Abstract
The angiotensin II type 2 (AT2) receptor has a role in promoting insulin sensitivity. However, the mechanisms underlying the AT2 receptor-induced facilitation of insulin are still not completely understood. Therefore, we investigated whether acute in vivo administration of AT2 receptor agonist compound 21 (C21) could activate insulin signaling molecules in insulin-target tissues. We report that, in male C57BL/6 mice, an acute (5 min, 0.25 mg/kg; i.v.) injection of C21 induces the phosphorylation of Akt and ERK1/2 at activating residues (Ser473 and Thr202/Tyr204, respectively) in both epididymal white adipose tissue (WAT) and heart tissue. In WAT, the extent of phosphorylation (p) of Akt and ERK1/2 induced by C21 was approximately 65% of the level detected after a bolus injection of a dose of insulin known to induce maximal activation of the insulin receptor (IR). In the heart, C21 stimulated p-Akt to a lesser extent than in WAT and stimulated p-ERK1/2 to similar levels to those attained by insulin administration. C21 did not modify p-IR levels in either tissue. We conclude that in vivo injection of the AT2 receptor agonist C21 activates Akt and ERK1/2 through a mechanism that does not involve the IR, indicating the participation of these enzymes in AT2R-mediated signaling.
1. Introduction
The renin-angiotensin system modulates insulin action mainly through the actions of its principal peptide angiotensin (Ang) II acting on its two subtypes of receptors, angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R), which belong to the G protein-coupled receptor (GPCRs) family [1,2,3,4,5]. Conditions of chronic elevation of Ang II are associated with insulin resistance. This negative effect is mediated by the AT1R. Inhibition of Ang II action through an AT1 receptor blockade with specific antagonists or reduction of its production through angiotensin converting enzyme inhibitors results in improvement of glucose homeostasis both in animal models of insulin resistance and/or type 2 diabetes [1,2,3,4,5]. In the last decade, it has been established that the AT2R exerts a positive effect on insulin sensitivity [6,7]. In general, targeting the AT2R with pharmacological tools clearly supports a favorable role in glucose metabolism and insulin function. This particularly applies to adipose tissue [6,7]. Pharmacological acute antagonism of the AT2R with the non-peptide antagonist PD123319 decreased glucose uptake and reduced Akt phosphorylation in rat skeletal muscle [8,9], while chronic blockade of the AT2R reduced insulin receptor signaling in terms of PI3K/Akt activation in the liver and adipose tissue [10], suggesting a physiological role for the AT2R. Stimulation of the AT2R using the established AT2R agonist C21 has been associated with improved insulin sensitivity in KK-Ay type 2 diabetic mice [11], in rats fed a high-fat/high-fructose diet [12], in healthy and streptozotocin (STZ)-diabetic rats and mice [13,14,15], in neonatal STZ-diabetic rats [16], in mice with high-fat diet (HFD)-induced obesity [17,18], in healthy, normal C57BL/6 mice [19] and in female diabetic db/db mice [20]. This physiological role of the AT2R was also corroborated by a study in AT2R-knockout (KO) mice, which showed that in these animals displayed higher STZ-induced glycemia coupled with lower pancreatic insulin levels [15]. However, overall, data from AT2R-KO are controversial and support a beneficial role only in female animals [21,22]. Despite this large amount of evidence for favorable metabolic effects exerted by the AT2R, the mechanisms by which these effects proceed are not known.
