Enhanced Membrane Incorporation of H289Y Mutant GluK1 Receptors from the Audiogenic Seizure-Prone GASH/Sal Model: Functional and Morphological Impacts on Xenopus Oocytes
Abstract
1. Introduction
2. Results
2.1. Assessing the Impact of the H289Y Mutation on the Function and Stability of GluK1Rs using Sequence-Homology Based Tools
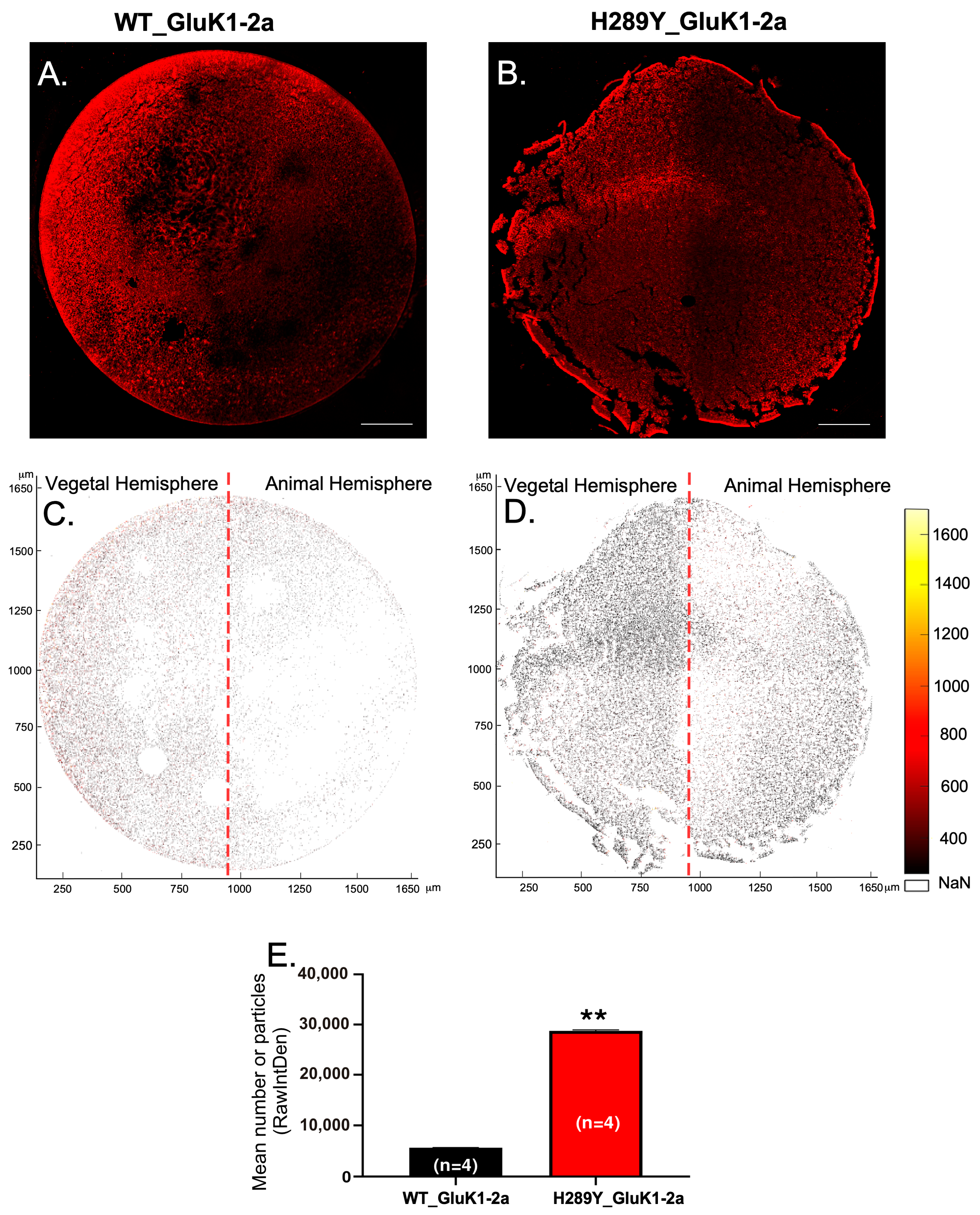
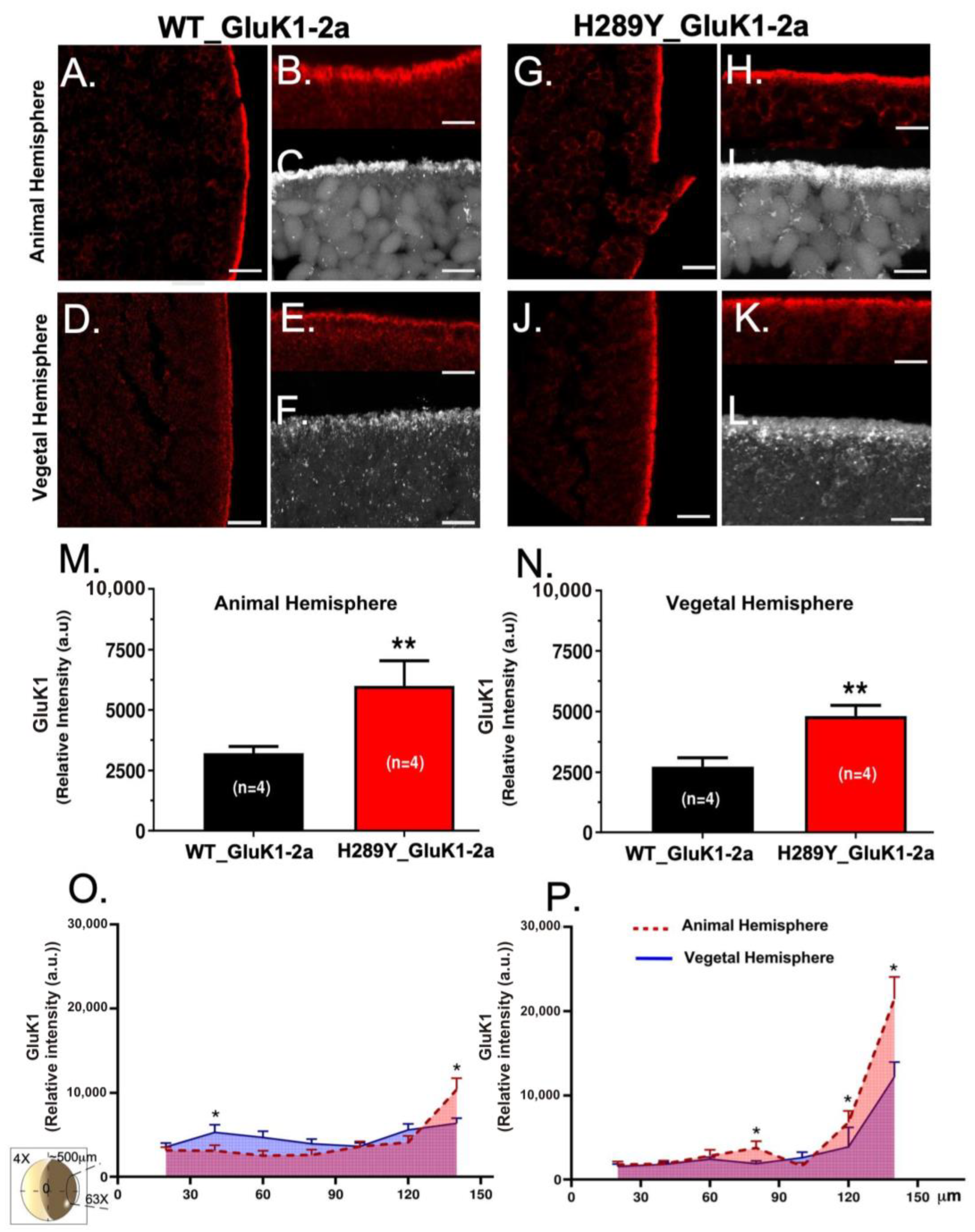
2.2. Expression and Localization of GluK1 Receptors in Xenopus Oocytes
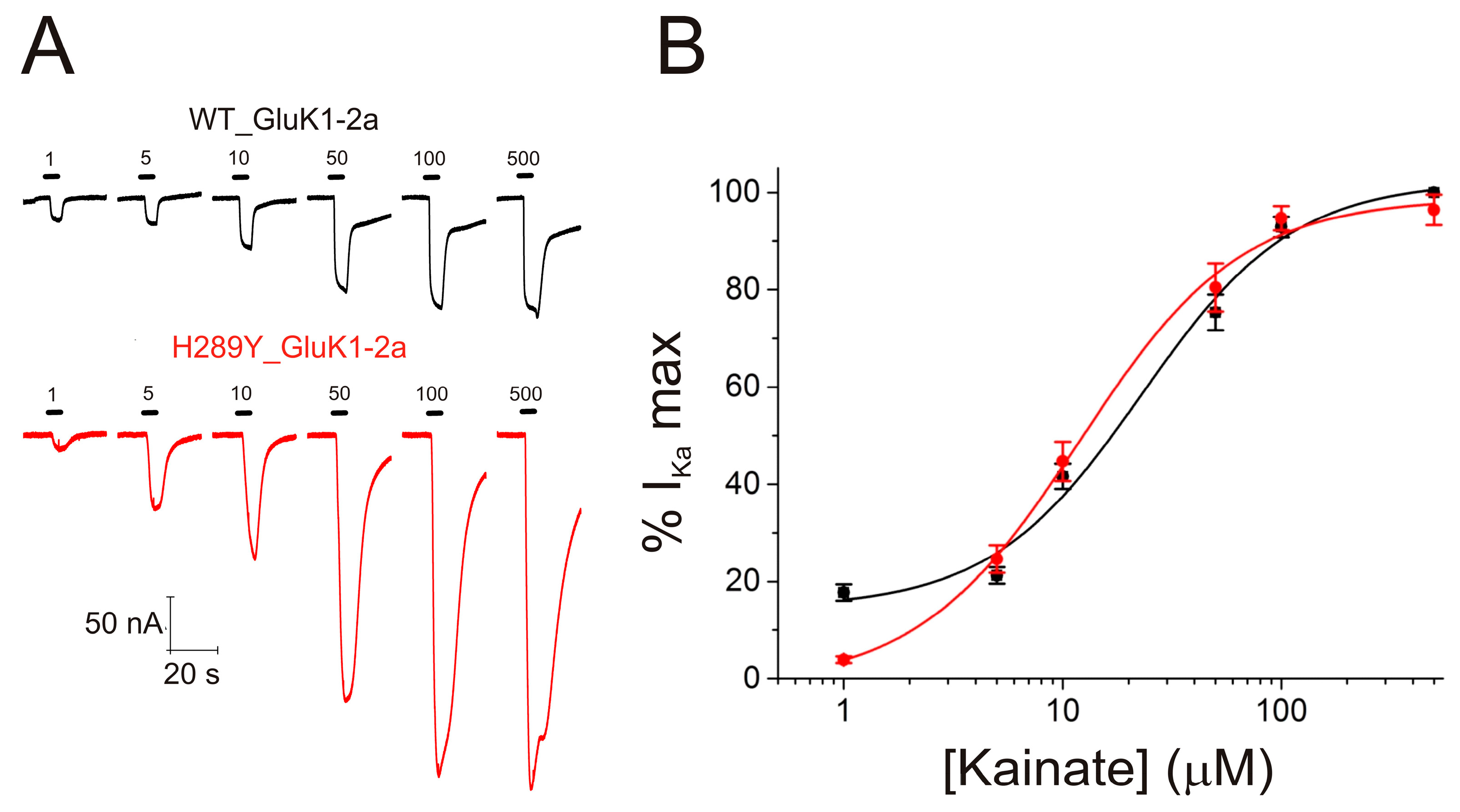
2.3. The H289Y Mutation Enhances Kainate Currents in Oocytes Expressing GluK1-2aRs
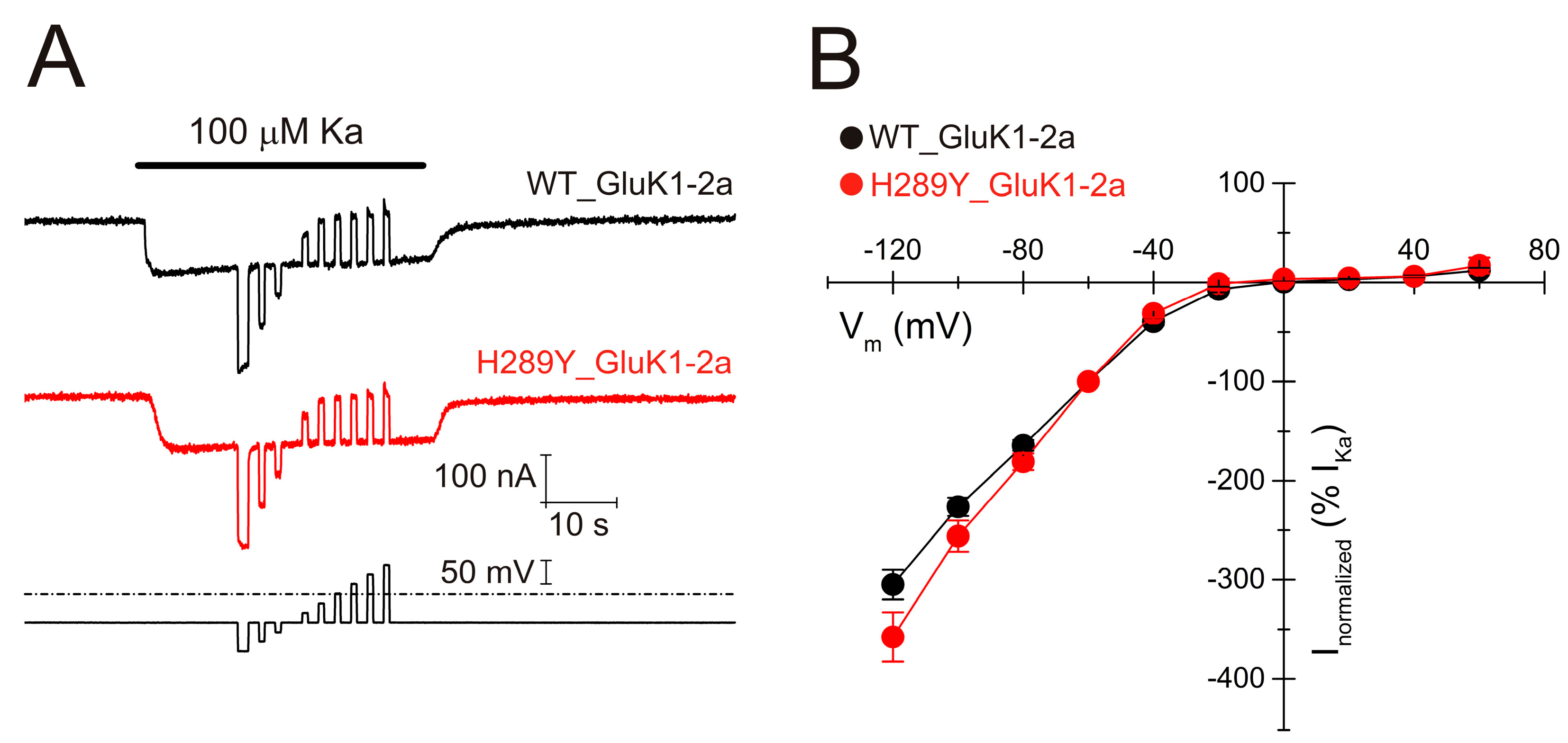
2.4. The H289Y Mutation Does Not Significantly Modify the Functional Properties of GluK1-2aRs
3. Discussion
4. Materials and Methods
4.1. In Silico Prediction Tools
4.2. Design of the Vector and Synthesis of mRNA Coding for Wild-Type and Mutant GluK1 Receptors
4.3. Oocyte Microinjection with mRNA Coding for GluK1 Receptors
4.4. Oocyte Preparation, Processing, Immunostaining, and Imaging
4.4.1. Immunohistochemistry
4.4.2. Observation and Study of Histological Samples
4.5. Mapping of WT and H289Y GluK1-2a Proteins in Xenopus Oocytes
4.5.1. Image Analysis
4.5.2. MATLAB Maps
4.6. Two-Electrode Voltage-Clamp Recordings in Xenopus Oocytes
4.7. Experimental Design and Data Analysis of Functional Experiments
4.8. Statistical Analysis
4.9. Drugs
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef]
- Lerma, J.; Paternain, A.V.; Rodríguez-Moreno, A.; López-García, J.C. Molecular Physiology of Kainate Receptors. Phys. Rev. 2001, 81, 971–998. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharm. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Watkins, J.C.; Jane, D.E. The glutamate story. Br. J. Pharm. 2006, 147, S100–S108. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharm. 2009, 56, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Contractor, A.; Mulle, C.; Swanson, G.T. Kainate receptors coming of age: Milestones of two decades of research. Trends Neurosci. 2011, 34, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.-F.; Ye, Y.; Xu, S.; Tao, W.; Zhao, S.; Jin, T.; Nicoll, R.A.; Shi, Y.S.; Sheng, N. Signal peptide represses GluK1 surface and synaptic trafficking through binding to amino-terminal domain. Nat. Commun. 2018, 9, 4879. [Google Scholar] [CrossRef]
- Karakas, E.; Simorowski, N.; Furukawa, H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009, 28, 3910–3920. [Google Scholar] [CrossRef]
- Rossmann, M.; Sukumaran, M.; Penn, A.C.; Veprintsev, D.B.; Babu, M.M.; Greger, I.H. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J. 2011, 30, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Sheng, N.; Shi, Y.S.; Nicoll, R.A. Amino-terminal domains of kainate receptors determine the differential dependence on Neto auxiliary subunits for trafficking. Proc. Natl. Acad. Sci. USA 2017, 114, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.; Noam, Y.; Nomura, T.; Yamasaki, M.; Yan, D.; Fernandes, H.B.; Zhang, P.; Howe, J.R.; Watanabe, M.; Contractor, A.; et al. Distinct Subunit Domains Govern Synaptic Stability and Specificity of the Kainate Receptor. Cell Rep. 2016, 16, 531–544. [Google Scholar] [CrossRef]
- Sommer, B.; Burnashev, N.; Verdoorn, T.A.; Keinänen, K.; Sakmann, B.; Seeburg, P.H. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992, 11, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Gregor, P.; O’Hara, B.F.; Yang, X.; Uhl, G.R. Expression and novel subunit isoforms of glutamate receptor genes GluR5 and GluR6. Neuroreport 1993, 4, 1343–1346. [Google Scholar] [CrossRef]
