Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells
Abstract
1. Introduction
2. Results
2.1. Subjects Characteristics and Protein–HNE Levels in Adipose Tissue
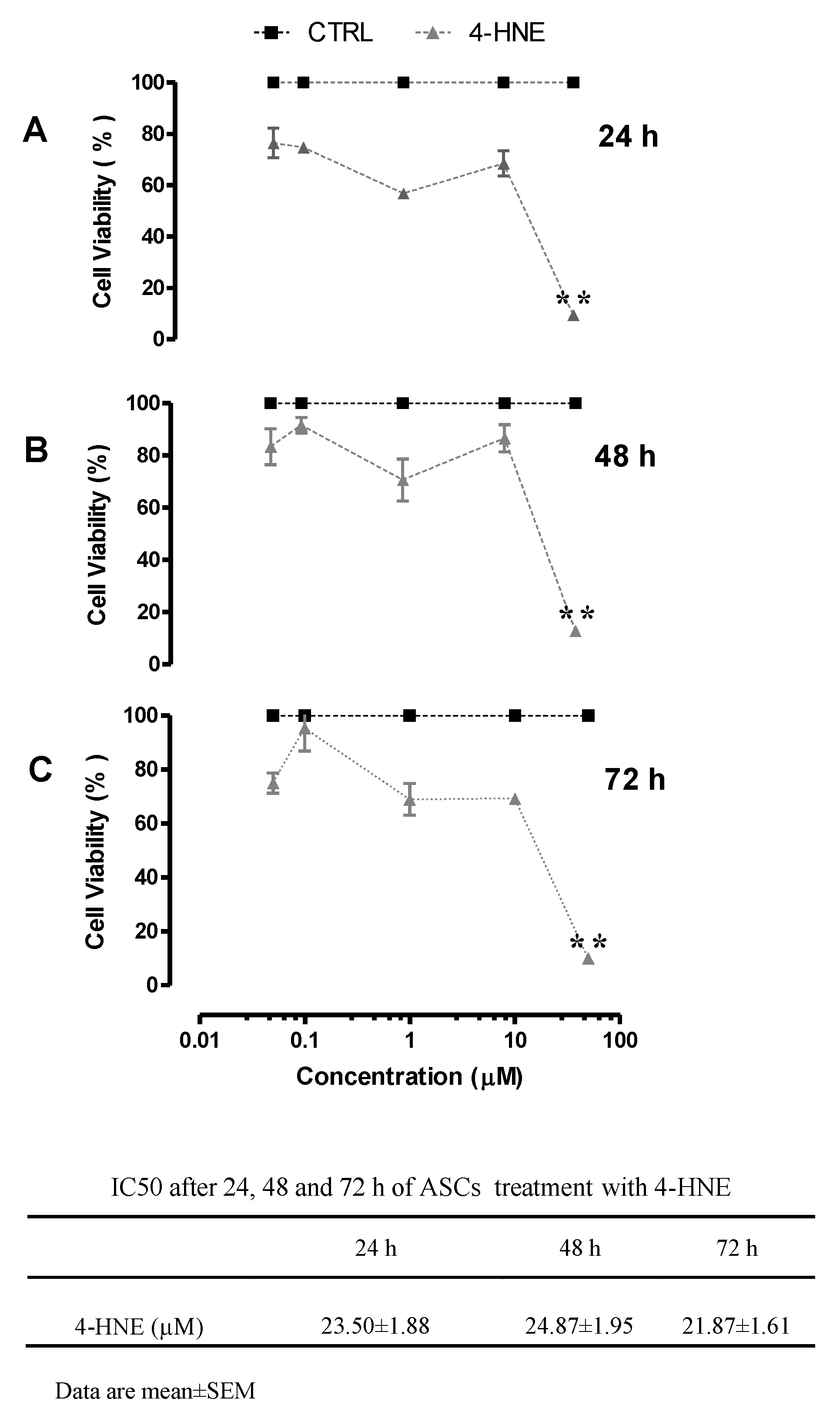
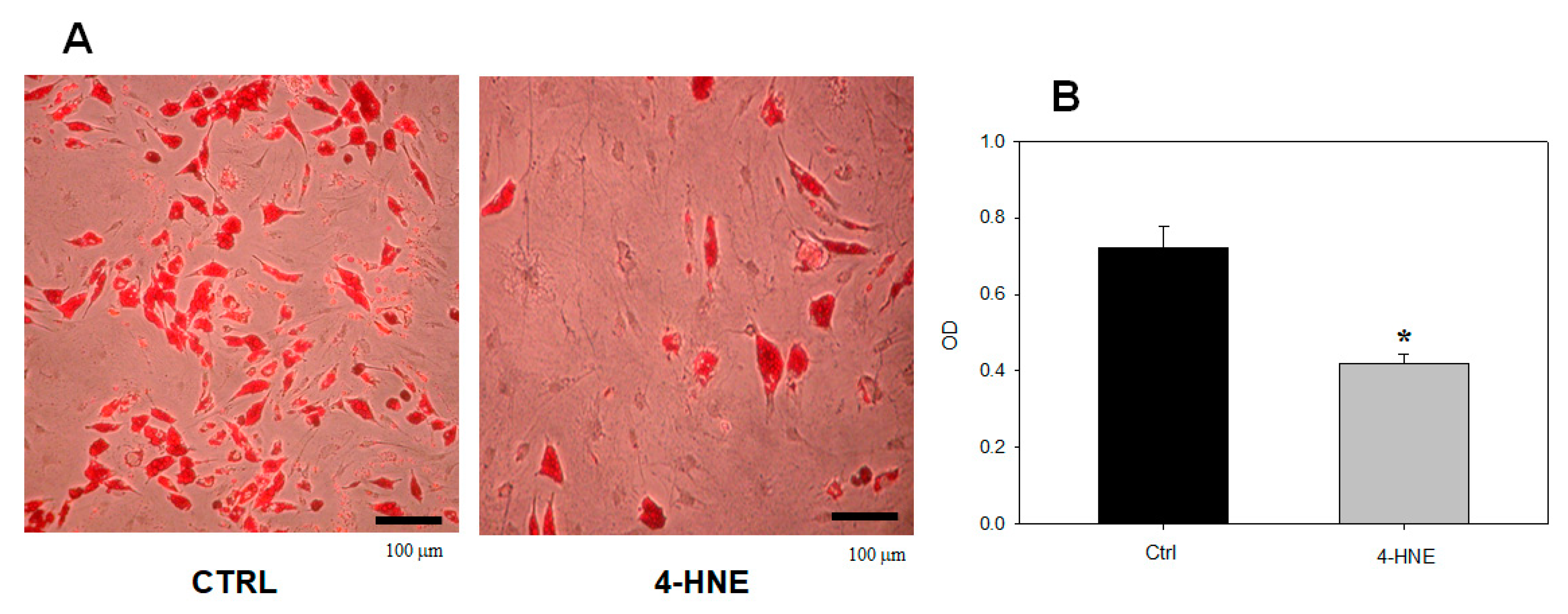
2.1.1. Effects of 4-HNE on ASC Viability and Differentiation
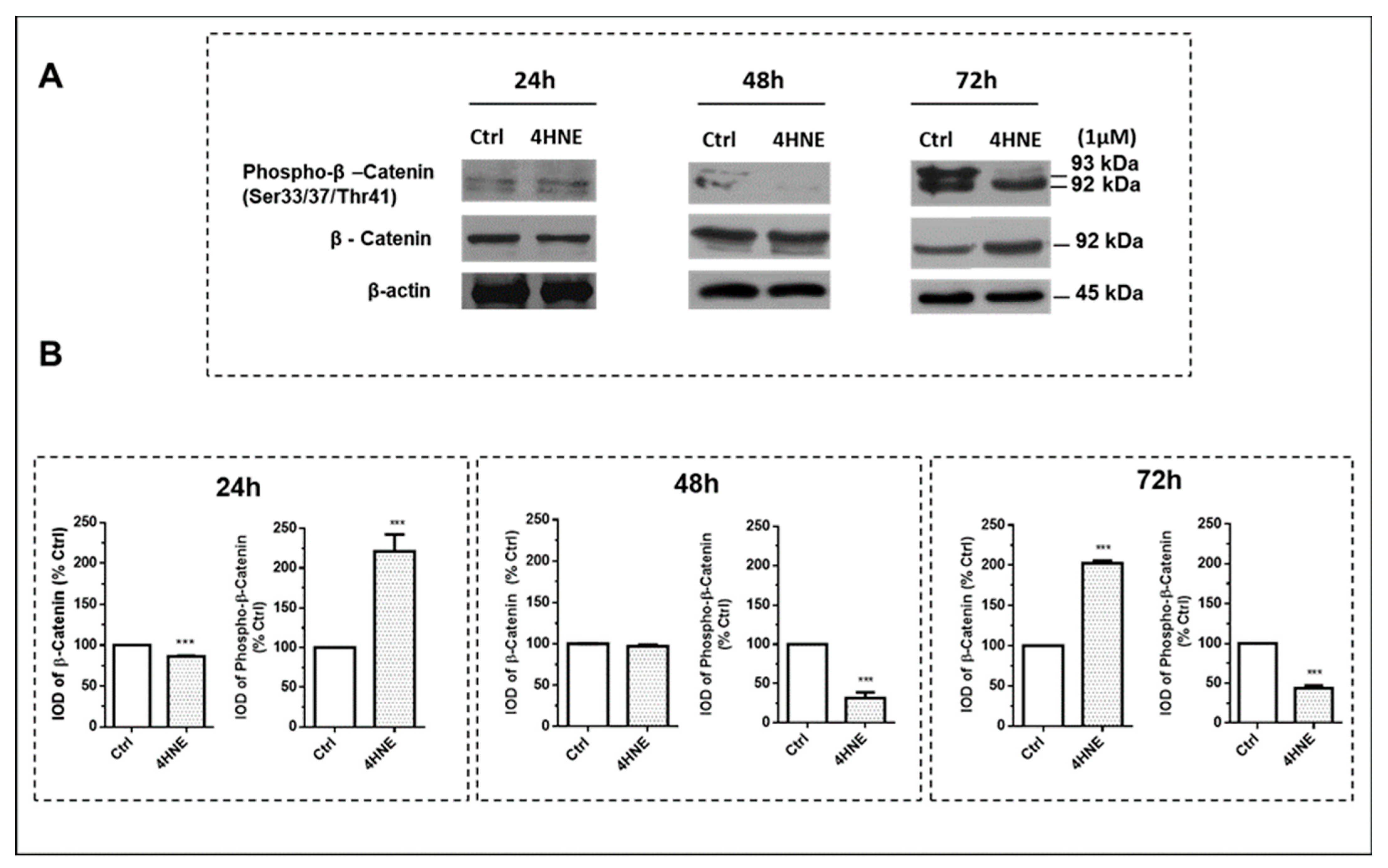
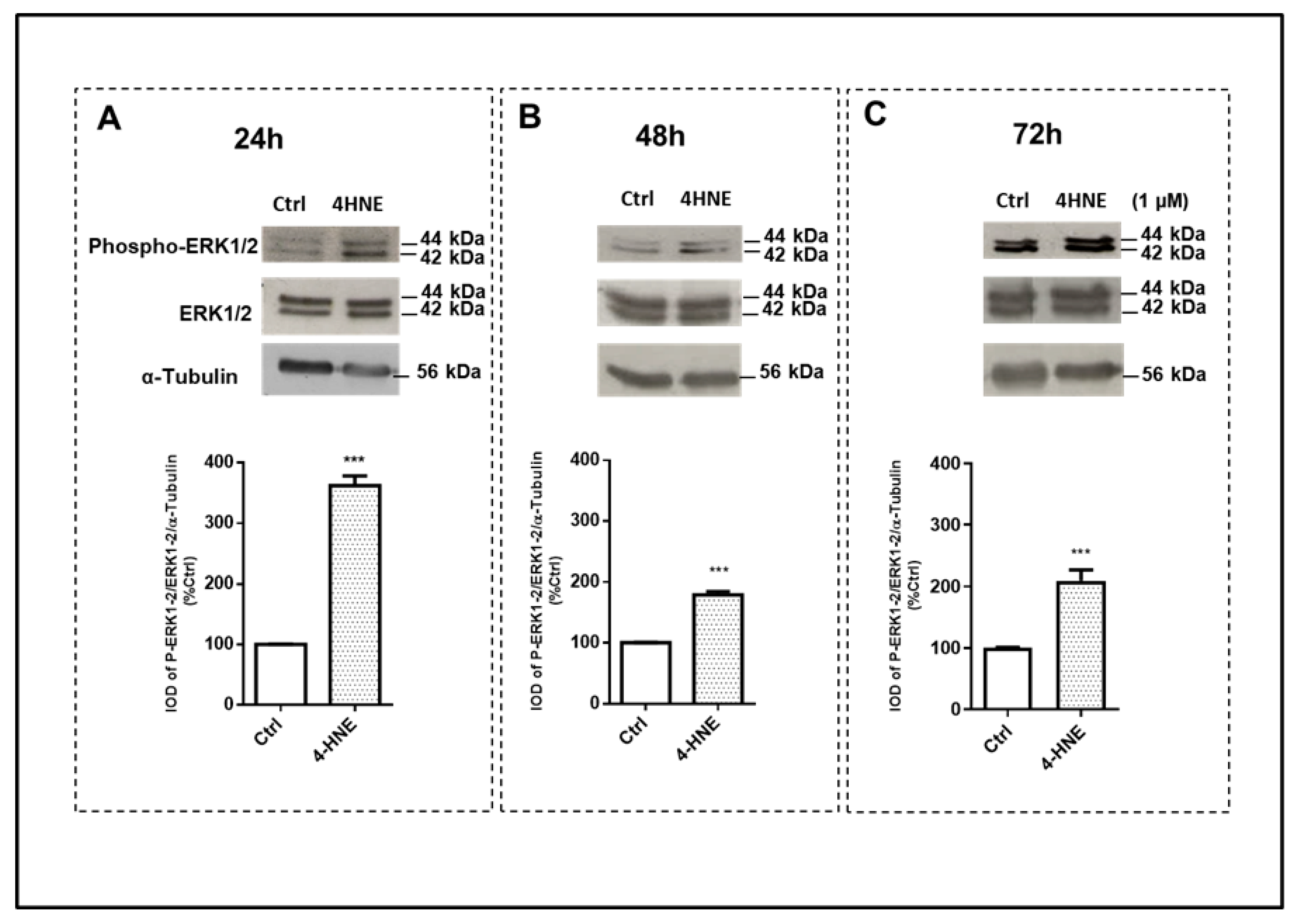
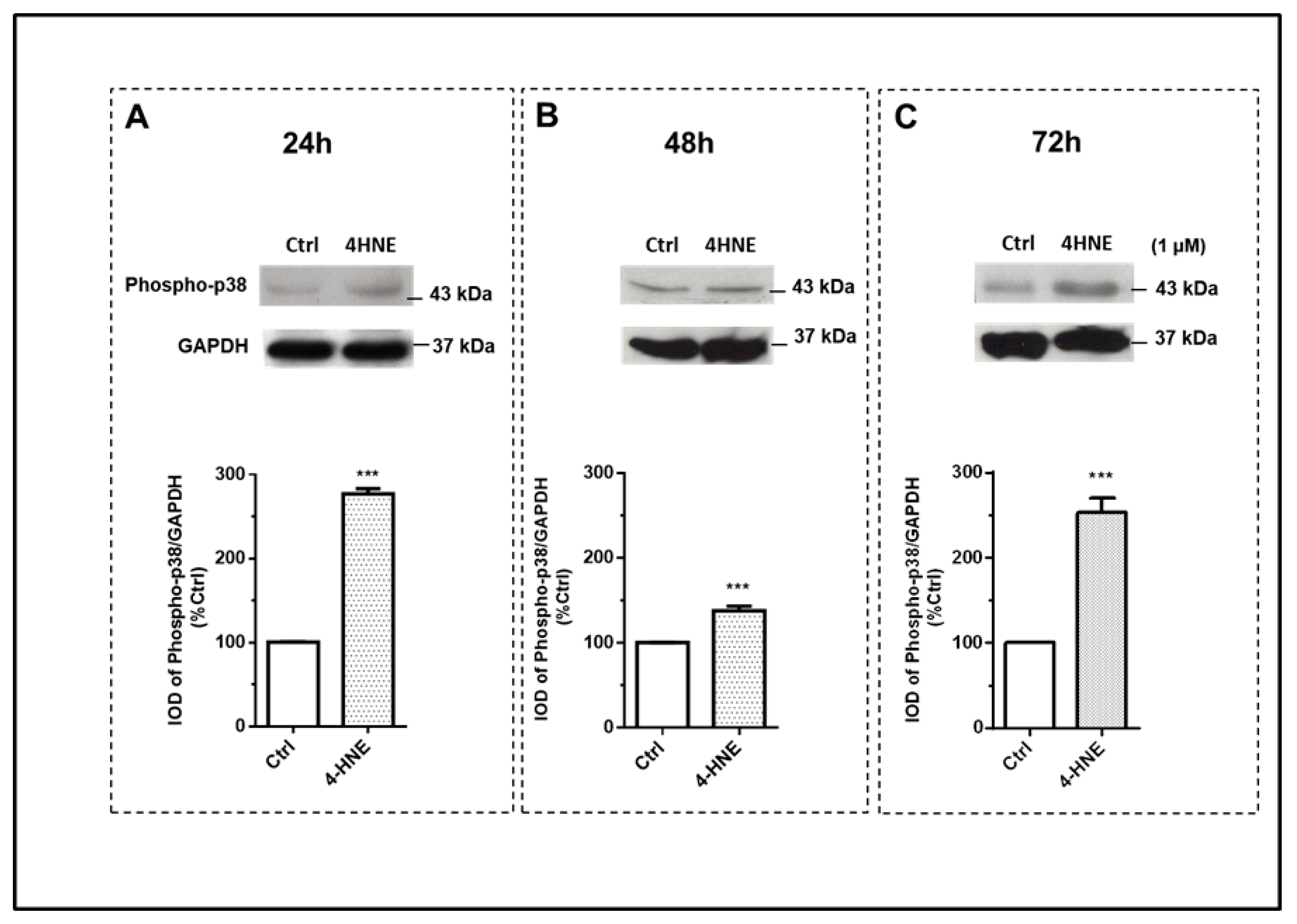
2.1.2. ROS Production and the Wnt/β-Catenin and MAPK Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Subjects
4.1.1. Body Composition and SCAAT Needle Biopsy
4.1.2. ASC Isolation, Cell Viability, and Adipogenic Differentiation
4.1.3. Western Blots
4.1.4. Intracellular ROS Production and Cell Viability
4.1.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb164970. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. Restricted Adipogenesis in Hypertrophic Obesity: The Role of WISP2, WNT, and BMP4. Diabetes 2013, 62, 2997–3004. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef]
- Sethi, J.K. Activatin’ human adipose progenitors in obesity. Diabetes 2010, 59, 2354–2357. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. Activation of canonical Wingless-type MMTV integration site family (WNT) signaling in mature adipocytes increases β-catenin levels and leads to cell dedifferentiation and insulin resistance. J. Biol. Chem. 2010, 285, 14031–14041. [Google Scholar] [CrossRef]