AT2R-induced intracellular signaling is atypical and different from the traditional modes of signaling displayed by many other GPCRs including the AT1R [6,7]. Initial AT2R signaling involves the association of an inhibitory G-protein (Gi) or AT2R-interacting protein (ATIP) with the AT2R [7]. These early associations lead to subsequent signaling via phosphatase, kinase, and PPARγ pathways. There is strong evidence for the involvement of kinases in the intermediate signaling of the AT2R [6,7]. In human aortic endothelial cells, incubation with C21 has been shown to induce a rapid phosphorylation of Akt and ERK1/2 at activating residues indicating a recruitment of these kinases by the AT2R [23,24]. There is evidence for the participation of Akt in AT2R-induced effects including improvement of insulin signaling [19,20], nitric oxide (NO) production [23,25], adipose fat browning [26], proximal tubule albumin endocytosis [27], osmotic cellular resistance [28] and antiproteinuric actions [29]. Participation of ERK1/2 has been reported in various AT2R-mediated actions such as neuronal differentiation [30], skeletal muscle regeneration [31] and eNOS-mediated vasodilation [32]. However, direct AT2R-mediated activation of either Akt or ERK1/2 has not been evidenced in vivo yet. Thus, the goal of the current work was to determine whether acute intravenous administration of the AT2R agonist C21 could result in phosphorylation of Akt and ERK1/2 in the metabolic tissues of the mouse in vivo. Our results extend the knowledge of the signaling pathways mediated by the AT2R and indicate that in vivo injection of C21 induces the activation of both Akt and ERK in mouse white adipose tissue (WAT) and heart tissue. These findings highlight the importance of these two kinases in AT2R-mediated signaling.
2. Results
2.1. C21 Induces the Phosphorylation of Akt and ERK1/2 in Mouse White Adipose Tissue (WAT)
For comparison, samples from C21-injected mice were run together with samples of WAT homogenates obtained from insulin (a known recruiter of both Akt and ERK1/2) or vehicle (saline)-injected animals. As compared to baseline values, a bolus injection of insulin known to attain maximal stimulation of the insulin receptor (IR) induced a significant increase in the phosphorylation of the IR at activating Tyr residues (Tyr1158/1162/1163) in WAT (1.6-fold increase; Figure 1A). Accordingly, phospho (p)-Akt-Ser473 levels and p-ERK1/2-Thr202/Tyr204 levels in WAT increased significantly after insulin injection (Figure 1B,C). While acute intravenous injection of C21 did not modify IR phosphorylation in WAT (Figure 1A), acute C21 injection induced a marked and significant increase in both p-Akt and p-ERK1/2 in mouse WAT (Figure 1B,C). The mean level of Akt phosphorylation attained 5 min after C21 administration was approximately 65% of that detected after insulin injection (Figure 1B), while the level of ERK1/2 phosphorylation was comparable to that induced by in vivo insulin administration (Figure 1C). The protein abundance of IR, Akt and ERK1/2 in WAT was not modified after either treatment with saline, insulin or C21 (Figure 1A–C).
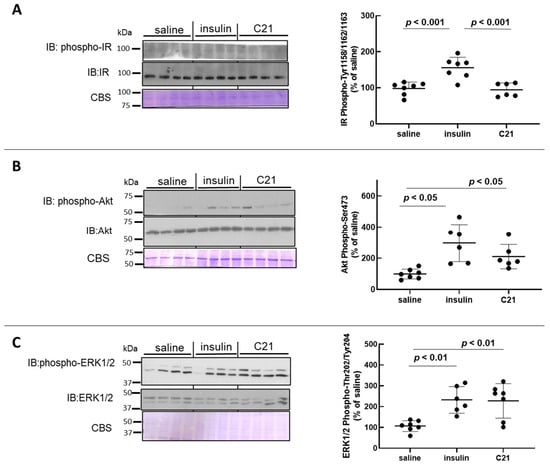
Figure 1.
C21 stimulates the phosphorylation of Akt and ERK1/2 in mouse adipose tissue. The phosphorylation level and total abundance of the insulin receptor (IR) (A), Akt (B) and ERK1/2 (C) were evaluated in epididymal adipose tissue homogenates by Western blot. Western blot membranes were stained with Coomassie Blue for loading control. The phosphorylation-to-protein ratio was calculated for each sample. Data are expressed as mean ± SEM (n = 6 for all groups). A representative image is presented. All analyses were carried out using GraphPad Prism 8.0.
