Increased RBP4 and Asprosin Are Novel Contributors in Inflammation Process of Periodontitis in Obese Rats
Abstract
:1. Introduction
2. Results
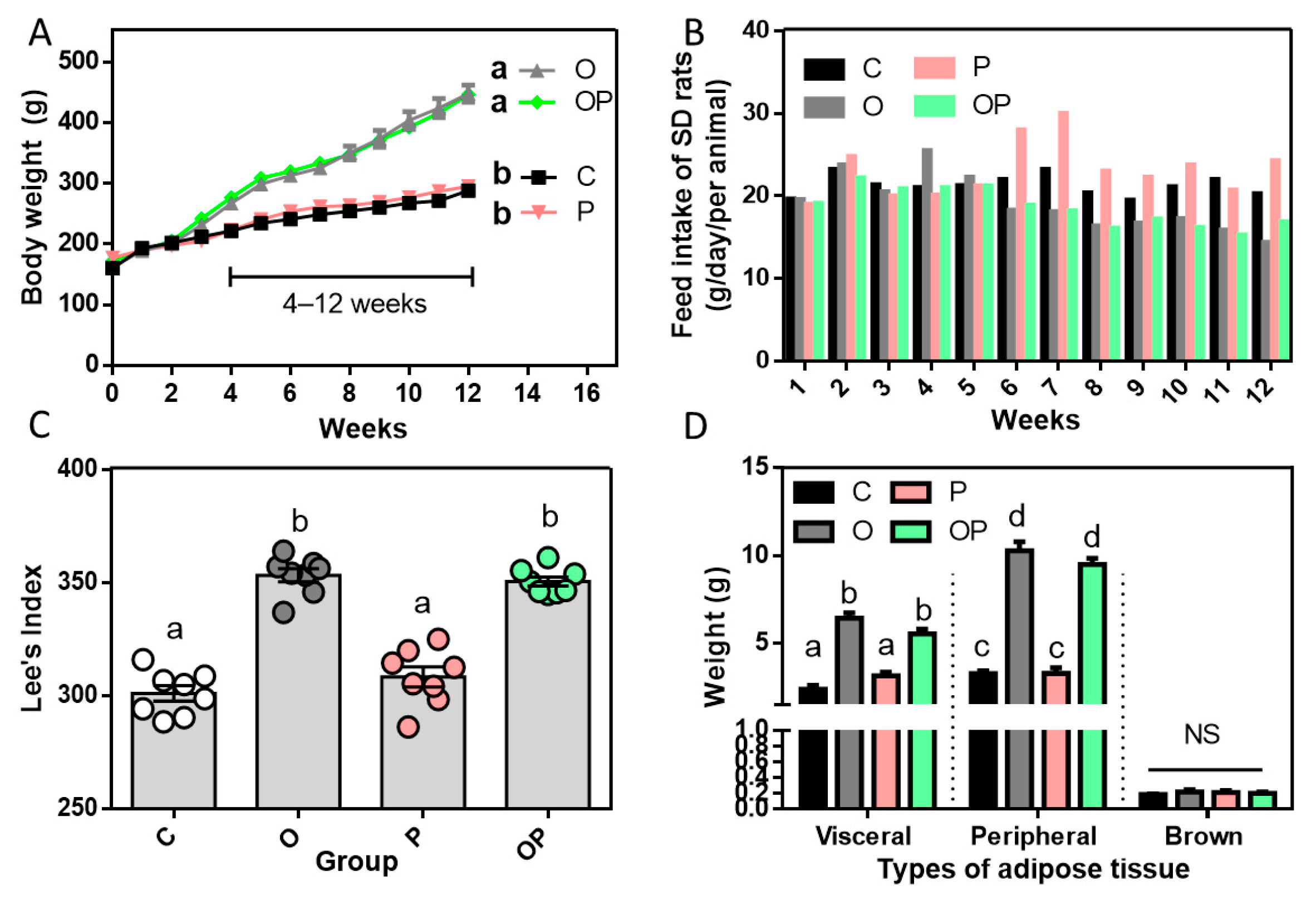
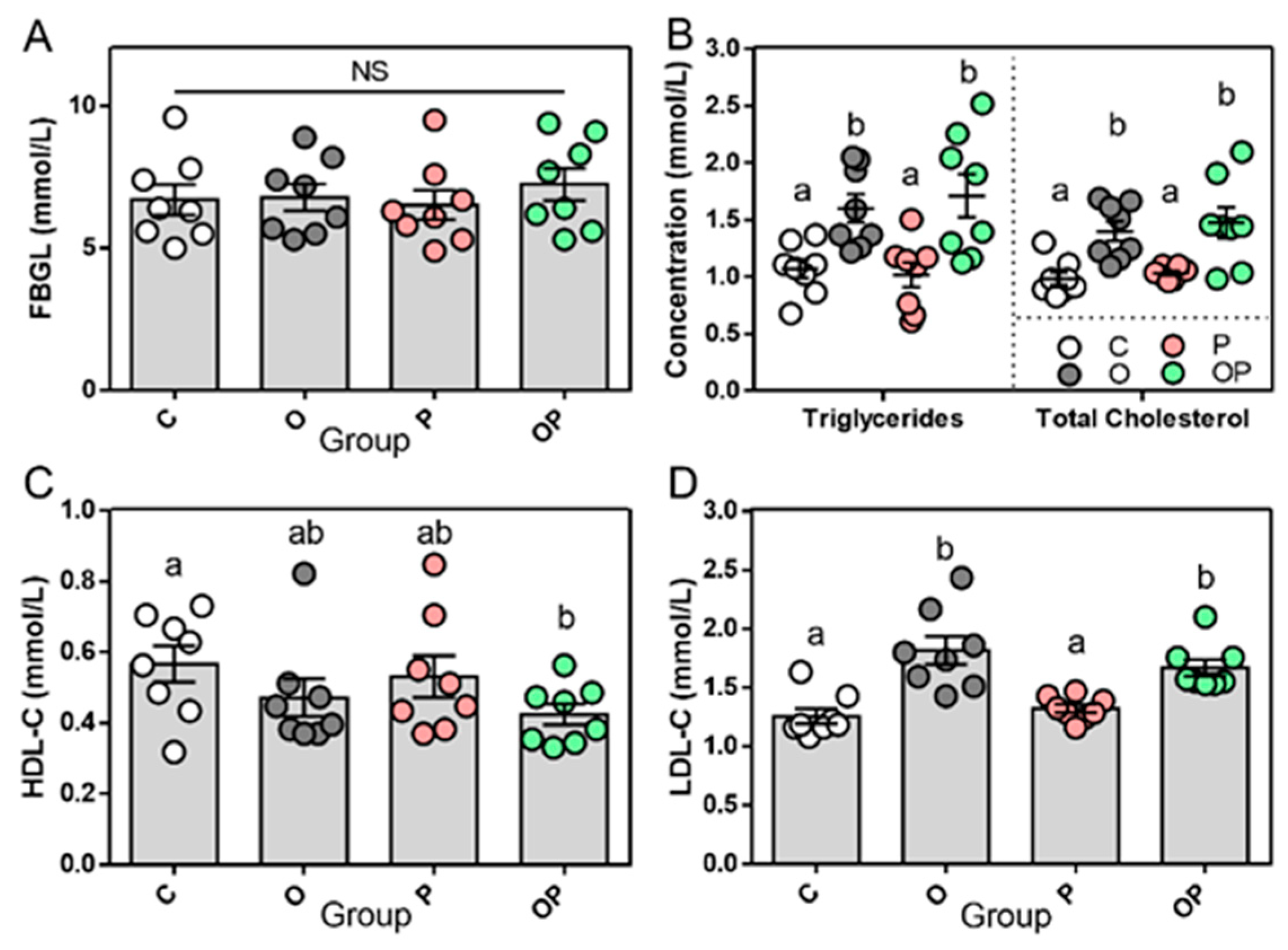
2.1. Establishment of Experimental Obesity Rat Model and Related Biochemical Data
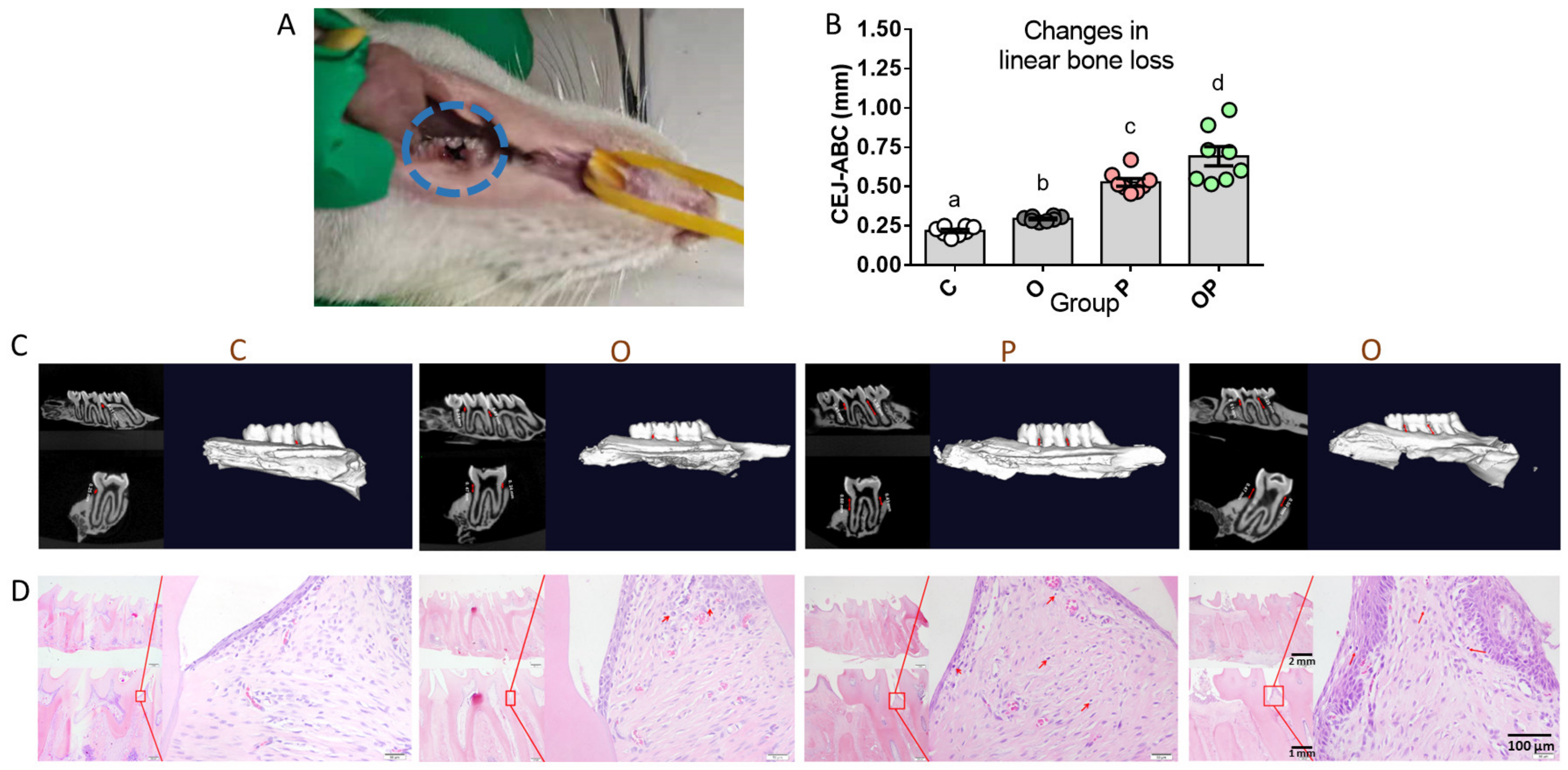
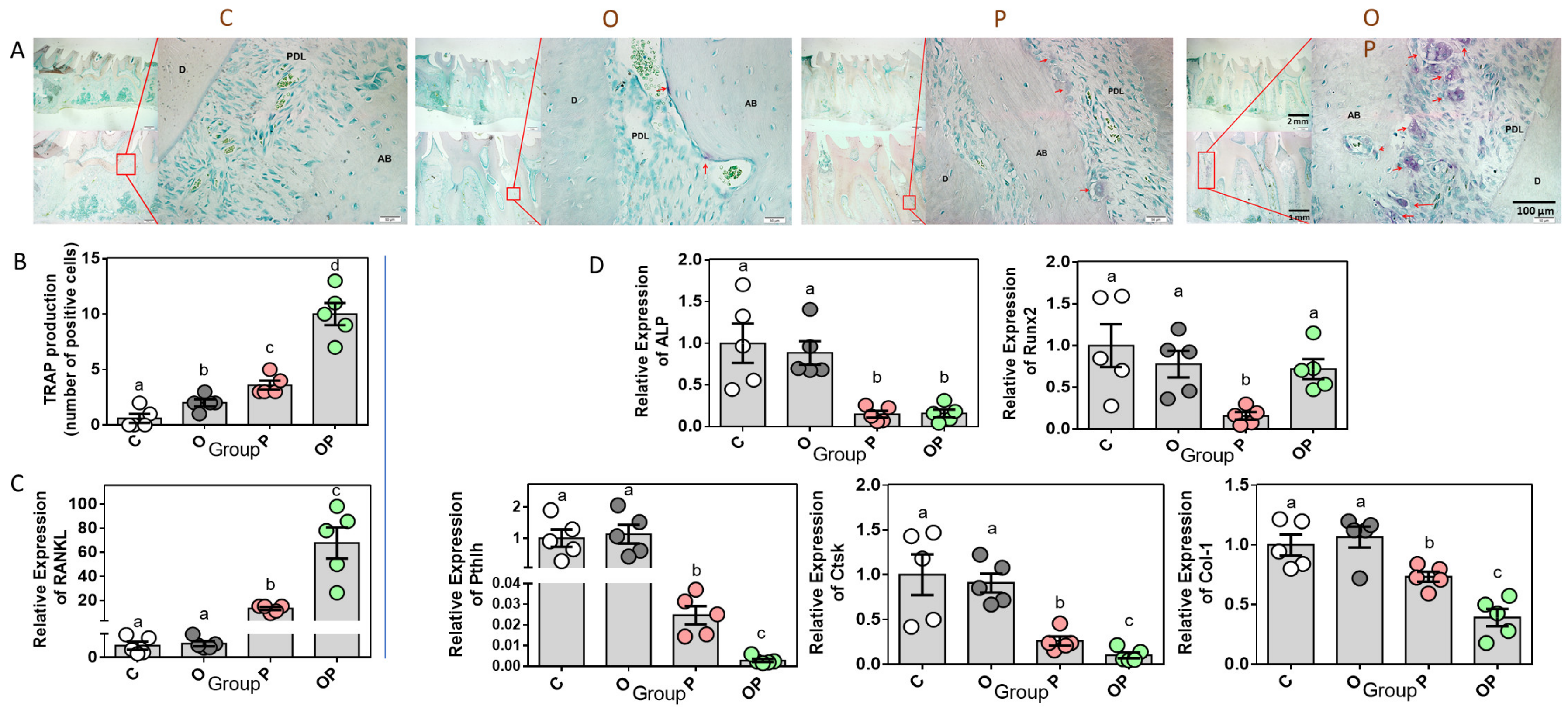
2.2. Establishment of Experimental Periodontitis Rat Model
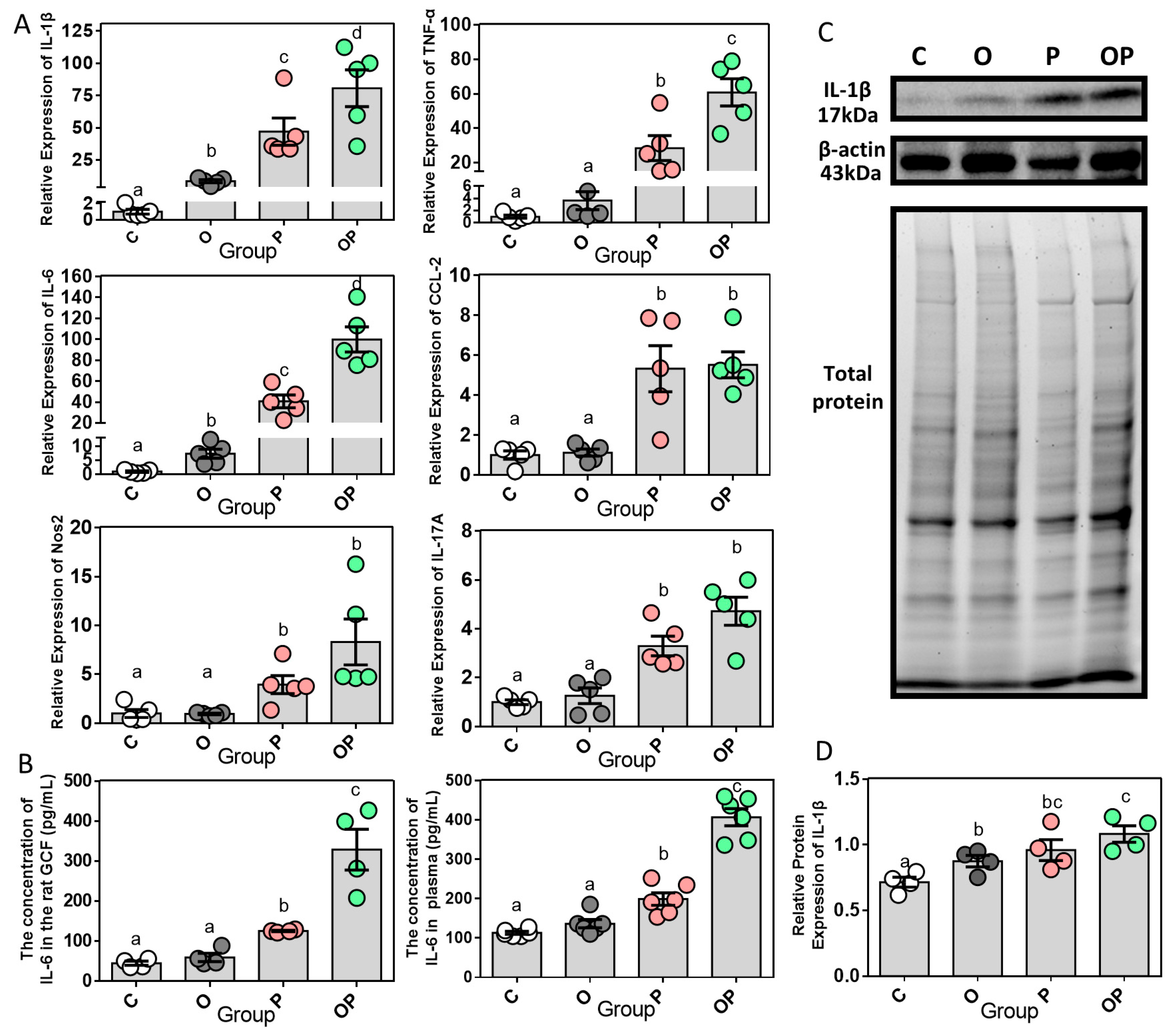
2.3. Expression of Periodontal and Systemic Inflammatory Cytokines
2.4. Expression of Bone Remodeling Biomarkers in Periodontium
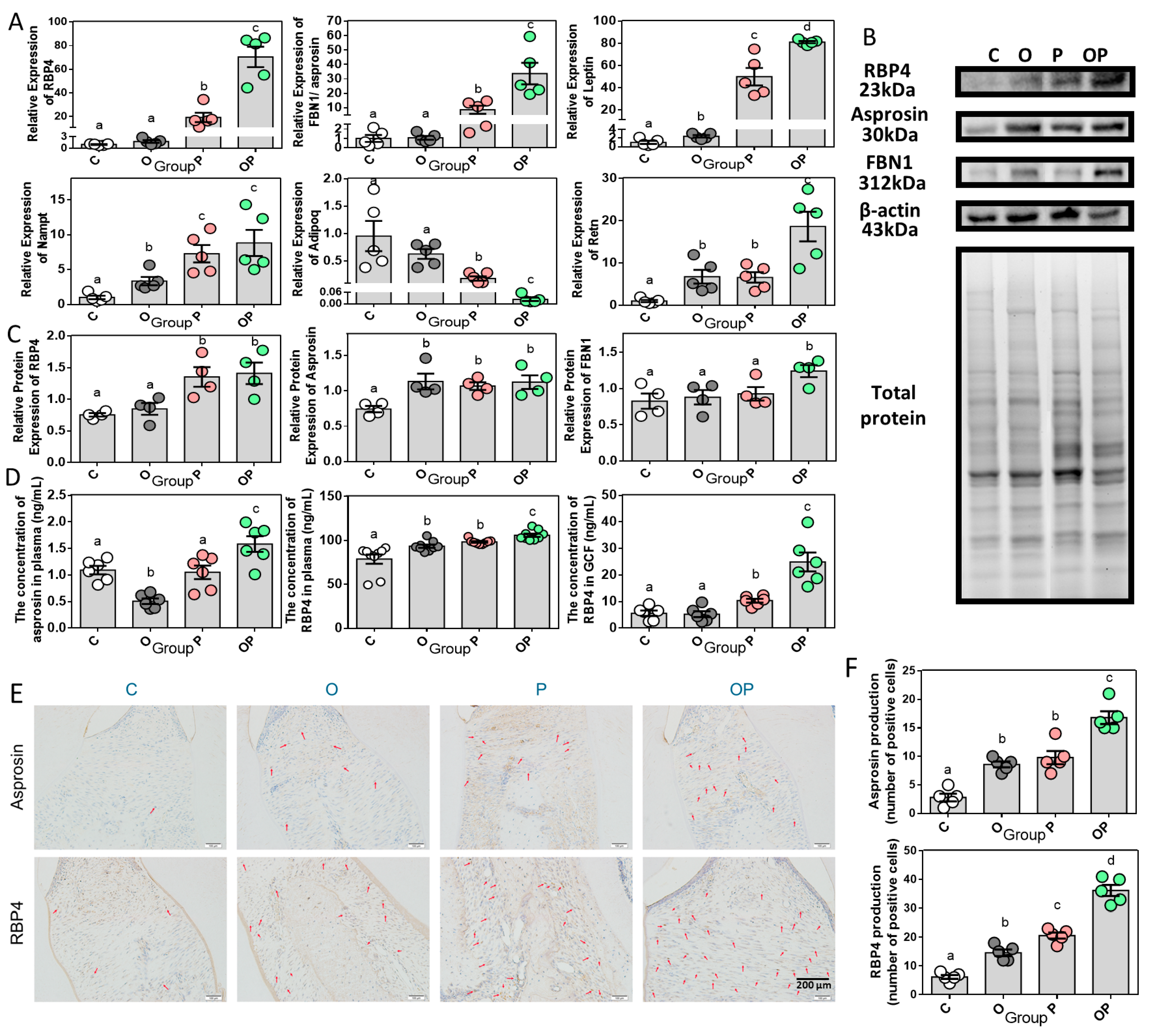
2.5. Adipokine Expression Pattern in Periodontitis and Obesity
2.6. Correlations of RBP4/Asprosin with Obesity and Periodontitis
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Metabolic Studies
4.3. Micro-Computed Tomography
4.4. Histological Evaluation
4.5. Immunohistochemistry
4.6. Quantitative Real-Time PCR Analysis
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xu, A.; Leung, W.K. Obesity, Bone Loss, and Periodontitis: The Interlink. Biomolecules 2022, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.E.; Zimmermann, G.S.; Figueiredo, L.C.; Souza Mde, C.; da Cruz, D.F.; Bastos, M.F.; da Silva, H.D.; Duarte, P.M. Local and serum levels of adipokines in patients with obesity after periodontal therapy: One-year follow-up. J. Clin. Periodontol. 