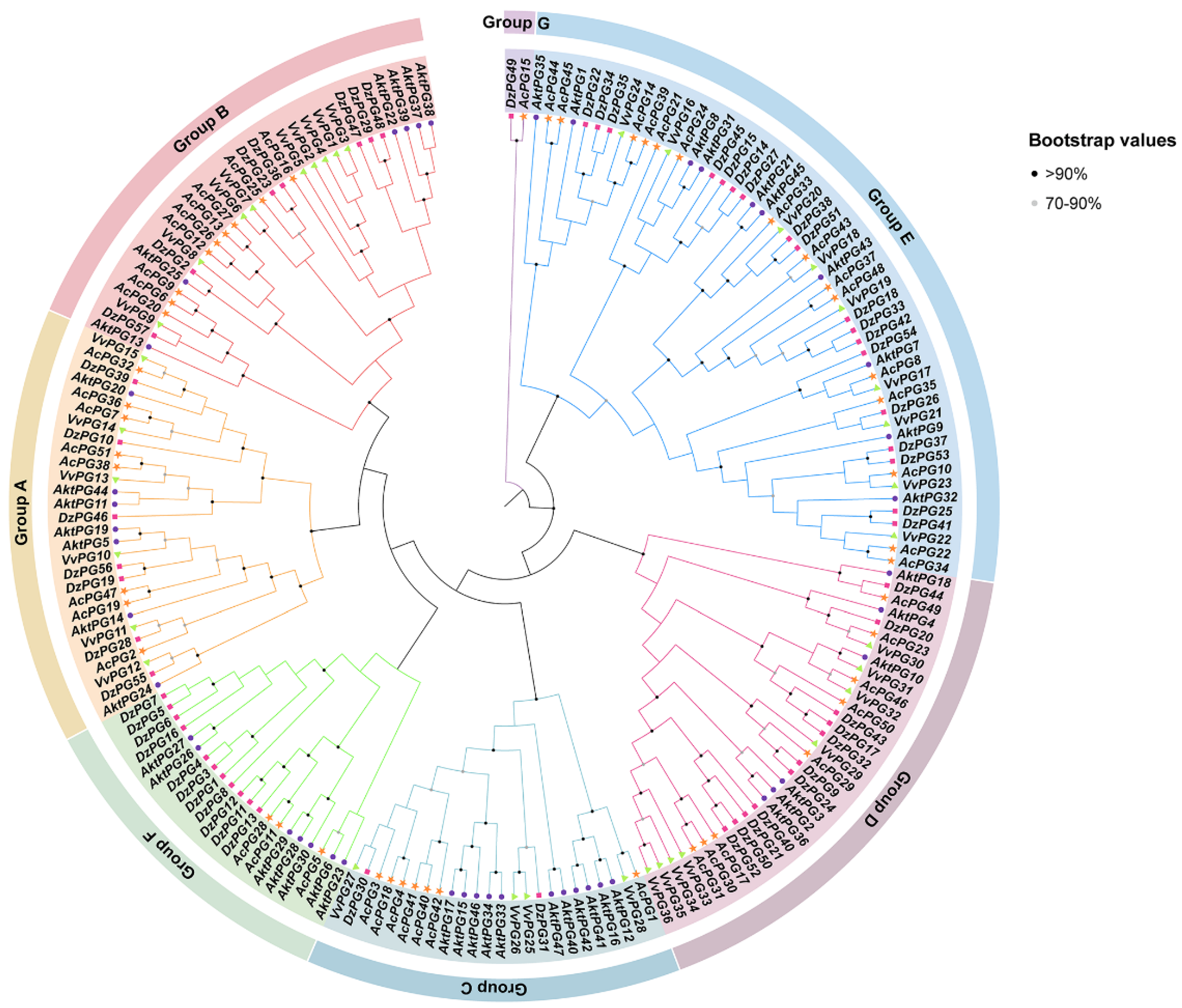
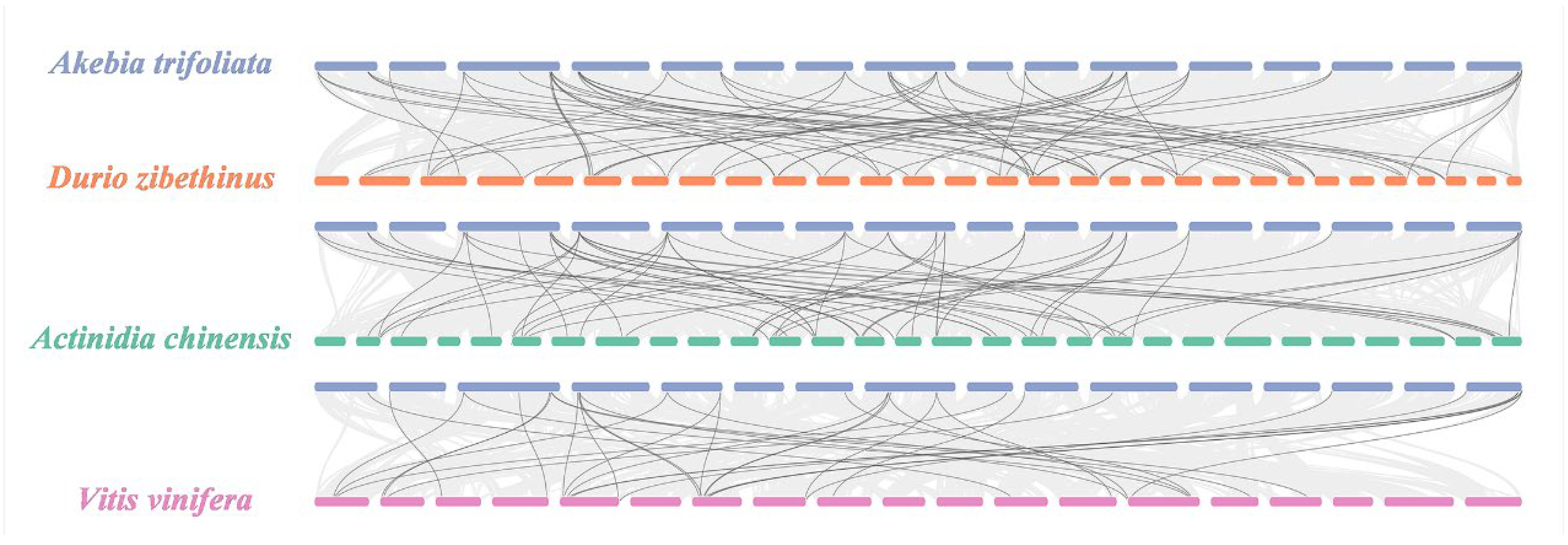
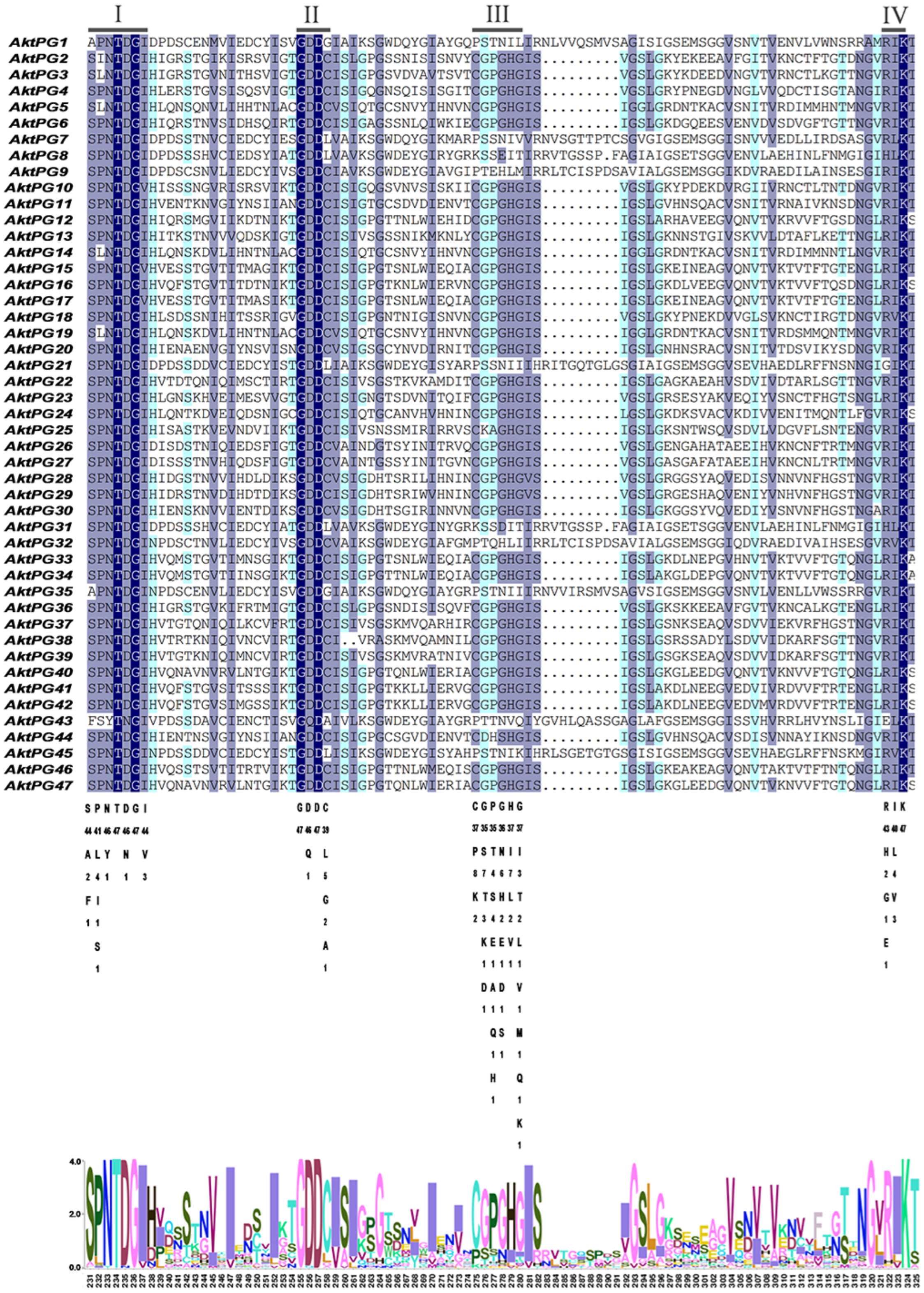

| Gene Name | Chromosome | Location | Gene Length (bp) | Exons | Duplication Type | Putative Protein | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (AA) | PI | Molecular Weight (kDa) | Instability Index | |||||||
| AktPG1 | 1 | 1950986 | 1963052 | 12,066 | 5 | WGD or Segmental | 491 | 8.62 | 55.26 | 45.59 |
| AktPG2 | 1 | 29549713 | 29551549 | 1836 | 4 | WGD or Segmental | 396 | 8.76 | 42.22 | 29.29 |
| AktPG3 | 1 | 30682795 | 30686212 | 3417 | 4 | Dispersed | 399 | 4.94 | 42.26 | 30.34 |
| AktPG4 | 2 | 579257 | 582555 | 3298 | 6 | Dispersed | 420 | 8.80 | 44.25 | 27.41 |
| AktPG5 | 3 | 3762763 | 3767823 | 5060 | 7 | WGD or Segmental | 520 | 7.54 | 56.88 | 43.59 |
| AktPG6 | 3 | 5777237 | 5785021 | 7784 | 8 | Dispersed | 408 | 7.56 | 44.56 | 36.70 |
| AktPG7 | 3 | 35682662 | 35687812 | 5150 | 5 | Dispersed | 487 | 7.13 | 53.12 | 36.22 |
| AktPG8 | 3 | 53310300 | 53316508 | 6208 | 7 | WGD or Segmental | 442 | 9.23 | 48.59 | 39.23 |
| AktPG9 | 3 | 54139078 | 54143861 | 4783 | 6 | WGD or Segmental | 484 | 4.75 | 53.24 | 43.41 |
| AktPG10 | 4 | 3864384 | 3867211 | 2827 | 5 | WGD or Segmental | 416 | 9.24 | 44.77 | 36.83 |