2.2. C21 Induces the Phosphorylation of Akt and ERK1/2 in Mouse Heart
As compared to baseline values, in vivo intravenous injection of insulin induced an approximate 3.5-fold increase in IR phosphorylation at activating residues Tyr1158/1162/1163 (Figure 2A) while, as expected, C21 did not modify IR phosphorylation in mouse heart tissue (Figure 2A).
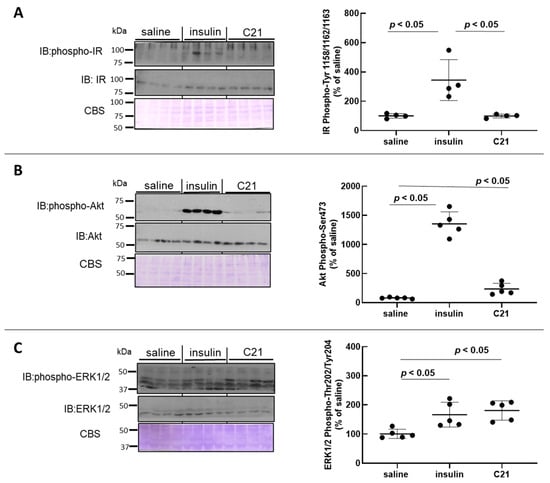
Figure 2.
C21 stimulates the phosphorylation of Akt and ERK1/2 in mouse heart. The phosphorylation level and total abundance of the insulin receptor (IR) (A), Akt (B) and ERK1/2 (C) were evaluated in heart homogenates by Western blot. Western blot membranes were stained with Coomassie Blue for loading control. The phosphorylation-to-protein ratio was calculated for each sample. Data are expressed as mean ± SEM (n = 6 for all groups). All analyses were carried out using GraphPad Prism 8.0.
Similarly to what was detected for WAT, in vivo C21 injection stimulated the phosphorylation levels of Akt at Ser473 by approximately 2.5–3-fold in heart tissue (Figure 2B). However, this stimulation was only a fraction of that attained after insulin administration using the same protocol. When heart homogenates were probed with an anti p-ERK1/2-Thr202/Tyr204 antibody, an approximate 1.8-fold increase over baseline values was detected for ERK1/2 phosphorylation in heart tissue after in vivo C21 injection (Figure 2C). The mean level of Akt phosphorylation attained 5 min after C21 administration was approximately 25% of that detected after insulin injection (Figure 2B), while the level of ERK1/2 phosphorylation was comparable to that induced by in vivo insulin administration (Figure 2C). The protein abundance of IR, Akt and ERK1/2 in heart tissue was not modified after either treatment with saline, insulin or C21 (Figure 2A–C).
3. Discussion
The AT2R is one of the main receptors within the protective arm of the RAS, others being MAS and insulin-regulated aminopeptidase [6,7]. Compared to other GPCRs of therapeutic significance, the development of drugs targeting the AT2R for therapeutic use of its protective and regenerative properties has been slow [6]. The difficulty in determining robust parameters for the detection of AT2R effects is likely a major reason for this delay. Since the signaling pathways afford AT2Rs the ability to exert protective actions in multiple disease states—sometimes in direct opposition to deleterious AT1R-mediated effects—the investigation of these pathways is a topic of importance. Recent reports have reinforced the notion that in vivo stimulation of the AT2R with C21 leads to major beneficial actions, including reduction of inflammation [33], attenuation of cardiac fibrosis [34], antagonism of the thromboxane receptor [35], enhancement of insulin sensitivity and amelioration of type-2 diabetes complications [6,19,20].
Intracellular signaling induced by the AT2R is atypical and remarkably it does not share a resemblance with traditional modes of signaling displayed by many other GPCRs, including the AT1R [6,35,36]. There is evidence that AT2R signaling events include the participation of phosphatases, kinases and PPAR pathways. In addition, accumulated evidence indicates that there is a large variety of AT2R-stimulated signal transduction pathways, with evidence for both G-protein-dependent and independent mechanisms, a common pattern for GPCRs [6,37,38]. Activation of protein phosphatases is a central intermediate step in AT2R signaling, regardless of whether the upstream signaling involves G-proteins or not [6,7,39].