- Barbon, A.; Barlati, S. Genomic organization, proposed alternative splicing mechanisms, and RNA editing structure of GRIK1. Cytogenet. Cell Genet. 2000, 88, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, C.; Park, J.S.; Niu, L. Channel-Opening Kinetic Mechanism of Wild-Type GluK1 Kainate Receptors and a C-Terminal Mutant. Biochemistry 2012, 51, 761–768. [Google Scholar] [CrossRef]
- Bowie, D. Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J. Biol. Chem. 2018, 293, 18789–18802. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Fièvre, S.; Gorlewicz, A.; Mulle, C. Kainate receptors in the hippocampus. Eur. J. Neurosci. 2014, 39, 1835–1844. [Google Scholar] [CrossRef]
- Sihra, T.S.; Flores, G.; Rodríguez-Moreno, A. Kainate Receptors. Neuroscientist 2014, 20, 29–43. [Google Scholar] [CrossRef]
- Lerma, J.; Marques, J.M. Kainate Receptors in Health and Disease. Neuron 2013, 80, 292–311. [Google Scholar] [CrossRef]
- Lucarini, N.; Verrotti, A.; Napolioni, V.; Bosco, G.; Curatolo, P. Genetic Polymorphisms and Idiopathic Generalized Epilepsies. Pediatr. Neurol. 2007, 37, 157–164. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Balista, P.A.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno-Junior, L.S.; Leite, J.P. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693. [Google Scholar] [CrossRef]
- Muñoz, L.J.; Carballosa-Gautam, M.M.; Yanowsky, K.; García-Atarés, N.; López, D.E. The genetic audiogenic seizure hamster from Salamanca: The GASH:Sal. Epilepsy Behav. 2017, 71 Pt B, 181–192. [Google Scholar] [CrossRef]
- Carballosa-Gonzalez, M.M.; Muñoz, L.J.; López-Alburquerque, T.; Pardal-Fernández, M.J.; Nava, E.; de Cabo, C.; Sancho, C.; López, D.E. EEG characterization of audiogenic seizures in the hamster strain GASH:Sal. Epilepsy Res. 2013, 106, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, S.M.; López-López, D.; Herrero-Turrión, M.J.; Gómez-Nieto, R.; Canal-Alonso, A.; Lopéz, D.E. Inferior Colliculus Transcriptome After Status Epilepticus in the Genetically Audiogenic Seizure-Prone Hamster GASH/Sal. Front. Neurosci. 