2015, 42, 409–431. [Google Scholar] [CrossRef]
- Al-Hamoudi, N.; Abduljabbar, T.; Mirza, S.; Al-Sowygh, Z.H.; Vohra, F.; Javed, F.; Akram, Z. Non-surgical periodontal therapy reduces salivary adipocytokines in chronic periodontitis patients with and without obesity. J. Investig. Clin. Dent. 2018, 9, e12314. [Google Scholar] [CrossRef]
- Groener, J.B.; Valkanou, A.; Kender, Z.; Pfeiffenberger, J.; Kihm, L.; Fleming, T.; Nawroth, P.P.; Kopf, S. Asprosin response in hypoglycemia is not related to hypoglycemia unawareness but rather to insulin resistance in type 1 diabetes. PLoS ONE 2019, 14, e0222771. [Google Scholar] [CrossRef]
- Kanoriya, D.; Pradeep, A.R.; Mallika, A.; Singhal, S.; Garg, V. Correlation of crevicular fluid and serum levels of retinol-binding protein 4 and leptin in chronic periodontitis and obesity. Clin. Oral Investig. 2016, 21, 2319–2325. [Google Scholar] [CrossRef]
- Jiri Mutu, Z.; Shilong, L.; Peng, N.; Yong’an, L.; Qinying, A.I.; Xiaojun, Y.; Hongning, L.; Yanhua, J.I.; Zhijun, Z. Efficacy of Kushen decoction on high-fat-diet-induced hyperlipidemia in