While the signaling pathways employed by the AT2R have been the focus of intense research efforts, the role of downstream kinase and phosphatase pathways on AT2R-mediated actions requires further investigation. Our results are indicative of the participation of both Akt and ERK1/2 in AT2R signaling in both white adipose tissue and heart tissue—tissues known to express the AT2R [6,7]. These results are in good agreement with previous reports indicating that stimulation of the AT2R using C21 induces Akt phosphorylation in human aortic endothelial cells (HAECs), an event that was linked to NO production [23]. More recently, the phosphorylation status of HAECs after stimulation with C21 was determined utilizing time-resolved quantitative phosphoproteomics, showing that AT2Rs stimulation induces the phosphorylation and dephosphorylation of 172 proteins, of which, a large proportion are involved in antiproliferation and apoptosis [24]. Computer-based kinase prediction found that both Akt and ERK1/2 take part in AT2R-signaling. Participation of these kinases in AT2R-mediated signaling in HAECs was confirmed by Western Blotting [39]. Our current findings are in excellent correlation with this study and indicate that these events also take place in vivo and thus they could be of physiological relevance. At present, it is, however, not known how the connection between the AT2R and these downstream kinases proceeds. Unlike most other GPCRs, the AT2R does not associate with β-arrestin [40]. Since physical interaction of the AT2R with other receptors such as AT1R, B2R and Mas and with several other binding proteins has been established [41], we hypothesize that these interactions could be relevant for current findings.
Of note, current results support the participation of the kinases Akt and ERK1/2 that has been reported in several AT2R-mediated actions such as improvement of insulin signaling [19,20], NO synthesis [23,24], adipose fat browning [25], proximal tubule albumin endocytosis [26], osmotic cellular resistance [27], antiproteinuric actions [28] and anti-fibrotic effects [37], for Akt, and neuronal differentiation [29] skeletal muscle regeneration [30], endothelial NO synthase-mediated vasodilation [31] and mitogen-activated protein kinase phosphatase activation [39], in the case of ERK1/2. Our previous reports involving pharmacological agonism or blockade of the AT2R and mice with global deletion of the AT2R [10,19,20,22] suggested that the presence of the AT2R in adipose tissue is critical to the role of this receptor in the control of insulin action and glucose homeostasis. Considering current findings, it is hypothesized that the kinases Akt and ERK1/2, known to participate in the control of metabolism, could have a role in AT2R-mediated metabolic actions in this tissue.
When analyzing the strengths of the study, we considered the following aspects: (a) results contribute to expanding the knowledge of AT2R-mediated signaling pathways, strongly supporting the participation of kinases aside from phosphatases; (b) the detection of Akt and ERK1/2 phosphorylation in mouse tissues through the use of phospho-specific antibodies make the results unequivocal; and (c) reported results are ascribed to AT2R agonism since C21 is a compound with proven specificity towards this receptor. Noteworthily, it must be mentioned that this study has several limitations. Namely: (a) the utilization of a single species, a single gender and a single dose of C21 at a one-time point is not enough to fully characterize the selectivity and efficacy of the in vivo activation of the analyzed kinases [42]; (b) analysis of Akt and ERK1/2 phosphorylation after co-infusion of C21 with an AT2R antagonist would be important to further corroborate that activation of the studied kinases is AT2R-mediated; (c) it would be of value to demonstrate that the actions originated by stimulation with C21 are not present in cells in which the AT2R is either absent or silenced.
In conclusion, current findings provide new information that contributes to the knowledge of AT2R-signaling, by the identification of functional AT2Rs in mouse adipose tissue and heart tissue and the demonstration of Akt and ERK1/2 phosphorylation upon in vivo activation of AT2Rs in these tissues.
