Improving the Thermostability of Thermomyces lanuginosus Lipase by Restricting the Flexibility of N-Terminus and C-Terminus Simultaneously via the 25-Loop Substitutions
Abstract
:1. Introduction
2. Results
2.1. Key Residues Selected for Improving TLL Thermostability
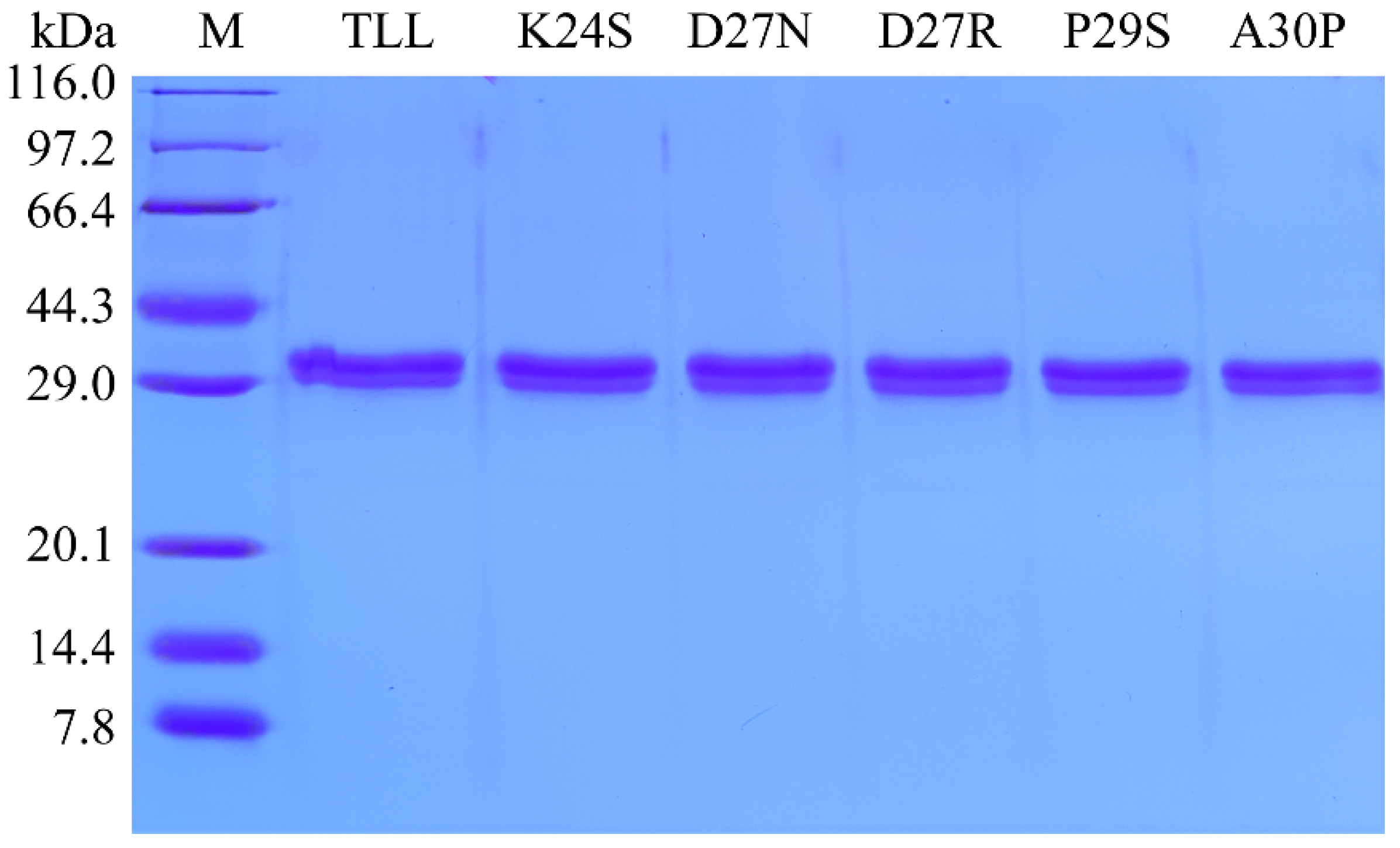
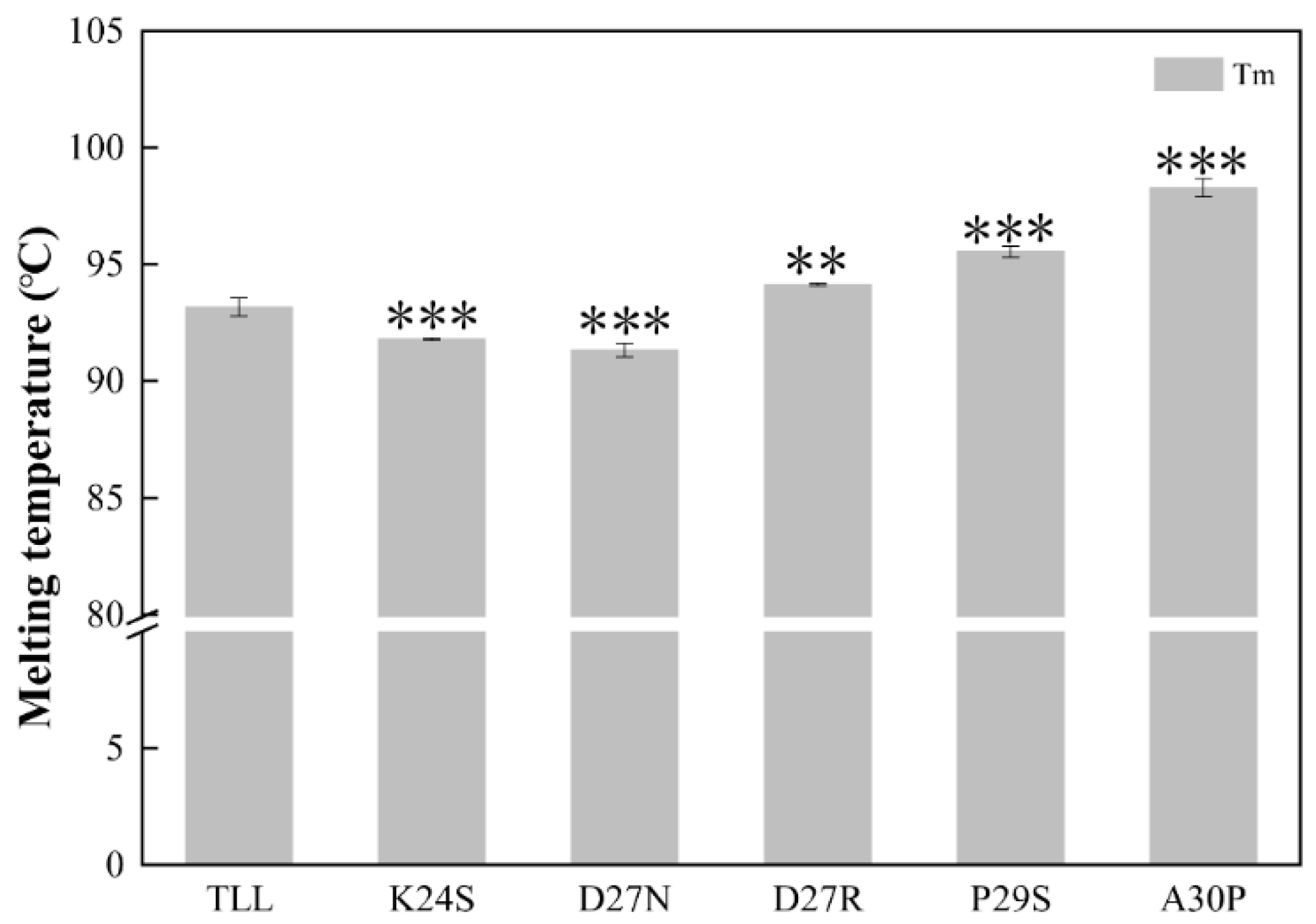
2.2. Construction and Characterization of TLL and the Five Single Variants
2.3. Kinetic and Productivity Analysis of the TLL and Five Variants
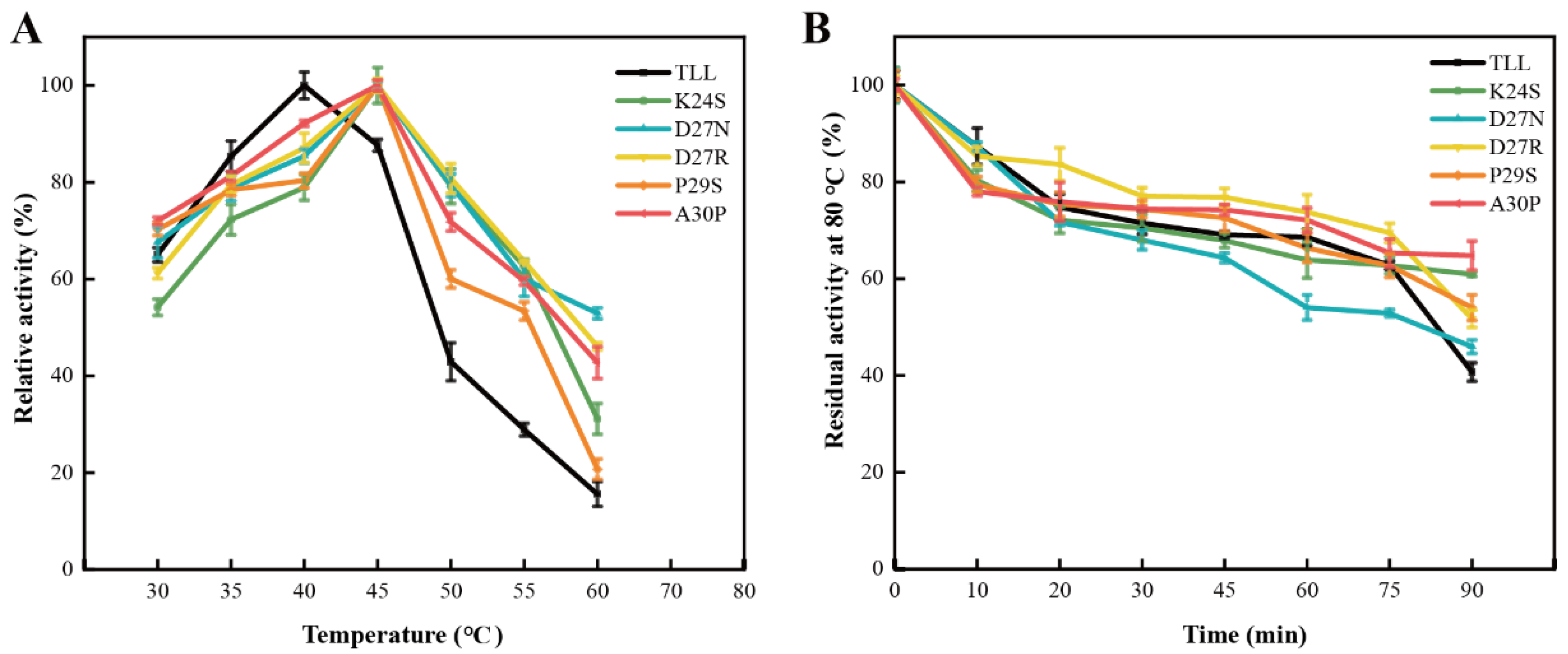
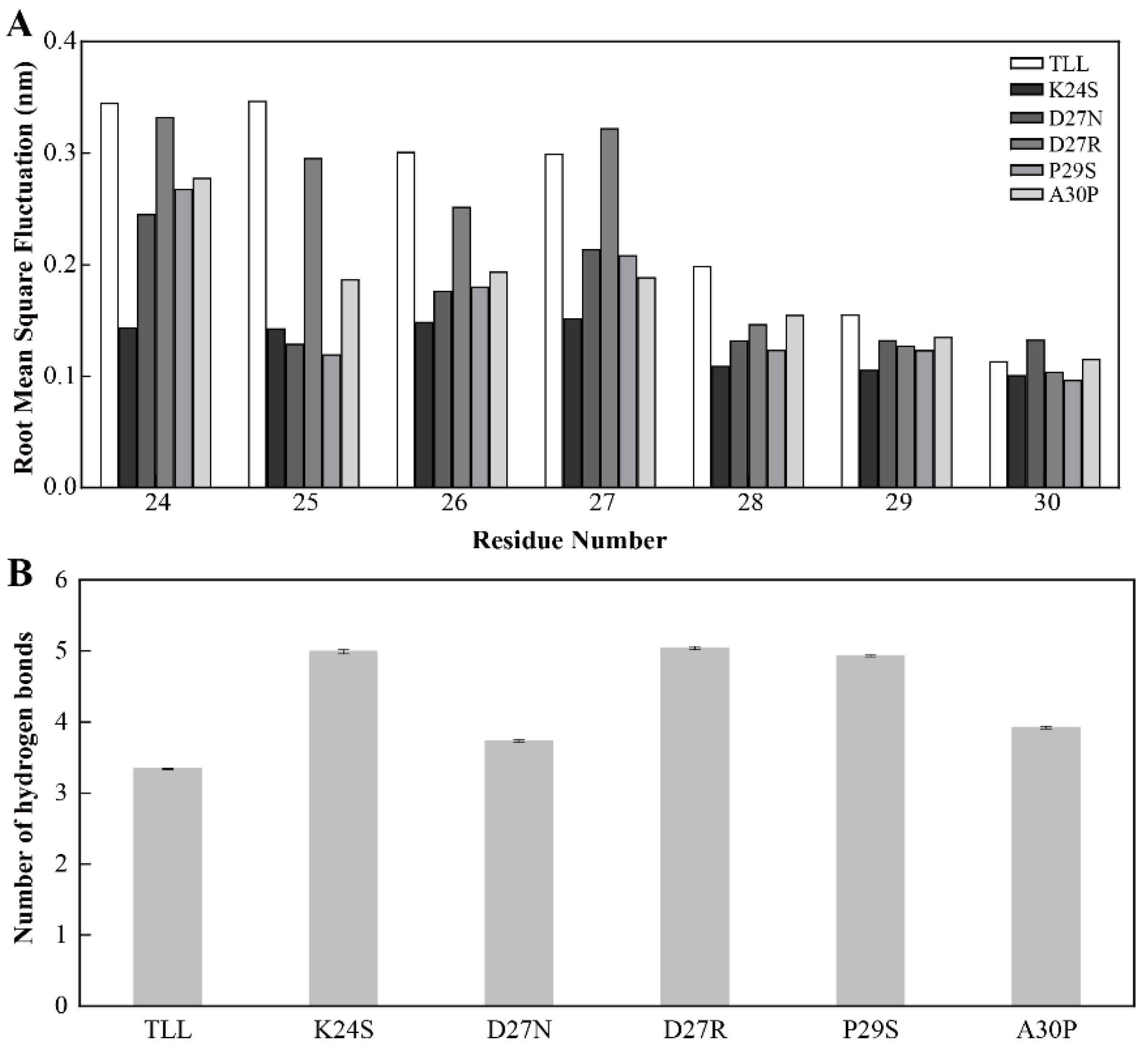
2.4. Stability of the NTR for TLL and Five Variants
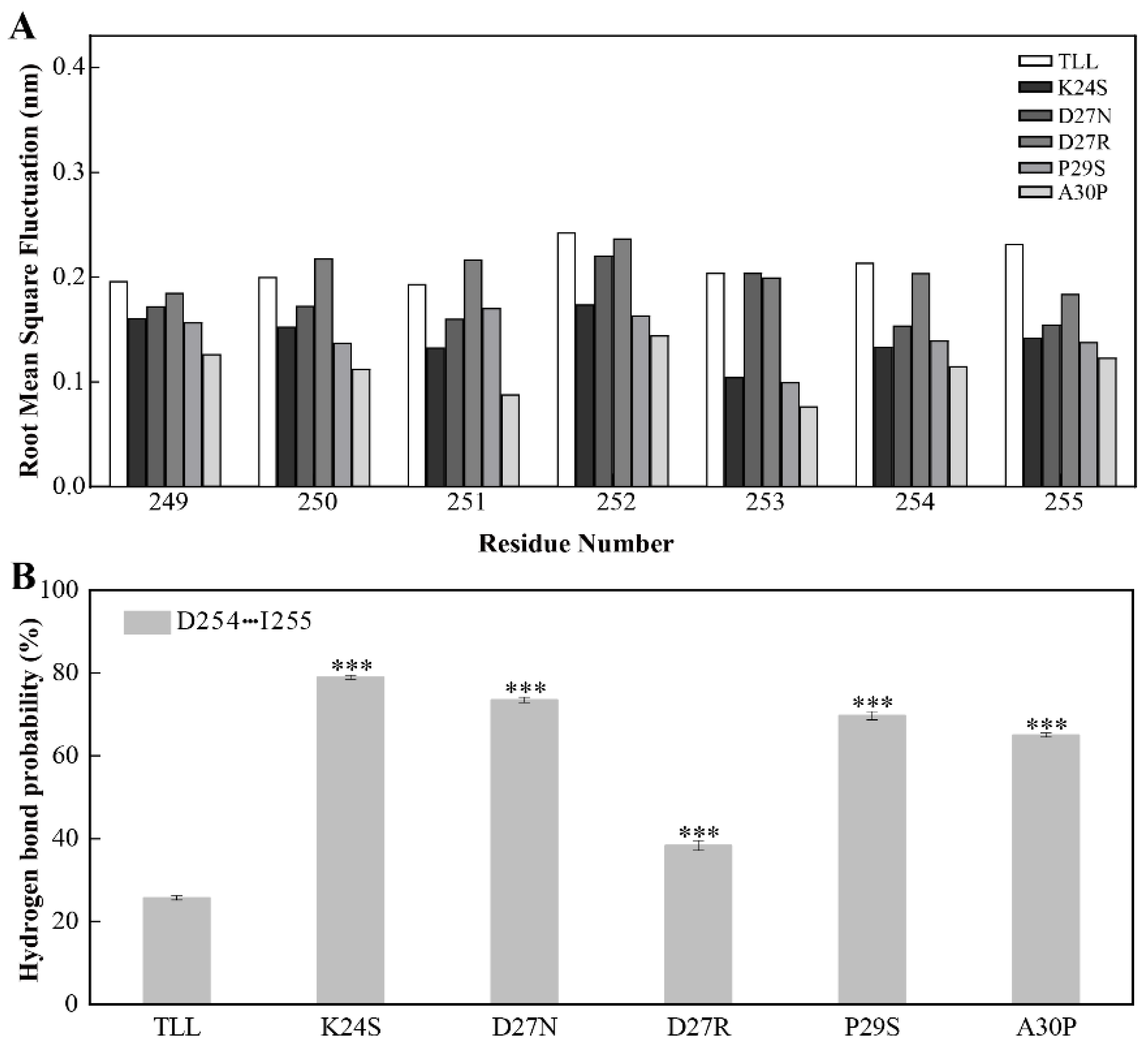
2.5. Stability of the CTR for TLL and Five Variants