rats. J. Tradit. Chin. Med. 2022, 42, 364–371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus white adipose tissue: A mini-review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S237–S248. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A. Periodontal disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; Del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Maher, J.J. Macronutrients and the Adipose-Liver Axis in Obesity and Fatty Liver. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 749–761. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Zhou, N.; Fu, Y.; Cheng, X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin. Chim. Acta 2019, 489, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, H.; Xiong, X.; Qiu, Y.; Liao, Y.; Chen, Y.; Zheng, Y.; Zheng, H. Plasma Asprosin Concentrations Are Increased in Individuals with Glucose Dysregulation and Correlated with Insulin Resistance and First-Phase Insulin Secretion. Mediators Inflamm. 2018, 2018, 9471583. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Deschner, J.; Eick, S.; Damanaki, A.; Nokhbehsaim, M. The role of adipokines in periodontal infection and healing. Mol. Oral Microbiol. 2014, 29, 258–269. [Google Scholar] [CrossRef]
- de Molon, R.S.; Park, C.H.; Jin, Q.; Sugai, J.; Cirelli, J.A. Characterization of ligature-induced experimental periodontitis. Microsc. Res. Tech. 2018, 81, 1412–1421. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128.e124. [Google Scholar] [CrossRef]
- Wang, M.; Xu, C.; Wu, X.; He, X.; Guo, Y.; Zhang, W.; Sun, Y. The expression of Runx2 in the pathogenesis of periodontitis. Oral Dis. 2023, in press. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, J.; Staels, B. Leptin. Lancet 1998, 351, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Handzlik-Orlik, G.; Okopien, B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 2012, 51, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, Y.; Gao, P.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Leptin Aggravates Periodontitis by Promoting M1 Polarization via NLRP3. J. Dent. Res. 2022, 101, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Bayani, M.; Tasorian, B.; Mahdian, S. The comparison of visfatin levels of gingival crevicular fluid in systemic lupus erythematosus and chronic periodontitis patients with healthy subjects. Clin. Rheumatol. 2019, 38, 3139–3143. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Jiang, C.; Zhang, H.; Gao, X.; Guo, Y.; Cao, Z. Visfatin regulates Pg LPS-induced proinflammatory/prodegradative effects in healthy and inflammatory periodontal cells partially via NF-κB pathway. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119042. [Google Scholar] [CrossRef]
- Tabari, Z.A.; Keshani, F.; Sharbatdaran, M.; Banishahabadi, A.; Nejatifard, M.; Ghorbani, H. Visfatin expression in gingival tissues of chronic periodontitis and aggressive periodontitis patients: An immunohistochemical analysis. Dent. Res. J. 2018, 15, 104–110. [Google Scholar]
- Jamaluddin, M.S.; Weakley, S.M.; Yao, Q.; Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012, 165, 622–632. [Google Scholar] [CrossRef]
- Suresh, S.; Mahendra, J.; Saketharaman, P.; Sivsankar, P.; Selvakumar, J.; Elangovan, R. Evaluation of Reactive Oxygen Metabolites, Resistin, and Red Complex Bacteria in Obese Subjects with or without Periodontitis. J. Contemp. Dent. Pract. 2022, 23, 703–708. [Google Scholar]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Kyriazi, E.; Tsiotra, P.C.; Boutati, E.; Ikonomidis, I.; Fountoulaki, K.; Maratou, E.; Lekakis, J.; Dimitriadis, G.; Kremastinos, D.T.; Raptis, S.A. Effects of adiponectin in TNF-α, IL-6, and IL-10 cytokine production from coronary artery disease macrophages. Horm. Metab. Res. 2011, 43, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, M.; Abad-Jiménez, Z.; Silvestre, F.J.; López-Domènech, S.; Márquez-Arrico, C.F.; Silvestre-Rangil, J.; Víctor, V.M.; Rocha, M. Effect of Non-Surgical Periodontal Treatment on Oxidative Stress Markers in Leukocytes and Their Interaction with the Endothelium in Obese Subjects with Periodontitis: A Pilot Study. J. Clin. Med. 2020, 9, 2117. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, M.; Silvestre, F.J.; Silvestre-Rangil, J.; López-Domènech, S.; Bañuls, C.; Rocha, M. Levels of serum retinol-binding protein 4 before and after non-surgical periodontal treatment in lean and obese subjects: An interventional study. J. Clin. Periodontol. 2018, 45, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; González-Calixto, C.; Flores-Alfaro, E.; Espinoza-Rojo, M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 2022, 26, 244. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Liu, H.; Mishra, I.; Duerrschmid, C.; Gao, P.; Xu, P.; Wang, C.; He, Y. Asprosin promotes feeding through SK channel-dependent activation of AgRP neurons. Sci. Adv. 2023, 9, eabq6718. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Aversa, T.; Currò, M.; Tropeano, A.; Pepe, G.; Alibrandi, A.; Ientile, R.; Wasniewska, M. Asprosin serum levels and glucose homeostasis in children with obesity. Cytokine 2021, 142, 155477. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Xie, X.; Du, C.; Zhao, Y.; Zhang, C.; Zhan, D.; Li, Z.; Ning, Q.; Luo, X. Decreased Circulating Levels of Asprosin in Obese Children. Horm. Res. Paediatr. 2019, 91, 271–277. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; Kim, H.U.; Park, T.; Park, J.; Kim, U.; Kim, M.K.; Jeong, J.H. Asprosin attenuates insulin signaling pathway through PKCδ-activated ER stress and inflammation in skeletal muscle. J. Cell. Physiol. 2019, 234, 20888–20899. [Google Scholar] [CrossRef]
- Svensson, A.M.; Hellerström, C.; Jansson, L. Diet-induced obesity and pancreatic islet blood flow in the rat: A preferential increase in islet blood perfusion persists after withdrawal of the diet and normalization of body weight. J. Endocrinol. 1996, 151, 507–511. [Google Scholar] [CrossRef]

| RBP4 a | Asprosin a | |||
|---|---|---|---|---|
| r | p | r | p | |
| CEJ-ABC | 0.9270 | <0.0001 | 0.7783 | <0.0001 |
| IL-1β a | 0.9610 | <0.0001 | 0.8322 | <0.0001 |
| TNF-α a | 0.9278 | <0.0001 | 0.9455 | <0.0001 |
| RANKL a | 0.9478 | <0.0001 | 0.9026 | <0.0001 |
| Col-1 a | −0.7921 | <0.0001 | −0.4855 | 0.0188 |