4. Materials and Methods
4.1. Experimental Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the School of Pharmacy and Biochemistry of the University of Buenos Aires. Adult (3–4 months old) C57BL/6 male mice were used. Animals were housed 3–5 per cage in a room with controlled light (12 h light: 12 h darkness cycle) and temperature (22 ± 2 °C). Mice had free access to a nutritionally balanced diet and tap water.
4.2. In Vivo Administration of C21and Tissue Collection
Compound 21 was obtained through Vicore Pharma AB (Göteborg, Sweden). The dose of C21 was calculated based on previous studies aimed at exploring vasodilation or insulin enhancement effects derived from in vivo AT2R stimulation [43,44]. With a molecular weight of 475.63 g/mol and the assumption of a blood volume of 1.8 mL in a 20-g mouse [45], the maximal blood concentration of C21 attained immediately after injection would be in the range of 8–10 µM, assuming that no degradation occurred during the timeframe of the experiment. At this concentration, C21 has been shown to evoke vasodilation and to facilitate insulin delivery to tissues [43,44]. The duration of the treatment was selected from previously published studies [23,24].
4.3. Western Blot
Western blotting procedures used in this study have been reported previously [19,20]. Information on all antibodies used is presented in Table S1. Adipose tissue and heart extracts were denatured, resolved by SDS-PAGE, transferred into PVDF membranes (Millipore Immobilon-FL; EMD Millipore, Billerica, MA, USA) and finally probed with specific antibodies: anti-phospho-Tyr 1158/1162/1163 insulin receptor β subunit (Millipore, Burlington, MA, USA), IR β subunit (GeneTex, Irvine, CA, USA), Akt, phospho-Ser 473 Akt, ERK1/2 or phospho-Thr202/Tyr204 ERK1/2 (Cell Signaling, Danvers, MA, USA). Immunoreactive bands were detected by chemiluminescence (Pierce™ ECL Plus Western Blotting Substrate, Thermo Fisher Scientific, Waltham, MA, USA). Protein loading control was performed by relativizing protein content to Coomassie Blue staining of PVDF membranes after blotting experiments as previously described [46]. The level of each protein evaluated was normalized to the area obtained from control samples to avoid sources of variation. Phosphorylation values were then related to calculated protein values for each protein analyzed (IR, Akt and ERK1/2). To assess the error of the control group, each individual control value was divided by average intensity obtained for the control group (saline-injected mice). The units shown in bar graphs were obtained by considering the average value of intensity of each specific band in the control group as 100% ± S.E.M). The molecular weight of proteins was estimated using pre-stained protein markers (Bio-Rad, Hercules, CA, USA).
4.4. Statistical Analysis
Data are presented as mean ± SEM. Comparisons were performed via one-way ANOVA with the post-hoc Tukey method for multiple groups using Prism software 8.0 (GraphPad, San Diego, CA, USA). Differences were considered statistically significant at p < 0.05.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242316839/s1.
Author Contributions
Conceptualization, F.P.D.; methodology, F.P.D., M.G.Z., J.A.N.P., B.B. and M.C.M.; formal analysis, F.P.D.; resources, F.P.D.; data curation, D.T.Q. and J.A.N.P.; writing—original draft preparation, F.P.D.; writing—review and editing, F.P.D., M.G.Z. and A.G.; funding acquisition, F.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Buenos Aires, grant number 20020170100116BA and the AGENCIA NACIONAL DE PROMOCIÓN CIENTÍFICA Y TECNOLÓGICA (ANPCyT), grant number PICT-2019-0051 to F.P.D.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee “CICUAL” of the School of Pharmacy and Biochemistry, University of Buenos Aires (Res. 1368/2018, date of approval 17 April 2018).