- Gustafson, B.; Eliasson, B.; Smith, U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: A link with osteogenesis. Diabetologia 2010, 53, 536–540. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yamamoto, H.; Sato, A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009, 19, 119–129. [Google Scholar] [CrossRef]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R., 3rd; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A. Wnt signalling and the control of cellular metabolism. Biochem. J. 2010, 427, 1–17. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. The Wnt inhibitor Dicckopf 1 and Bone Morphogenetic Protein 4 rescue adipogenesis in hypertrophic obesity in human. Diabetes 2012, 61, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Isakson, P.; Gustafson, B.; Smith, U. Wnt-signaling is maintained and adipogenesis inhibited by TNFalpha but not MCP-1 and resistin. Biochem. Biophys. Res. Commun. 2007, 357, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lagathu, C.; Christodoulides, C.; Virtue, S.; Cawthorn, W.P.; Franzin, C.; Kimber, W.A.; Nora, E.D.; Campbell, M.; Medina-Gomez, G.; Cheyette, B.N.; et al. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes 2009, 58, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Grunberg, J.R.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. The Novel Secreted Adipokine WNT1-inducible Signaling Pathway Protein 2 (WISP2) Is a Mesenchymal Cell Activator of Canonical WNT. J. Biol. Chem. 2014, 289, 6899–6907. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Acosta, V.M.; Bauch, E.; Ledbetter, M.P.; Waxman, A.; Bouchard, L.S.; Budker, D. Temperature dependence of the nitrogen-vacancy magnetic resonance in diamond. Phys. Rev. Lett. 2010, 104, 070801. [Google Scholar] [CrossRef]