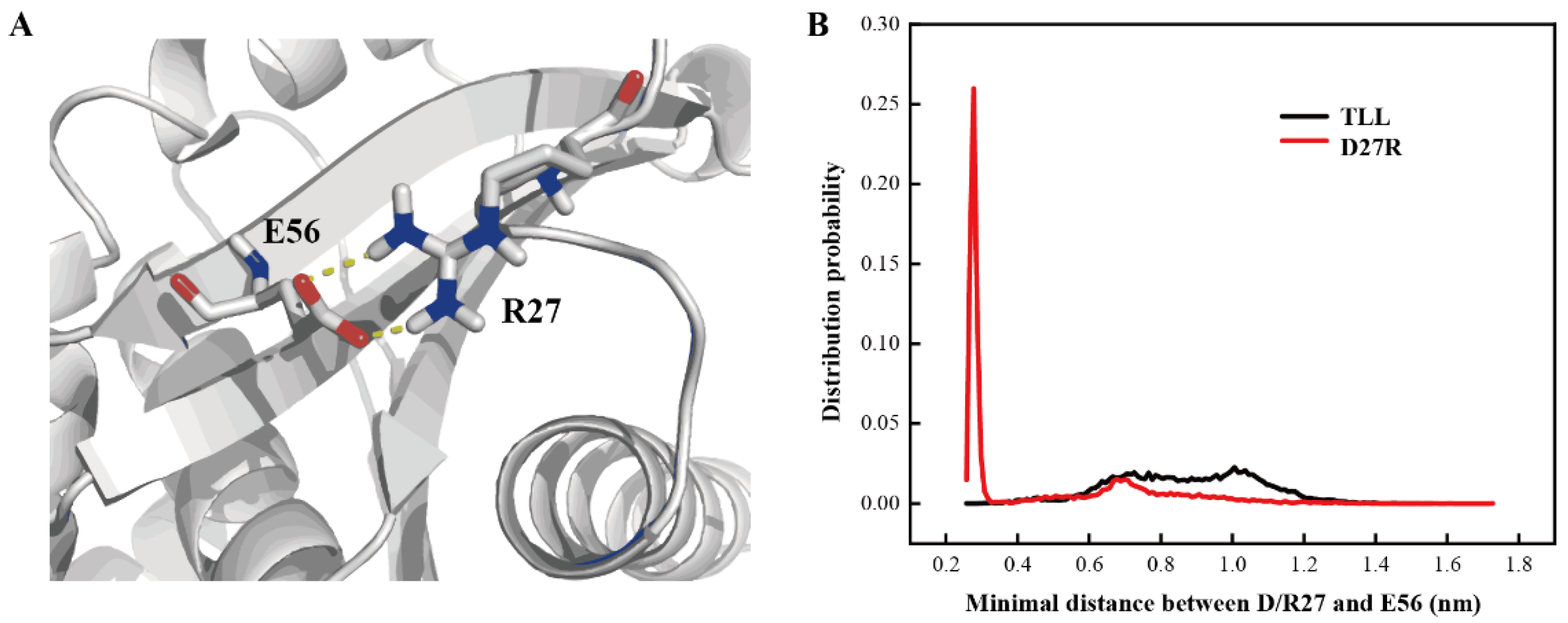
2.6. Additional Salt-Bridge Interaction Formed in D27R
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Gene Cloning and Site-Directed Mutagenesis
4.3. Protein Expression and Purification
4.4. Assessment of Lipase Activity
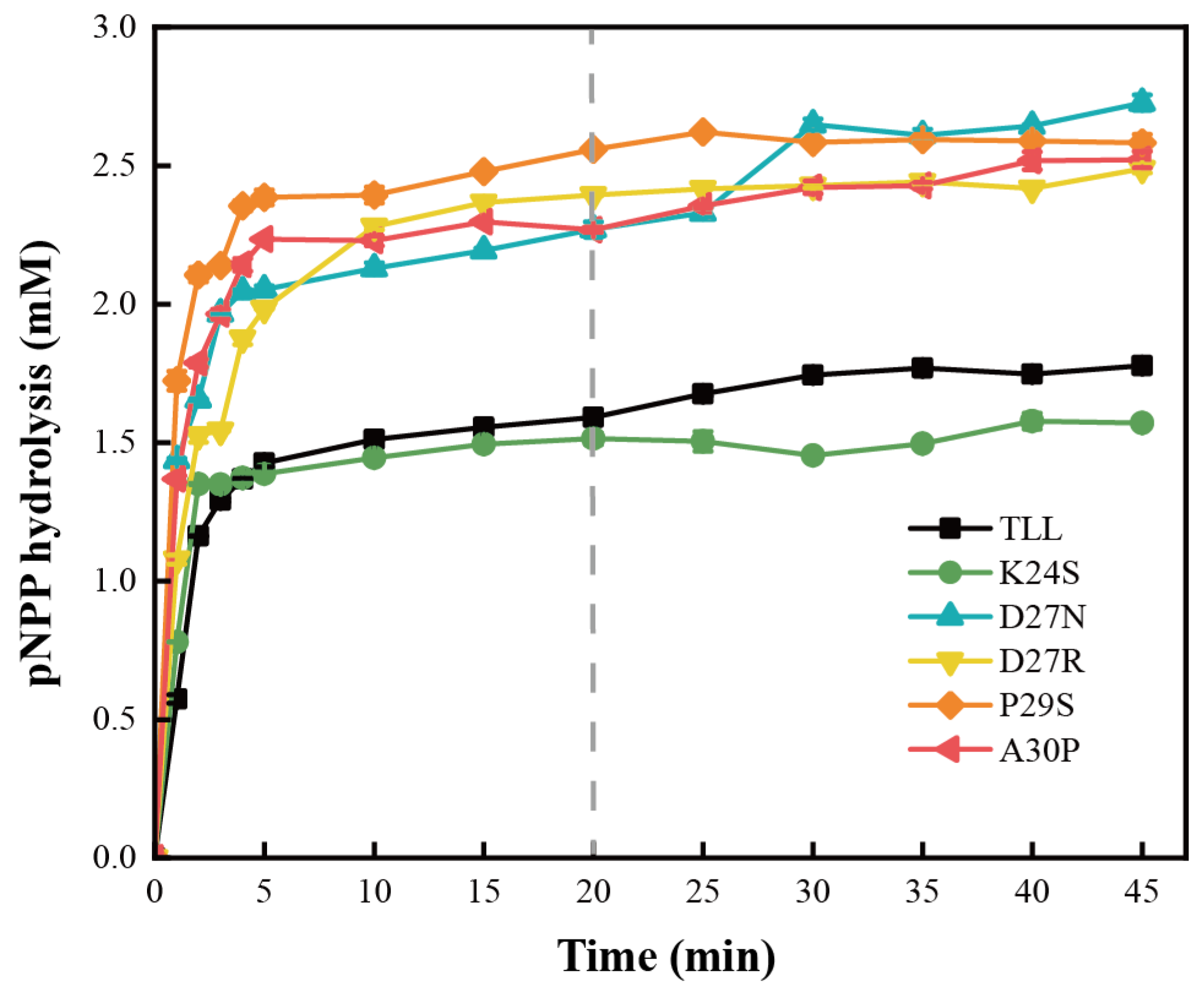
4.5. Assessment of Lipase Productivity
4.6. Prediction of the Mutagenesis Sites via B-Factor Comparison
4.7. MD Simulation Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Salgado, C.A.; dos Santos, C.I.A.; Vanetti, M.C.D. Microbial lipases: Propitious biocatalysts for the food industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Kumar, R.; Goomber, S.; Kaur, J. Engineering lipases for temperature adaptation: Structure function correlation. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140261. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094. [Google Scholar] [CrossRef]
- Veno, J.; Ahmad Kamarudin, N.H.; Mohamad Ali, M.S.; Masomian, M.; Rahman, R. Directed Evolution of Recombinant C-Terminal Truncated Staphylococcus epidermidis Lipase AT2 for the Enhancement of Thermostability. Int. J. Mol. Sci. 2017, 18, 2202. [Google Scholar] [CrossRef]
- Huang, J.; Dai, S.; Chen, X.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Alteration of Chain-Length Selectivity and Thermostability of Rhizopus oryzae Lipase via Virtual Saturation Mutagenesis Coupled with Disulfide Bond Design. Appl. Environ. Microbiol. 2023, 89, e0187822. [Google Scholar] [CrossRef]
- Huang, Z.; Ni, D.; Chen, Z.; Zhu, Y.; Zhang, W.; Mu, W. Application of molecular dynamics simulation in the field of food enzymes: Improving the thermal-stability and catalytic ability. Crit. Rev. Food Sci. Nutr. 2023, 24, 1–13. [Google Scholar] [CrossRef]
- Xu, Z.; Cen, Y.-K.; Zou, S.-P.; Xue, Y.-P.; Zheng, Y.-G. Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 2020, 40, 83–98. [Google Scholar] [CrossRef]
- Reetz, M.T. What are the Limitations of Enzymes in Synthetic Organic Chemistry? Chem. Rec. 2016, 16, 2449–2459. [Google Scholar] [CrossRef]
- Xie, Y.; An, J.; Yang, G.; Wu, G.; Zhang, Y.; Cui, L.; Feng, Y. Enhanced Enzyme Kinetic Stability by Increasing Rigidity within the Active Site. J. Biol. Chem. 2014, 289, 7994–8006. [Google Scholar] [CrossRef]