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ang | Angiotensin |
| AT1R | Angiotensin II receptor type 1 |
| AT2R | Angiotensin II receptor type 2 |
| ATIP | AT2R-interacting protein |
| C21 | Compound 21 |
| GPCR | G protein-coupled receptor |
| IR | Insulin receptor |
| KO | Knockout |
| NO | Nitric oxide |
| PPAR | Peroxisome proliferator-activated receptor |
| NO | Nitric oxide |
| STZ | Streptozotocin |
| WAT | White adipose tissue |
References
- Folli, F.; Kahn, C.R.; Hansen, H.; Bouchie, J.L.; Feene, E.P. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J. Clin. Investig. 1997, 100, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J. Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R974–R980. [Google Scholar] [CrossRef] [PubMed]
- Shiuchi, T.; Iwai, M.; Li, H.S.; Wu, L.; Min, L.J.; Li, J.M.; Okumura, M.; Cui, T.X.; Horiuchi, M. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 2004, 43, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.A.; Esnault, V.L.; Van Obberghen, E. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E435–E449. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Giani, J.F.; Dominici, F.P.; Turyn, D.; Toblli, J.E. Long-term treatment with an angiotensin II receptor blocker decreases adipocyte size and improves insulin signaling in obese Zucker rats. J. Hypertens. 2009, 27, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Widdop, R.E.; Sturrock, E.D.; Lubbe, L.; Hussain, T.; Kaschina, E.; Unger, T.; Hallberg, A.; Carey, R.M.; Sumners, C. The angiotensin AT2 receptor: From a binding site to a novel therapeutic target. Pharmacol. Rev. 2022, 74, 1051–1135. [Google Scholar] [CrossRef]
- Fatima, N.; Patel, S.N.; Hussain, T. Angiotensin II type 2 receptor: A target for protection against hypertension, metabolic dysfunction, and organ remodeling. Hypertension 2021, 77, 1845–1856. [Google Scholar] [CrossRef]
- Chai, W.; Wang, W.; Dong, Z.; Cao, W.; Liu, Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 2011, 60, 2939–2946. [Google Scholar] [CrossRef]
- Chai, W.; Wang, W.; Liu, J.; Barrett, E.J.; Carey, R.M.; Cao, W.; Liu, Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 2010, 55, 523–530. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Burghi, V.; Miquet, J.G.; Cervino, I.A.; Quiroga, D.T.; Mazziotta, L.; Dominici, F.P. Chronic blockade of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice. Peptides 2017, 88, 37–45. [Google Scholar] [CrossRef]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.-J.; Ogimoto, A.; Dahlöf, B.; Steckelings, U.M.; Unger, T.; et al. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARγ activation. PLoS ONE 2012, 7, e483. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Pinard, S.; Guimond, M.-O.; Labbé, S.M.; Roberge, C.; Baillargeon, J.-P.; Langlois, M.F.; Alterman, M.; Wallinder, C.; Hallberg, A.; et al. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high fat/high-fructose diet-induced insulin resistance in rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E197–E210. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yu, L.; Gao, L. Activation of angiotensin type 2 receptors partially ameliorates streptozotocin-induced diabetes in male rats by islet protection. Endocrinology 2014, 155, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zucker, I.H.; Gao, L. Angiotensin type 2 receptor in pancreatic islets of adult rats: A novel insulinotropic mediator. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1281–E1291. [Google Scholar] [CrossRef]