| AktPG11 | 4 | 4589231 | 4591100 | 1869 | 6 | WGD or Segmental | 466 | 4.67 | 50.71 | 41.14 |
| AktPG12 | 4 | 5079077 | 5080727 | 1650 | 4 | WGD or Segmental | 390 | 9.27 | 42.28 | 32.39 |
| AktPG13 | 5 | 3716964 | 3722125 | 5161 | 10 | Dispersed | 449 | 5.33 | 48.69 | 31.54 |
| AktPG14 | 5 | 3742665 | 3746619 | 3954 | 7 | WGD or Segmental | 516 | 6.85 | 56.43 | 47.09 |
| AktPG15 | 5 | 27256023 | 27257798 | 1775 | 5 | Tandem | 366 | 5.35 | 38.32 | 23.56 |
| AktPG16 | 5 | 27294394 | 27296597 | 2203 | 4 | WGD or Segmental | 388 | 8.80 | 41.64 | 31.14 |
| AktPG17 | 5 | 27307604 | 27336337 | 28,733 | 8 | Proximal | 753 | 5.71 | 79.99 | 23.59 |
| AktPG18 | 5 | 34397399 | 34401928 | 4529 | 6 | WGD or Segmental | 410 | 8.16 | 43.94 | 35.70 |
| AktPG19 | 7 | 28823177 | 28826730 | 3553 | 7 | WGD or Segmental | 478 | 7.17 | 51.78 | 44.81 |
| AktPG20 | 8 | 13692797 | 13695055 | 2258 | 6 | WGD or Segmental | 463 | 6.29 | 50.55 | 42.41 |
| AktPG21 | 8 | 15213064 | 15216086 | 3022 | 6 | WGD or Segmental | 466 | 4.97 | 51.03 | 36.10 |
| AktPG22 | 8 | 41653829 | 41660180 | 6351 | 10 | WGD or Segmental | 446 | 6.08 | 48.93 | 38.24 |
| AktPG23 | 8 | 46547108 | 46550654 | 3546 | 9 | Dispersed | 371 | 7.52 | 40.24 | 32.55 |
| AktPG24 | 9 | 23425160 | 23428978 | 3818 | 8 | Dispersed | 474 | 9.11 | 51.99 | 44.54 |