- Zhang, H.; Sang, J.; Zhang, Y.; Sun, T.; Liu, H.; Yue, R.; Zhang, J.; Wang, H.; Dai, Y.; Lu, F.; et al. Rational design of a Yarrowia lipolytica derived lipase for improved thermostability. Int. J. Biol. Macromol. 2019, 137, 1190–1198. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Xu, Y.; Yu, X. Enhancing the thermostability of Rhizopus chinensis lipase by rational design and MD simulations. Int. J. Biol. Macromol. 2020, 160, 1189–1200. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Amira, M.; Benmabrouk, S.; Ahmed, F.; Sayari, A. Fungal Lipases as Biocatalysts: A Promising Platform in Several Industrial Biotechnology Applications. Biotechnol. Bioeng. 2022, 119, 3370–3392. [Google Scholar] [CrossRef]
- Zhu, E.; Xiang, X.; Wan, S.; Miao, H.; Han, N.; Huang, Z. Discovery of the Key Mutation Site Influencing the Thermostability of Thermomyces lanuginosus Lipase by Rosetta Design Programs. Int. J. Mol. Sci. 2022, 23, 8963. [Google Scholar] [CrossRef]
- Farrokh, P.; Yakhchali, B.; Karkhane, A.A. Rational Design of K173A Substitution Enhances Thermostability Coupled with Catalytic Activity of Enterobacter sp. Bn12 Lipase. J. Mol. Microbiol. Biotechnol. 2014, 24, 262–269. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Karbalaei-Heidari, H.R.; Khajeh, K. Production of the renewable extremophile lipase: Valuable biocatalyst with potential usage in food industry. Food Bioprod. Process. 