- Koulis, C.; Chow, B.S.M.; McKelvey, M.; Steckelings, U.M.; Unger, T.; Thallas-Bonke, V.; Thomas, M.C.; Cooper, M.E.; Jandeleit-Dahm, K.A.; Allen, T.J. AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension 2015, 65, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Li, X.Y.; Leung, P.S. Angiotensin II type 2 receptor activation with Compound 21 augments islet function and regeneration in streptozotocin-induced neonatal rats and human pancreatic progenitor cells. Pancreas 2017, 46, 395–404. [Google Scholar] [CrossRef]
- Nag, S.; Khan, M.A.; Samuel, P.; Ali, Q.; Hussain, T. Chronic angiotensin AT2R activation prevents high-fat diet-induced adiposity and obesity in female mice independent of estrogen. Metabolism 2015, 64, 814–825. [Google Scholar] [CrossRef]
- Nag, S.; Patel, S.; Mani, S.; Hussain, T. Role of angiotensin type 2 receptor in improving lipid metabolism and preventing adiposity. Mol. Cell. Biochem. 2019, 461, 195–204. [Google Scholar] [CrossRef]
- Quiroga, D.T.; Muñoz, M.C.; Gil, C.; Pffeifer, M.; Toblli, J.E.; Steckelings, U.M.; Giani, J.F.; Dominici, F.P. Chronic administration of the angiotensin type 2 receptor agonist C21 improves insulin sensitivity in C57BL/6 mice. Physiol. Rep. 2018, 6, e13824. [Google Scholar] [CrossRef]
- Dominici, F.P.; Veiras, L.C.; Shen, J.Z.Y.; Bernstein, E.A.; Quiroga, D.T.; Steckelings, U.M.; Bernstein, K.E.; Giani, J.F. Activation of AT2 receptors prevents diabetic complications in female db/db mice by NO-mediated mechanisms. Br. J. Pharmacol. 2020, 177, 4766–4781. [Google Scholar] [CrossRef]
- Samuel, P.; Khan, M.A.; Nag, S.; Inagami, T.; Hussain, T. Angiotensin AT2 receptor contributes towards gender bias in weight gain. PLoS ONE 2013, 8, e48425. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, D.T.; Miquet, J.G.; Gonzalez, L.; Sotelo, A.I.; Muñoz, M.C.; Geraldes, P.M.; Giani, J.F.; Dominici, F.P. Mice lacking angiotensin type 2 receptor exhibit a sex specific attenuation of insulin sensitivity. Mol. Cell. Endocrinol. 2019, 498, 110587. [Google Scholar] [CrossRef] [PubMed]
- Peluso, A.A.; Bertelsen, J.B.; Andersen, K.; Mortsensen, T.P.; Hansen, P.B.; Sumners, C.; Bader, M.; Santos, R.A.; Steckelings, U.M. Identification of protein phosphatase involvement in the AT2 receptor-induced activation of endothelial nitric oxide synthase. Clin. Sci. 2018, 132, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Peluso, A.A.; Kempf, S.J.; Verano-Braga, T.; Rodrigues-Ribeiro, L.; Johansen, L.E.; Hansen, M.R.; Kitlen, G.; Haugaard, A.H.; Sumners, C.; Ditzel, H.J.; et al. Quantitative phosphoproteomics of the angiotensin AT2-Receptor signaling network identifies HDAC1 (histone-deacetylase-1) and p53 as mediators of antiproliferation and apoptosis. Hypertension 2022, 79, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Hiromi, H.; Katsutoshi, Y.; Masaoki, T.; Hiroshi, O. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension 2005, 45, 967–973. [Google Scholar]
- Than, A.; Xu, S.; Li, R.; Leow, M.K.-S.; Sun, L.; Chen, P. Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Signal Transduct. Target Ther. 2017, 2, 17022. [Google Scholar] [CrossRef] [PubMed]
- Caruso-Neves, C.; Kwon, S.-H.; Guggino, W.B. Albumin endocytosis in proximal tubule cells is modulated by angiotensin II through an AT2 receptor-mediated protein kinase B activation. Proc. Natl. Acad. Sci. USA 2005, 102, 17513–17518. [Google Scholar] [CrossRef]
- Guimarães-Nobre, C.C.; Mendonça-Reis, E.; Passinho-da-Costa, L.; Miranda-Alves, L.; Berto-Junior, H.C. Signaling pathway in the osmotic resistance induced by angiotensin II AT2 receptor activation in human erythrocytes. Rep. Biochem. Mol. Biol. 2021, 10, 314–326. [Google Scholar] [CrossRef]
- Kulkarni, K.; Patel, S.; Ali, R.; Hussain, P. Angiotensin II type 2 receptor activation preserves megalin in the kidney and prevents proteinuria in high salt diet fed rats. Sci. Rep. 2023, 13, 4277. [Google Scholar] [CrossRef]