| AktPG25 | 10 | 529791 | 535457 | 5666 | 9 | Dispersed | 290 | 8.24 | 31.81 | 43.07 |
| AktPG26 | 10 | 592482 | 598293 | 5811 | 8 | Tandem | 417 | 6.34 | 45.67 | 35.91 |
| AktPG27 | 10 | 606091 | 628366 | 22,275 | 9 | Tandem | 374 | 7.88 | 39.75 | 27.74 |
| AktPG28 | 10 | 644584 | 647904 | 3320 | 8 | Tandem | 389 | 7.06 | 42.12 | 36.69 |
| AktPG29 | 10 | 651049 | 655078 | 4029 | 8 | Tandem | 378 | 8.71 | 41.58 | 46.00 |
| AktPG30 | 10 | 655783 | 660884 | 5101 | 10 | Tandem | 423 | 8.14 | 45.57 | 36.50 |
| AktPG31 | 11 | 13500656 | 13506726 | 6070 | 5 | WGD or Segmental | 449 | 6.55 | 49.18 | 40.01 |
| AktPG32 | 11 | 21482203 | 21488172 | 5969 | 6 | WGD or Segmental | 479 | 6.07 | 52.11 | 34.72 |
| AktPG33 | 11 | 31436526 | 31440085 | 3559 | 5 | Tandem | 378 | 7.96 | 39.89 | 18.05 |
| AktPG34 | 11 | 31477315 | 31479413 | 2098 | 4 | Tandem | 390 | 9.05 | 41.41 | 19.24 |
| AktPG35 | 12 | 325546 | 331005 | 5459 | 5 | WGD or Segmental | 490 | 9.45 | 55.23 | 45.09 |
| AktPG36 | 13 | 1986765 | 1989582 | 2817 | 4 | WGD or Segmental | 394 | 9.10 | 42.04 | 26.42 |
| AktPG37 | 14 | 463984 | 471132 | 7148 | 10 | WGD or Segmental | 455 | 9.37 | 50.01 | 33.82 |
| AktPG38 | 14 | 473659 | 481805 | 8146 | 9 | Tandem | 327 | 9.36 | 35.84 | 35.60 |
