This Special Issue, the third dedicated to reproductive immunology and pregnancy, is another review of the latest trends in research topics in this field. The dynamic development of immunopathology of the reproductive system creates new opportunities to verify hypotheses regarding the etiopathogenesis of endometriosis and infertility, among other conditions [1].
While estrogens themselves do not cause endometriosis, the disease is classified as estrogen-dependent, in which cells with characteristics very similar to endometrial cells form ectopic foci outside the uterine cavity. Because endometriosis cells may express estrogen receptors (ERα, Erβ, GPER) and progesterone (P4) receptors (PR-A, PR-B), their growth, cyclic proliferation, and breakdown are similar to the processes that occur in the endometrium [2]. This leads to significant complications, mainly due to the chronic inflammatory response. Endometriosis affects 10–15% of women of reproductive age and is associated with chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility [3,4].
The pathogenesis of endometriosis is multifactorial [5,6]. There are several theories, including the widely accepted implantation theory, which assumes the occurrence of retrograde transport of viable endometrial cells with retained abilities for pelvic cavity attachment, proliferation, differentiation, and subsequent invasion into the surrounding tissue [7]. Achievements in the field of immunobiology and embryology have made it possible to supplement implantation theory with knowledge about the significant contribution of stem cells, leading to the development of the stem cell theory of endometriosis [8]. A population of stem cells in the uterus helps the endometrium regenerate after shedding each month during menstruation. These stem cells divide and produce more endometrial tissue to replace what is lost during menstruation. Accordingly, the most abundant cells in the endometrium are endometrial stromal cells (EnSCs) [8,9]. These cells constitute a particular population with clonogenic activity that resembles the properties of mesenchymal stem/stromal cells (MSCs). Thus, a significant role of stem cell-based dysfunction in the formation of initial endometrial lesions is suspected. There is increasing evidence that the role of epigenetic mechanisms and processes in endometriosis has been underestimated [5,10,11,12]. This conclusion is based on the fact that heritable phenotype changes that do not interfere with the DNA sequence are common triggers for hormonal, immunological, and inflammatory disorders, which play a key role in the formation of endometriotic foci [7].
The instability of estrogen/P4 homeostasis, which leads to excessive estrogen exposure and P4 resistance, is strongly reflected in endometriotic tissue as changes in the expression of transcription factors of the estrogen and P4 signaling pathways [9].
It is well known that endometriosis co-occurs in individuals with autoimmune diseases more often than in the general population [13]. This includes individuals with systemic lupus erythematosus (SLE), Hashimoto’s autoimmune thyroiditis, multiple sclerosis (MS), diabetes mellitus type 1, rheumatoid arthritis (RA), Graves’ disease, vitiligo, and celiac disease (CD), among others [13,14,15]. In autoimmune diseases, the immune system mistakenly recognizes its own tissues as immunologically foreign and then induces cellular immunity mechanisms based on T lymphocytes (T cells), macrophages, and natural killer (NK) cells to destroy them. The results of recent studies indicate significant disturbances in the function of these cells in women with endometriosis. For example, an increased number and high activity of regulatory T cells (Tregs) and macrophages are found in the peritoneal fluid of women with endometriosis. In relation to T cells, maintenance of forkhead box P3 (Foxp3) protein—the master regulatory protein involved in Treg-mediated immune system responses—seems crucial to ensure a balanced immune response [16]. Significant differences between autoimmunity and endometriosis in relation to the activity of Tregs and autoreactive effector CD4+ T helper (Th)1 and Th17 subsets are simplified in Figure 1.
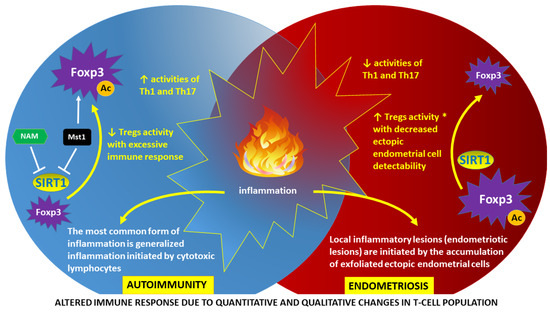
Figure 1.