- Stroth, U.; Blume, A.; Mielke, K.; Unger, T. Angiotensin AT2 receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res. Mol. Brain Res. 2000, 78, 175–180. [Google Scholar] [CrossRef]
- Yoshida, T.; Huq, T.S.; Delafontaine, P. Angiotensin Type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J. Biol. Chem. 2014, 289, 26239–26248. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, G.N.; Lobato, N.S.; Filgueira, F.P.; Akamine, E.H.; Aragão, D.S.; Casarini, D.E.; Carvalho, M.H.C.; Fortes, Z.B. Upregulation of ERK1/2-eNOS via AT2 receptors decreases the contractile response to angiotensin II in resistance mesenteric arteries from obese rats. PLoS ONE 2014, 9, e106029. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.K.; Irvine, J.C.; Shihata, W.A.; Dragoljevic, D.; Lumsden, N.; Huet, O.; Barnes, T.; Unger, T.; Steckelings, U.M.; Jennings, G.L.; et al. Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br. J. Pharmacol. 2016, 173, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Stella, A.; Zerbini, G.; Pelucchi, S.; Zatti, G.; di Gioia, C.R.T. Angiotensin type 2 and Mas receptor activation prevents myocardial fibrosis and hypertrophy through the reduction of inflammatory cell infiltration and local sympathetic activity in angiotensin II-dependent hypertension. Int. J. Mol. Sci. 2021, 22, 13678. [Google Scholar] [CrossRef] [PubMed]
- Fredgart, M.H.; Leurgans, T.M.; Stenelo, M.; Nybo, M.; Bloksgaard, M.; Lindblad, L.; De Mey, J.G.R.; Steckelings, U.M. The angiotensin AT2-receptor agonist compound 21 is an antagonist for the thromboxane TP-receptor—Implications for preclinical studies and future clinical use. Peptides 2023, 164, 170990. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Sumners, C.; Peluso, A.A.; Haugaard, A.H.; Bertelsen, J.B.; Steckelings, U.M. Anti-fibrotic mechanisms of angiotensin AT2-receptor stimulation. Acta Physiol. 2019, 227, e13280. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Shchepinova, M.M.; Hanyaloglu, A.C.; Frost, G.S.; Tate, E.W. Chemical biology of non-canonical G protein-coupled receptor signaling: Toward advanced therapeutics. Curr. Opin. Chem. Biol. 2020, 56, 98–110. [Google Scholar] [CrossRef]
- Turu, G.; Szidonya, L.; Gáborik, Z.; Buday, L.; Spät, A.; Clark, A.J.L.; Hunyady, L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 2006, 580, 41–45. [Google Scholar] [CrossRef]
- Colin, M.; Delaitre, D.; Foulquier, S.; Dupuis, F. The AT1/AT2 receptor equilibrium is a cornerstone of the regulation of the renin angiotensin system beyond the cardiovascular system. Molecules 2023, 28, 5481. [Google Scholar] [CrossRef] [PubMed]
- Goutaudier, R.; Coizet, V.; Carcenac, C.; Carnicella, S. Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. PLoS ONE 2020, 15, e0238156. [Google Scholar] [CrossRef] [PubMed]
- Bosnyak, S.; Welungoda, I.K.; Hallberg, A.; Alterman, M.; Widdop, R.E.; Jones, E.S. Stimulation of angiotensin AT2 receptors by the nonpeptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br. J. Pharmacol. 2010, 159, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yuan, Z.; Wang, N.; Carey, R.M.; Aylor, K.W.; Chen, L.; Zhou, X.; Liu, Z. Direct activation of angiotensin II type 2 receptors enhances muscle microvascular perfusion, oxygenation, and insulin delivery in male rats. Endocrinology 2018, 159, 685–695. [Google Scholar] [CrossRef]
- Riches, A.C.; Sharp, J.G.; Brynmor Thomas, D.; Vaughan Smith, S. Blood volume determination in the mouse. J. Physiol. 1973, 228, 279–284. [Google Scholar] [CrossRef]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).