| AktPG39 | 14 | 485377 | 488688 | 3311 | 9 | Tandem | 443 | 8.82 | 48.61 | 31.00 |
| AktPG40 | 16 | 287716 | 289512 | 1796 | 4 | WGD or Segmental | 388 | 9.35 | 41.86 | 34.11 |
| AktPG41 | 16 | 389335 | 391791 | 2456 | 4 | Proximal | 391 | 9.52 | 42.34 | 25.09 |
| AktPG42 | 16 | 405390 | 407880 | 2490 | 4 | Proximal | 394 | 9.67 | 42.89 | 29.64 |
| AktPG43 | 16 | 30413390 | 30419071 | 5681 | 6 | Dispersed | 457 | 5.14 | 48.75 | 38.24 |
| AktPG44 | 16 | 32206491 | 32211639 | 5148 | 7 | WGD or Segmental | 463 | 4.98 | 49.95 | 46.13 |
| AktPG45 | 16 | 32296843 | 32299964 | 3121 | 6 | WGD or Segmental | 466 | 5.34 | 51.38 | 35.44 |
| AktPG46 | Contig00874 | 8841 | 24460 | 15,619 | 8 | Dispersed | 778 | 7.15 | 82.83 | 19.74 |
| AktPG47 | Contig00909 | 12723 | 14521 | 1798 | 4 | Dispersed | 388 | 9.35 | 41.89 | 34.11 |
| Motif | Wide | Best Possible Match | PG Domain |
|---|---|---|---|
| Motif1 | 41 | ITAPGDSPNTDGIHYQSSTNVVIEDSVIKTGDDCISIGSGT | I, II |
| Motif2 | 29 | GYVRNITFENITMNNVQNPIIIDQNYCPH | |
| Motif3 | 29 | EAGVSNVTVKNVVFTGTTNGVRIKTWQGG | IV |
| Motif4 | 21 | VWIENINCGPGHGISIGSLGK | III |
| Motif5 | 29 | ISNVTYKNIKGTSATEVAIKFDCSKSVPC | |
| Motif6 | 29 | PTGPTSLRFYNSKNIVISGLTSINSPQFH | |
| Motif7 | 11 | GTIDGQGSVWW | |
| Motif8 | 50 | LTGSFNLTSHMTLFLDKGAVILGSQDEKHWPLIDPLPSYGRGRELPGGRY | |