2020, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Casado, E.; Gómez-Nieto, R.; de Pereda, J.M.; Muñoz, L.J.; Jara-Acevedo, M.; López, D.E. Analysis of gene variants in the GASH/Sal model of epilepsy. PLoS ONE 2020, 15, e0229953. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, R.H.; Goldman, D. Genomics and variation of ionotropic glutamate receptors. Ann. N. Y. Acad. Sci. 2003, 1003, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z.J.; Liu, L.; Xu, H.Q.; Shi, Y.W.; Yi, Y.H.; He, N.; Liao, W.P. Epilepsy-associated genes. Seizure 2017, 44, 11–20. [Google Scholar] [CrossRef] [PubMed]
- International League Against Epilepsy Consortium on Complex Epilepsies. Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat. Commun. 2018, 9, 5269. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Hildmann, T.; Kretz, R.; Fürst, R.; Sailer, U.; Bauer, G.; Schmitz, B.; Beck-Mannagetta, G.; Wienker, T.F.; Janz, D. Allelic association of juvenile absence epilepsy with a GluR5 kainate receptor gene (GRIK1) polymorphism. Am. J. Med. Genet. 1997, 74, 416–421. [Google Scholar] [CrossRef]
- Friedman, D.; Kannan, K.; Faustin, A.; Shroff, S.; Thomas, C.; Heguy, A.; Serrano, J.; Snuderl, M.; Devinsky, O. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom. Med. 2018, 3, 9. [Google Scholar] [CrossRef]
- Ivorra, I.; Alberola-Die, A.; Cobo, R.; González-Ros, J.M.; Morales, A. Xenopus Oocytes as a Powerful Cellular Model to Study Foreign Fully-Processed Membrane Proteins. Membranes 2022, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Nie, M.; Zhang, A.; Wen, J.; Noel, S.D.; Lee, V.; Carroll, R.S.; Kaiser, U.B. Computational