Altered immune response as a result of epigenetically determined dysfunction of regulatory T cells (Tregs): autoimmunity vs. endometriosis. Tregs are essential for maintaining immune homeostasis. Forkhead box P3 (Foxp3) is the master regulatory protein involved in Treg development and function [17]. The activity of Foxp3 is a derivative of the degree of acetylation (Ac), which is directly related to the presence of sirtuin-1 (SIRT1). SIRT1 functions as a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase linked to cellular energy and redox status. Deacetylation of Foxp3 in autoimmunity may be inhibited by nicotinamide (NAM), a byproduct of an SIRT1-catalyzed reaction. Another mechanism of SIRT1 inhibition in autoimmunity involves phosphorylation of SIRT1 by mammalian sterile 20-like kinase 1 (Mst1) protein kinase with subsequent protein p53 acetylation and transactivation, resulting in apoptosis induction and decreased cell proliferation [18]. A high level of Foxp3 acetylation in autoimmunity leads to a decrease in the activity of Tregs and, consequently, to an increase in the activity of autoreactive effector CD4+ T helper (Th)1 and Th17 subsets [19]. Conversely, in endometriosis, a low level of Foxp3 acetylation with uninhibited deacetylating function of SIRT1 leads to an increase in Tregs activity (*) and subsequent reduction in Th1 and Th17 activities [20]. Therefore, the detection of ectopic locations of endometrial cells (endometriotic foci) by the immune system is impaired. An inherent symptom of both autoimmunity and endometriosis is inflammation, although with different pathogenesis and characteristics [16].
Dysfunctional natural killer (NK) cells may also contribute to the impaired recognition of ectopic foci in endometriosis and the lack of effective removal of endometrial cells by the immune system. Reis et al. (2022) [21] reviewed, summarized, and updated the previous literature on NK cells and endometriosis, focusing on the current state of knowledge about the role of NK receptors (NKRs).
The overexpression of NK cell inhibitory receptors (KIRs), such as CD158a+, KIR2DL1, CD94/NKG2A, PD-1, NKB1, and EB6, and inhibitory ligands, namely, PD-L1, HLA-E, HLA-G, and HLA-I, may play an important role in the pathogenesis of endometriosis.
Moreover, the early onset of preeclampsia may also be caused by immune checkpoint disorders, with the presence of a population of NK cells with abnormal KIR expression causing a predisposition to inadequate uterine spiral artery remodeling and shallow trophoblast invasion [22]. It has been shown that immunological interactions in the maternal–fetal system occurring in early pregnancy are not only moderated by T lymphocytes but also by NK cells, which may be because decidual NK cells are the largest population of immune system cells in the uterus during early pregnancy [23]. It has been shown that immunological interactions in the maternal–fetal system occurring in early pregnancy are moderated mainly by NK cells, not T cells, which may be because decidual NK cells are the largest population of immune system cells in the uterus during early pregnancy [23].
Fertility disorders related to abnormal functioning of the immune system, increasing the risk of autoimmune diseases, are a constantly growing and supplemented pool of causes of infertility [24]. Antiphospholipid syndrome (APLS) is characterized by thrombosis and/or recurrent pregnancy loss coexisting with the presence of circulating autoantibodies that are directed against phospholipid-binding proteins (antiphospholipid or aPL antibodies) [25]. Minimal vasculitis combined with complement consumption in patients experiencing infertility may be an underlying mechanism for impaired implantation because aPL antibodies regulate the inflammatory response [26]. Recently, a novel autoantibody against a complex of β2-glycoprotein I and human leukocyte antigen class II molecules (β2-GPI/HLA-DR) has been reported to be an independent autoantibody associated with APLS [27]. In addition, human leukocyte antigen G (HLA-G), expressed on trophoblastic cell surfaces, seems to be one of the main molecules involved in the modulation of both local and systemic maternal immune responses. It was demonstrated that HLA-G 3’UTR polymorphisms and haplotypes may be involved in unexplained recurrent spontaneous abortion (URSA) and may be a predictor of pregnancy outcome [28].
Alpha-enolase (enolase 1, ENO1) is a multifunctional protein that acts as a key glycolytic enzyme in the cytoplasm and a receptor for plasminogen expressed on the cell surface. In euthyroid females with autoimmune thyroiditis, serum levels of autoantibodies against strong epitopes of α-enolase may be treated as good predictive markers for pregnancy loss [29].
Adipokines are cell-signaling molecules (cytokines) produced in adipose tissue and are involved in metabolic, endocrinological, vascular, and immunogenic processes. The obesity pandemic, also occurring among pregnant women, has undoubtedly contributed to the increased interest in the study of adipokines in recent years. Many of these studies aimed to explain the relationship between the concentrations of specific adipokines (e.g., fatty acid binding protein 4, FABP4), especially in pregnant women with weight disorders, and the course of pregnancy and the risk of complications, such as gestational diabetes mellitus (GDM), preeclampsia (PE), fetal growth restriction (FGR), and macrosomia [30,31,32].