| Asprosin b | 0.4555 | 0.0289 | 0.7144 | 0.0001 |
| RBP4 b | 0.8783 | <0.0001 | 0.8417 | <0.0001 |
| IL-6 b | 0.9206 | <0.0001 | 0.7775 | <0.0001 |
| RBP4 c | 0.8550 | <0.0001 | 0.8052 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y.; Tan, Y.; Luo, X.; Jia, R. Increased RBP4 and Asprosin Are Novel Contributors in Inflammation Process of Periodontitis in Obese Rats. Int. J. Mol. Sci. 2023, 24, 16739. https://doi.org/10.3390/ijms242316739
Zhang Y, Zhang Y, Tan Y, Luo X, Jia R. Increased RBP4 and Asprosin Are Novel Contributors in Inflammation Process of Periodontitis in Obese Rats. International Journal of Molecular Sciences. 2023; 24(23):16739. https://doi.org/10.3390/ijms242316739
Chicago/Turabian StyleZhang, Yuwei, Yifei Zhang, Yutian Tan, Xiao Luo, and Ru Jia. 2023. "Increased RBP4 and Asprosin Are Novel Contributors in Inflammation Process of Periodontitis in Obese Rats" International Journal of Molecular Sciences 24, no. 23: 16739. https://doi.org/10.3390/ijms242316739
APA StyleZhang, Y., Zhang, Y., Tan, Y., Luo, X., & Jia, R. (2023). Increased RBP4 and Asprosin Are Novel Contributors in Inflammation Process of Periodontitis in Obese Rats. International Journal of Molecular Sciences, 24(23), 16739. https://doi.org/10.3390/ijms242316739