Analysis of Missense Variants of G Protein-Coupled Receptors Involved in the Neuroendocrine Regulation of Reproduction. Neuroendocrinology 2016, 103, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Klein, C.; Hollmann, M. Xenopus laevis oocytes endogenously express all subunits of the ionotropic glutamate receptor family. J. Mol. Biol. 2009, 390, 182–195. [Google Scholar] [CrossRef]
- Coussen, F. Molecular determinants of kainate receptor trafficking. Neuroscience 2009, 158, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jaskolski, F.; Coussen, F.; Mulle, C. Subcellular localization and trafficking of kainate receptors. Trends Pharmacol. Sci. 2005, 26, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Riley, N.J.; Garcia, E.P.; Sanders, J.M.; Swanson, G.T.; Marshall, J. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J. Neurosci. 2003, 23, 6608–6616. [Google Scholar] [CrossRef]
- Yan, S.; Sanders, J.M.; Xu, J.; Zhu, Y.; Contractor, A.; Swanson, G.T. A C-terminal determinant of GluR6 kainate receptor trafficking. J. Neurosci. 2004, 24, 679–691. [Google Scholar] [CrossRef]
- Eusebi, F.; Palma, E.; Amici, M.; Miledi, R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: A potent tool for expanding functional information. Prog. Neurobiol. 2009, 88, 32–40. [Google Scholar] [CrossRef]
- Kusano, K.; Miledi, R.; Stinnakre, J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. 1982, 328, 143–170. [Google Scholar] [CrossRef]
- Martinez-Torres, A.; Miledi, R. Expression of gamma-aminobutyric acid rho 1 and rho 1 Delta 450 as gene fusions with the green fluorescent protein. Proc. Natl. Acad. Sci. USA 2001, 98, 1947–1951. [Google Scholar] [CrossRef]