- Murdolo, G.; Piroddi, M.; Tortoioli, C.; Bartolini, D.; Schmelz, M.; Luchetti, F.; Canonico, B.; Papa, S.; Zerbinati, C.; Iuliano, L.; et al. Free Radical-derived Oxysterols: Novel Adipokines Modulating Adipogenic Differentiation of Adipose Precursor Cells. J. Clin. Endocrinol. Metab. 2016, 101, 4974–4983. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Gummersbach, C.; Hemmrich, K.; Kroncke, K.D.; Suschek, C.V.; Fehsel, K.; Pallua, N. New aspects of adipogenesis: Radicals and oxidative stress. Differentiation 2009, 77, 115–120. [Google Scholar] [CrossRef]
- Schroder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.M.; Pearson, K.J.; Gizard, F.; Zhao, Y.; Qing, H.; Jones, K.L.; Cohn, D.; Heywood, E.B.; de Cabo, R.; Bruemmer, D. Oxidative stress accumulates in adipose tissue during aging and inhibits adipogenesis. PLoS ONE 2011, 6, e18532. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Sasson, S. Lipid peroxidation of poly-unsaturated fatty acids in normal and obese adipose tissues. Arch. Physiol. Biochem. 2011, 117, 131–139. [Google Scholar] [CrossRef]