The most well-studied gestational trophoblastic disease (GTD) is hydatidiform mole (also known as a molar pregnancy), which is characterized by an overgrown villous trophoblast with cystic “swollen” villi that develop inside the uterus after conception. The peculiarity of this rare disease, which occurs with distinct geographical variations in approximately 1 in 1200 pregnancies, is that the tumor essentially originates from the pregnancy tissue and not from the mother’s tissue [33]. A mole is classified as partial when the cells are triploid and contain two copies of the paternal genome and one copy of the maternal genetic material, and—in the absence of the maternal genome—as complete (contains two sets of the paternal chromosomes) [33]. This feature was used in the differential diagnosis of mole by examining paternally imprinted genes. Paternally imprinted genes are those that are expressed only when inherited from the mother, while the father’s allele is silenced by DNA methylation [34]. Because complete hydatidiform mole lacks a maternal genomic component, there is no expression of paternally imprinted genes in this pathological tissue. Since approximately 40 parentally imprinted genes have been identified in humans, the number of genetic markers in complete hydatidiform mole is constantly increasing. To date, the diagnostic utility has been confirmed with immunohistochemistry of cyclin-dependent kinase inhibitor 1C (p57, encoded by CDKN1C imprinted gene), retinoblastoma transcriptional corepressor 1 (RB1), and pleckstrin homology-like domain family A member 2 (IPL/TSSC3) [35,36,37,38,39].
Lactation in mammals completes the reproductive process, although milk production generally interferes with fertility by inhibiting ovulation. In addition to its reproductive functions, prolactin—the main hormone responsible for breast development during pregnancy and lactation—is also involved in metabolism, osmoregulation, immunomodulation, and behavior. Lactation greatly changes the mother’s metabolism, redistributing the blood supply and increasing the demand for nutrients. The lack of a balanced diet in breastfeeding women carries a high risk of macro- and micronutrient deficiencies and makes it difficult to maintain a healthy body weight due to metabolic disorders. In general, apart from the obvious benefits for the baby, lactation also has a positive effect on the body of the breastfeeding mother. Some cancers, type 2 diabetes, high blood pressure, and nonalcoholic fatty liver disease (NAFLD) are less common among women who breastfeed [40]. However, in a situation unique to humans, i.e., industrial milk production in cows, intense and maximally prolonged lactation has an adverse effect mainly on liver function and the immune system. Cheng et al. (2023) [41] demonstrated that the functionality of circulating leukocytes in dairy cows is suppressed after calving, with negative energy balance as a risk factor. Moreover, the leukocytes of multiparous and primiparous cows responded differently to the diets across age, nutrient supply, and immunity, affecting health and subsequent fertility.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Pašalić, E.; Tambuwala, M.M.; Hromić-Jahjefendić, A. Endometriosis: Classification, pathophisiology, and treatment options. Pathol. Res. Pract. 2023, 251, 154847. [Google Scholar] [CrossRef]
- Szukiewicz, D. Aberrant epigenetic regulation of estrogen and progesterone signaling at the level of endometrial/endometriotic tissue in the pathomechanism of endometriosis. Vitam. Horm. 2023, 122, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, J.; García-Velasco, J.A. Endometriosis and Reproduction: What We Have Learned. Yale J. Biol. Med. 2020, 93, 571–577. [Google Scholar] [PubMed]
- Shan, J.; Li, D.J.; Wang, X.Q. Towards a Better Understanding of Endometriosis-Related Infertility: A Review on How Endometriosis Affects Endometrial Receptivity. Biomolecules 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Monnin, N.; Fattet, A.J.; Koscinski, I. Endometriosis: Update of Pathophysiology, (Epi) Genetic and Environmental Involvement. Biomedicines 2023, 11, 978. [Google Scholar] [CrossRef]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef]