- Palma, E.; Mileo, A.M.; Martinez-Torres, A.; Eusebi, F.; Miledi, R. Some properties of human neuronal alpha 7 nicotinic acetylcholine receptors fused to the green fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Miledi, R.; Sumikawa, K. Synthesis of cat muscle acetylcholine receptors by Xenopus oocytes. Biomed. Res. 1982, 3, 390–399. [Google Scholar] [CrossRef]
- Meyerson, J.R.; Chittori, S.; Merk, A.; Rao, P.; Han, T.H.; Serpe, M.; Mayer, M.L.; Subramaniam, S. Structural basis of kainate subtype glutamate receptor desensitization. Nature 2016, 537, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.; Weiss, B.; Ogden, A.M.; Knauss, J.L.; Oler, J.; Ho, K.; Large, T.H.; Bleakman, D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 2004, 46, 793–806. [Google Scholar] [CrossRef]
- Chen, T.-S.; Huang, T.-H.; Lai, M.-C.; Huang, C.-W. The Role of Glutamate Receptors in Epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef]
- Ojanen, S.; Kuznetsova, T.; Kharybina, Z.; Voikar, V.; Lauri, S.E.; Taira, T. Interneuronal GluK1 kainate receptors control maturation of GABAergic transmission and network synchrony in the hippocampus. Mol. Brain 2023, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Moya, R.; Sihra, T.S.; Rodríguez-Moreno, A. Kainate Receptors: Role in Epilepsy. Front. Mol. Neurosci. 2018, 11, 217. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, S.M.; Herrero-Turrión, M.J.; García-Peral, C.; Gómez-Nieto, R. Delving into the Significance of the His289Tyr Single-Nucleotide Polymorphism in the Glutamate Ionotropic Receptor Kainate-1 (Grik1) Gene of a Genetically Audiogenic Seizure Model. Front. Mol. Neurosci. 2023, in press. [Google Scholar]
- Hayes, D.M.; Braud, S.; Hurtado, D.E.; McCallum, J.; Standley, S.; Isaac, J.T.; Roche, K.W. Trafficking and surface expression of the glutamate receptor subunit, KA2. Biochem. Biophys. Res. Commun. 2003, 310, 8–13. [Google Scholar] [CrossRef]