- Mattson, M.P. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol. 2009, 44, 625–633. [Google Scholar] [CrossRef]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulere, L.; Lagarde, M.; Soulage, C.O. The Lipid Peroxidation By-Product 4-Hydroxy-2-Nonenal (4-HNE) Induces Insulin Resistance in Skeletal Muscle through Both Carbonyl and Oxidative Stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Ingram, K.H.; Hill, H.; Moellering, D.R.; Hill, B.G.; Lara-Castro, C.; Newcomer, B.; Brandon, L.J.; Ingalls, C.P.; Penumetcha, M.; Rupp, J.C.; et al. Skeletal Muscle Lipid Peroxidation and Insulin Resistance in Humans. J. Clin. Endocrinol. Metab. 2012, 97, E1182–E1186. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, J.; Gu, D.; Li, S.; Shen, C.; Song, Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS ONE 2013, 8, e70663. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, K.K.; Lee, K.; Gao, G.; Lyons, T.J.; Kowluru, R.; Ma, J.X. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia 2011, 54, 459–468. [Google Scholar] [CrossRef]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef]
- Hill, B.G.; Haberzettl, P.; Ahmed, Y.; Srivastava, S.; Bhatnagar, A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 2008, 410, 525–534. [Google Scholar] [CrossRef]
- Murdolo, G.; Smith, U. The dysregulated adipose tissue: A connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2006, 16 (Suppl. S1), S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Almuraikhy, S.; Al-Khelaifi, F.; Al-Jaber, M.; Bashah, M.; Mazloum, N.A.; Zarkovic, K.; Zarkovic, N.; Waeg, G.; Kafienah, W.; et al. Combined metformin and insulin treatment reverses metabolically impaired omental adipogenesis and accumulation of 4-hydroxynonenal in obese diabetic patients. Redox Biol. 2017, 12, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Korac, A.; Srdic-Galic, B.