| Motif9 | 16 | DFGAVGDGKTDDTKAF | |
| Motif10 | 15 | TYLVKPTIFSGPCKS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Chen, W.; Guan, J.; Zhu, J.; Zhang, Q.; Yang, H.; Yang, H.; Zhong, S.; Chen, C.; Tan, F.; et al. Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata. Int. J. Mol. Sci. 2023, 24, 16973. https://doi.org/10.3390/ijms242316973
Yi X, Chen W, Guan J, Zhu J, Zhang Q, Yang H, Yang H, Zhong S, Chen C, Tan F, et al. Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata. International Journal of Molecular Sciences. 2023; 24(23):16973. https://doi.org/10.3390/ijms242316973
Chicago/Turabian StyleYi, Xiaoxiao, Wei Chen, Ju Guan, Jun Zhu, Qiuyi Zhang, Huai Yang, Hao Yang, Shengfu Zhong, Chen Chen, Feiquan Tan, and et al. 2023. "Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata" International Journal of Molecular Sciences 24, no. 23: 16973. https://doi.org/10.3390/ijms242316973
APA StyleYi, X., Chen, W., Guan, J., Zhu, J., Zhang, Q., Yang, H., Yang, H., Zhong, S., Chen, C., Tan, F., Ren, T., & Luo, P. (2023). Genome-Wide Analysis of the Polygalacturonase Gene Family Sheds Light on the Characteristics, Evolutionary History, and Putative Function of Akebia trifoliata. International Journal of Molecular Sciences, 24(23), 16973. https://doi.org/10.3390/ijms242316973