- Nasu-Nishimura, Y.; Hurtado, D.; Braud, S.; Tang, T.T.; Isaac, J.T.; Roche, K.W. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J. Neurosci. 2006, 26, 7014–7021. [Google Scholar] [CrossRef]
- Ren, Z.; Riley, N.J.; Needleman, L.A.; Sanders, J.M.; Swanson, G.T.; Marshall, J. Cell Surface Expression of GluR5 Kainate Receptors Is Regulated by an Endoplasmic Reticulum Retention Signal. J. Biol. Chem. 2003, 278, 52700–52709. [Google Scholar] [CrossRef]
- Vivithanaporn, P.; Yan, S.; Swanson, G.T. Intracellular trafficking of KA2 kainate receptors mediated by interactions with coatomer protein complex I (COPI) and 14-3-3 chaperone systems. J. Biol. Chem. 2006, 281, 15475–15484. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kieserman, E.; Gray, R.S.; Park, T.J.; Wallingford, J. Whole-Mount Fluorescence Immunocytochemistry on Xenopus Embryos. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.; Dunsby, C.; De Beule, P.A.; Owen, D.M.; Anand, U.; Lanigan, P.M.; Benninger, R.K.; Davis, D.M.; Neil, M.A.; Anand, P.; et al. Multifocal multiphoton excitation and time correlated single photon counting detection for 3-D fluorescence lifetime imaging. Opt. Express 2007, 15, 12548. [Google Scholar] [CrossRef] [PubMed]
- Paulina, R.H.; Manuel, K.P. Produccion de Anticuerpos Policlonales Contra el Silenciador de la Transcripcion del Elemento Represor (Rest) de Xenopus laevis. Bachelor’s Thesis, Universidad de Chile, Santiago de Chile, Chile, 2005. [Google Scholar]
- Sánchez-Benito, D.; Hyppolito, M.A.; Alvarez-Morujo, A.J.; López, D.E.; Gómez-Nieto, R. Morphological and molecular correlates of altered hearing sensitivity in the genetically audiogenic seizure-prone hamster GASH/Sal. Hear Res. 2020, 392, 107973. [Google Scholar] [CrossRef]