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Vucetic, M.; Markelic, M.; Velickovic, K.; Golic, I.; et al. Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism 2014, 63, 661–671. [Google Scholar] [CrossRef]
- Dasuri, K.; Ebenezer, P.; Fernandez-Kim, S.O.; Zhang, L.; Gao, Z.; Bruce-Keller, A.J.; Freeman, L.R.; Keller, J.N. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: Implications for obesity and metabolic syndrome. Free Radic. Res. 2013, 47, 8–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E879–E886. [Google Scholar] [CrossRef]
- Awasthi, Y.C.; Yang, Y.; Tiwari, N.K.; Patrick, B.; Sharma, A.; Li, J.; Awasthi, S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic. Biol. Med. 2004, 37, 607–619. [Google Scholar] [CrossRef]
- De Fano, M.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Malara, M.; Galli, F.; Murdolo, G. Adipose Tissue Plasticity in Response to Pathophysiological Cues: A Connecting Link between Obesity and Its Associated Comorbidities. Int. J. Mol. Sci. 2022, 23, 5511. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binetruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Ueda, A.; Nishibata, Y.; Okura, H.; Ichinose, A.; Matsuyama, A.; Hayakawa, T. Human adipose tissue-derived multilineage progenitor cells exposed to oxidative stress induce neurite outgrowth in PC12 cells through p38 MAPK signaling. BMC Cell Biol. 2012, 13, 21. [Google Scholar] [CrossRef]