- Pais, A.S.; Almeida-Santos, T. Recent insights explaining susceptibility to endometriosis-From genetics to environment. WIREs Mech. Dis. 2023, 15, e1624. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.R.; Carvalhos, C.A.; Figueiredo-Dias, M. The Emerging Role of Menstrual-Blood-Derived Stem Cells in Endometriosis. Biomedicines 2022, 11, 39. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Stangret, A.; Ruiz-Ruiz, C.; Olivares, E.G.; Soriţău, O.; Suşman, S.; Szewczyk, G. Estrogen- and Progesterone (P4)-Mediated Epigenetic Modifications of Endometrial Stromal Cells (EnSCs) and/or Mesenchymal Stem/Stromal Cells (MSCs) in the Etiopathogenesis of Endometriosis. Stem Cell Rev. Rep. 2021, 17, 1174–1193. [Google Scholar] [CrossRef]
- Hon, J.X.; Wahab, N.A.; Karim, A.K.A.; Mokhtar, N.M.; Mokhtar, M.H. MicroRNAs in Endometriosis: Insights into Inflammation and Progesterone Resistance. Int. J. Mol. Sci. 2023, 24, 15001. [Google Scholar] [CrossRef]
- Szukiewicz, D. Insight into the Potential Mechanisms of Endocrine Disruption by Dietary Phytoestrogens in the Context of the Etiopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 12195. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, M.; Karimi, M.; Rajaei, S. The landscape of non-coding RNAs in the immunopathogenesis of Endometriosis. Front. Immunol. 2023, 14, 1223828. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Jiang, X.Y.; Xu, H.L.; Chen, G.; Wang, S.L.; Zhang, H.P.; Hong, L.; Jin, Q.Q.; Yao, H.; Zhang, W.Y.; et al. Autoimmune Disease-Related Hub Genes are Potential Biomarkers and Associated with Immune Microenvironment in Endometriosis. Int. J. Gen. Med. 2023, 16, 2897–2921. [Google Scholar] [CrossRef]
- Salmeri, N.; Gennarelli, G.; Vanni, V.S.; Ferrari, S.; Ruffa, A.; Rovere-Querini, P.; Pagliardini, L.; Candiani, M.; Papaleo, E. Concomitant Autoimmunity in Endometriosis Impairs Endometrium-Embryo Crosstalk at the Implantation Site: A Multicenter Case-Control Study. J. Clin. Med. 2023, 12, 3557. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D. Epigenetic regulation and T-cell responses in endometriosis-something other than autoimmunity. Front. Immunol. 2022, 13, 943839. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020, 10, 3100. [Google Scholar] [CrossRef]
- Yuan, F.; Xie, Q.; Wu, J.; Bai, Y.; Mao, B.; Dong, Y.; Bi, W.; Ji, G.; Tao, W.; Wang, Y.; et al. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J. Biol. Chem. 2011, 286, 6940–6945. [Google Scholar] [CrossRef]
- Akimova, T.; Xiao, H.; Liu, Y.; Bhatti, T.R.; Jiao, J.; Eruslanov, E.; Singhal, S.; Wang, L.; Han, R.; Zacharia, K.; et al. Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells. Mucosal Immunol. 2014, 7, 1209–1220. [Google Scholar] [CrossRef]
- Chopyak, V.V.; Koval, H.D.; Havrylyuk, A.M.; Lishchuk-Yakymovych, K.A.; Potomkina, H.A.; Kurpisz, M.K. Immunopathogenesis of endometriosis—A novel look at an old problem. Cent. Eur. J. Immunol. 2022, 47, 109–116. [Google Scholar] [CrossRef]
- Reis, J.L.; Rosa, N.N.; Ângelo-Dias, M.; Martins, C.; Borrego, L.M.; Lima, J. Natural Killer Cell Receptors and Endometriosis: A Systematic Review. Int. J. Mol. Sci. 2022, 24, 331. [Google Scholar] [CrossRef]
- Szereday, L.; Nagy, D.U.; Csiszar, B.; Kevey, D.; Feik, T.; Meggyes, M. Examination of the TIGIT, CD226, CD112, and CD155 Immune Checkpoint Molecules in Peripheral Blood Mononuclear Cells in Women Diagnosed with Early-Onset Preeclampsia. Biomedicines 2021, 9, 1608. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Mohamed Khosroshahi, L.; Parhizkar, F.; Kachalaki, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Immune checkpoints and reproductive immunology: Pioneers in the future therapy of infertility related Disorders? Int. Immunopharmacol. 2021, 99, 107935. [Google Scholar] [CrossRef]
- Bustamante, J.G.; Goyal, A.; Singhal, M. Antiphospholipid Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430980/ (accessed on 1 November 2023).