- Alberola-Die, A.; Encinar, J.A.; Cobo, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ivorra, I.; Morales, A. Peimine, an Anti-Inflammatory Compound from Chinese Herbal Extracts, Modulates Muscle-Type Nicotinic Receptors. Int. J. Mol. Sci. 2021, 22, 11287. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Rodríguez, S.M.; Ivorra, I.; Espinosa, J.; Vegar, C.; Herrero-Turrión, M.J.; López, D.E.; Gómez-Nieto, R.; Alberola-Die, A. Enhanced Membrane Incorporation of H289Y Mutant GluK1 Receptors from the Audiogenic Seizure-Prone GASH/Sal Model: Functional and Morphological Impacts on Xenopus Oocytes. Int. J. Mol. Sci. 2023, 24, 16852. https://doi.org/10.3390/ijms242316852
Díaz-Rodríguez SM, Ivorra I, Espinosa J, Vegar C, Herrero-Turrión MJ, López DE, Gómez-Nieto R, Alberola-Die A. Enhanced Membrane Incorporation of H289Y Mutant GluK1 Receptors from the Audiogenic Seizure-Prone GASH/Sal Model: Functional and Morphological Impacts on Xenopus Oocytes. International Journal of Molecular Sciences. 2023; 24(23):16852. https://doi.org/10.3390/ijms242316852
Chicago/Turabian StyleDíaz-Rodríguez, Sandra M., Isabel Ivorra, Javier Espinosa, Celia Vegar, M. Javier Herrero-Turrión, Dolores E. López, Ricardo Gómez-Nieto, and Armando Alberola-Die. 2023. "Enhanced Membrane Incorporation of H289Y Mutant GluK1 Receptors from the Audiogenic Seizure-Prone GASH/Sal Model: Functional and Morphological Impacts on Xenopus Oocytes" International Journal of Molecular Sciences 24, no. 23: 16852. https://doi.org/10.3390/ijms242316852
APA StyleDíaz-Rodríguez, S. M., Ivorra, I., Espinosa, J., Vegar, C., Herrero-Turrión, M. J., López, D. E., Gómez-Nieto, R., & Alberola-Die, A. (2023). Enhanced Membrane Incorporation of H289Y Mutant GluK1 Receptors from the Audiogenic Seizure-Prone GASH/Sal Model: Functional and Morphological Impacts on Xenopus Oocytes. International Journal of Molecular Sciences, 24(23), 16852. https://doi.org/10.3390/ijms242316852