- Acosta, J.R.; Douagi, I.; Andersson, D.P.; Backdahl, J.; Ryden, M.; Arner, P.; Laurencikiene, J. Increased fat cell size: A major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 2016, 59, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Jernas, M.; Palming, J.; Sjoholm, K.; Jennische, E.; Svensson, P.A.; Gabrielsson, B.G.; Levin, M.; Sjogren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Zhang, H.; Morgan, T.E.; Forman, H.J. Age-related alteration in HNE elimination enzymes. Arch. Biochem. Biophys. 2021, 699, 108749. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, M.A.; Zacarias-Flores, M.; Arronte-Rosales, A.; Correa-Munoz, E.; Mendoza-Nunez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Taleb-Belkadi, O.; Chaib, H.; Zemour, L.; Fatah, A.; Chafi, B.; Mekki, K. Lipid profile, inflammation, and oxidative status in peri- and postmenopausal women. Gynecol. Endocrinol. 2016, 32, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Murdolo, G.; Hammarstedt, A.; Schmelz, M.; Jansson, P.A.; Smith, U. Acute hyperinsulinemia differentially regulates interstitial and circulating adiponectin oligomeric pattern in lean and insulin-resistant, obese individuals. J. Clin. Endocrinol. Metab. 2009, 94, 4508–4516. [Google Scholar] [CrossRef]