- Regan, L.; Rai, R.; Saravelos, S.; Li, T.C. Royal College of Obstetricians and Gynaecologists. Recurrent MiscarriageGreen-top Guideline No. 17. BJOG 2023, 130, e9–e39. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Ueda, Y.; Tanimura, K.; Arase, H.; Yamada, H.; Saegusa, J. Association of anti-β2-glycoprotein I/HLA-DR complex antibody with arterial thrombosis in female patients with systemic rheumatic diseases. Arthritis Res. Ther. 2023, 25, 195. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Lin, Z.; Ye, J.; Zhou, L.; Xi, J.; Cai, W. Frequency of HLA-G UTR-1/UTR-3/UTR-7 in women with. Exp. Ther. Med. 2022, 24, 729. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Y.; Wang, H.; Sun, W.; Lu, Y.; Shan, Z.; Teng, W.; Li, J. A Predictive Role of Autoantibodies Against the Epitope aa168-183 of ENO1 in the Occurrence of Miscarriage Related to Thyroid Autoimmunity. Front. Immunol. 2022, 13, 890502. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Li, Y.; Dong, K.; Sun, Y.; Ma, A.; Yang, X. Regulative effect of maternal serum fatty acid-binding protein 4 on insulin resistance and the development of gestational diabetes mellitus. Prostaglandins Leukot. Essent. Fatty Acids 2020, 163, 102213. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, N.; Blüher, M.; Stepan, H.; Stumvoll, M.; Ebert, T.; Tönjes, A.; Schrey-Petersen, S. Adipokines in Pregnancy: A Systematic Review of Clinical Data. Biomedicines 2023, 11, 1419. [Google Scholar] [CrossRef]
- Vorobjova, T.; Tagoma, A.; Talja, I.; Janson, H.; Kirss, A.; Uibo, R. FABP4 and I-FABP Levels in Pregnant Women Are Associated with Body Mass Index but Not Gestational Diabetes. J. Diabetes Res. 2022, 2022, 1089434. [Google Scholar] [CrossRef]
- Ghassemzadeh, S.; Farci, F.; Kang, M. Hydatidiform Mole. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459155/ (accessed on 1 November 2023).
- Khambata, K.; Begum, S.; Raut, S.; Mohan, S.; Irani, D.; Singh, D.; Bansal, V.; Patil, A.; Balasinor, N.H. DNA methylation biomarkers to identify epigenetically abnormal spermatozoa in male partners from couples experiencing recurrent pregnancy loss. Epigenetics 2023, 18, 2252244. [Google Scholar] [CrossRef]
- Giacometti, C.; Bellan, E.; Ambrosi, A.; Dei Tos, A.P.; Cassaro, M.; Ludwig, K. While there is p57, there is hope. The past and the present of diagnosis in first trimester abortions: Diagnostic dilemmas and algorithmic approaches. A review. Placenta 2021, 116, 31–37. [Google Scholar] [CrossRef]
- Saxena, A.; Frank, D.; Panichkul, P.; Van den Veyver, I.B.; Tycko, B.; Thaker, H. The product of the imprinted gene IPL marks human villous cytotrophoblast and is lost in complete hydatidiform mole. Placenta 2003, 24, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Matsuda, T.; Hirakawa, T.; Ueda, K.; Inoue, T.; Miyanari, Y.; Asanoma, K.; Nakano, H.; Wake, N. Differential diagnosis between complete and partial mole by TSSC3 antibody completely correlates to DNA diagnosis. Diagn. Mol. Pathol. 2005, 14, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Buiting, K.; Kanber, D.; Horsthemke, B.; Lohmann, D. Imprinting of RB1 (the new kid on the block). Brief. Funct. Genom. 2010, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.M.; Fitzgerald, B.; McCarthy, T.V.; Coulter, J.; O’Donoghue, K. Advances in the diagnosis and early management of gestational trophoblastic disease. BMJ Med. 2022, 1, e000321. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Sinn, D.H.; Oh, J.H.; Goh, M.J.; Kim, K.; Kang, W.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. The Association Between Breastfeeding and Nonalcoholic Fatty Liver Disease in Parous Women: A Nation-wide Cohort Study. Hepatology 2021, 74, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; McLaughlin, D.L.; Little, M.W.; Ferris, C.; Salavati, M.; Ingvartsen, K.L.; Crowe, M.A.; Wathes, D.C.; The GplusE Consortium. Proportion of Concentrate in the Diet of Early Lactation Dairy Cows Has Contrasting Effects on Circulating Leukocyte Global Transcriptomic Profiles, Health and Fertility According to Parity. Int. J. Mol. Sci. 2022, 24, 39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).