- Smith, U.; Gogg, S.; Johansson, A.; Olausson, T.; Rotter, V.; Svalstedt, B. Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. Faseb J. 2001, 15, 215–220. [Google Scholar] [CrossRef]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Thomou, T.; DePonte, M.; Koo, A.; Forse, R.A.; Chinnappan, D.; Martin-Ruiz, C.; von Zglinicki, T.; et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 2006, 55, 2571–2578. [Google Scholar] [CrossRef]
- Astruc, M.; Roussillon, S.; Defay, R.; Descomps, B.; Crastes de Paulet, A. DNA and cholesterol biosynthesis in synchronized embryonic rat fibroblasts. II. Effects of sterol biosynthesis inhibitors on cell division. Biochim. Biophys. Acta 1983, 17, 11–18. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Miyake, N.; Hiai, H.; Hagiwara, M.; Kawakishi, S.; Osawa, T.; Uchida, K. The monclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995, 359, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Torquato, P.; Barola, C.; Russo, A.; Rychlicki, C.; Giusepponi, D.; Bellezza, G.; Sidoni, A.; Galarini, R.; Svegliati-Baroni, G.; et al. Nonalcoholic fatty liver disease impairs the cytochrome P-450-dependent metabolism of alpha-tocopherol (vitamin E). J. Nutr. Biochem. 2018, 47, 120–131. [Google Scholar] [CrossRef] [PubMed]
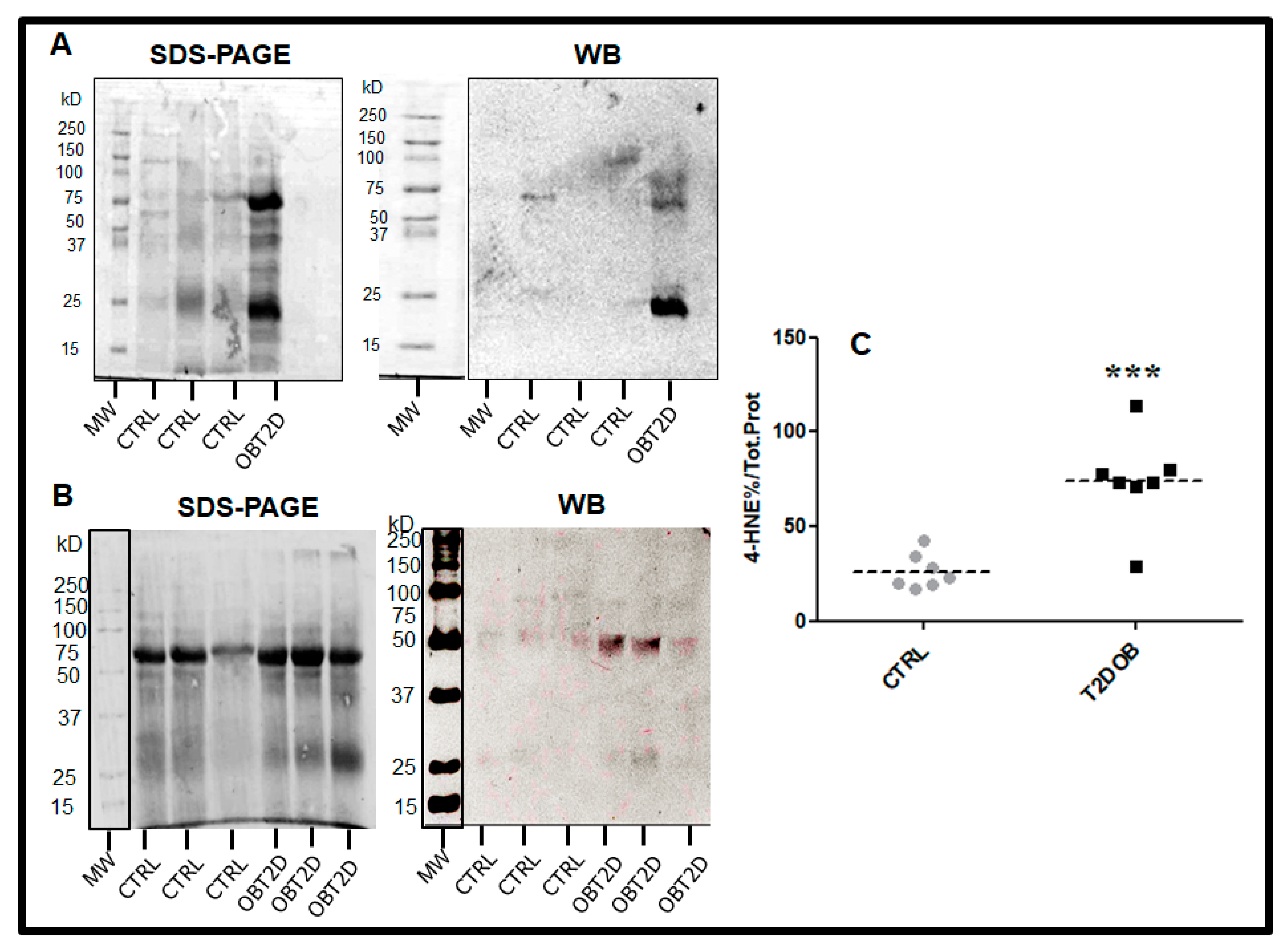

| CTRL | OBT2D | p Value | |
|---|---|---|---|
| n | 7 | 7 | |
| Sex (M/F) | 4/3 | 5/2 | |
| Age (yr) | 39 ± 3.5 | 60 ± 3.8 | <0.01 |
| BMI (kg/m2) | 23.2 ± 0.9 | 35.6 ± 1.9 | <0.0001 |
| Waist circumference (cm) | 79.1 ± 3.14 | 109.1 ± 3.8 | <0.001 |
| WHR | 0.86 ± 0.04 | 1.2 ± 0.09 | <0.001 |
| Total FM (kg) | 14.1 ± 2.6 | 45.3 ± 3.1 | <0.001 |
| Trunk FM (kg) | 8.4 ± 0.7 | 22.5 ± 1.1 | <0.001 |
| FFM (kg) | 58.2 ± 2.1 | 65.9 ± 2.3 | 0.01 |
| HbA1c (%) | 4.6 ± 0.4 | 7.7 ± 0.7 | <0.001 |
| Adipocyte cell diameter (μm) | 70.5 ± 3.2 | 105 ± 1 | <0.001 |
| Independent Variables | β | p Value | Adjusted R2 |
|---|---|---|---|
| Adipocyte cell diameter | 0.807 | <0.001 <0.001 | 0.622 |
| Sex | −0.109 | 0.889 | |
| Age | −0.045 | 0.858 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdolo, G.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Piroddi, M.; Galli, F. Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells. Int. J. Mol. Sci. 2023, 24, 16645. https://doi.org/10.3390/ijms242316645
Murdolo G, Bartolini D, Tortoioli C, Vermigli C, Piroddi M, Galli F. Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells. International Journal of Molecular Sciences. 2023; 24(23):16645. https://doi.org/10.3390/ijms242316645
Chicago/Turabian StyleMurdolo, Giuseppe, Desirée Bartolini, Cristina Tortoioli, Cristiana Vermigli, Marta Piroddi, and Francesco Galli. 2023. "Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells" International Journal of Molecular Sciences 24, no. 23: 16645. https://doi.org/10.3390/ijms242316645
APA StyleMurdolo, G., Bartolini, D., Tortoioli, C., Vermigli, C., Piroddi, M., & Galli, F. (2023). Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells. International Journal of Molecular Sciences, 24(23), 16645. https://doi.org/10.3390/ijms242316645