2017, 102, 153–166. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, H.; Zhang, J.; Wang, P.; Liu, S.; Liu, G.; Wu, L. Effects of Asn-33 glycosylation on the thermostability of Thermomyces lanuginosus lipase. J. Appl. Microbiol. 2014, 117, 151–159. [Google Scholar] [CrossRef]
- Qu, P.; Li, D.; Lazim, R.; Xu, R.; Xiao, D.; Wang, F.; Li, X.; Zhang, Y. Improved thermostability of Thermomyces lanuginosus lipase by molecular dynamics simulation and in silico mutation prediction and its application in biodiesel production. Fuel 2022, 327, 125039. [Google Scholar] [CrossRef]
- Gupta, P.; Nipunta; Dutt, K.; Saran, S.; Saxena, R.K. Binary immobilization: A newer approach for immobilizing lipase from a Thermophilic sp. of Thermomyces lanuginosus. Indian J. Biochem. Biophys. 2019, 56, 358–362. [Google Scholar]
- Bernal, C.; Rodríguez, K.; Martínez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Ringe, D.; Petsko, G.A. Chapter 19. Study of Protein Dynamics by X-ray Diffraction. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 131, pp. 389–433. [Google Scholar]
- Parthasarathy, S.; Murthy, M.R.N. Protein thermal stability: Insights from atomic displacement parameters (B values). Protein Eng. Des. Sel. 2000, 13, 9–13. [Google Scholar] [CrossRef]
- Yu, H.; Huang, H. Engineering proteins for thermostability through rigidifying flexible sites. Biotechnol. Adv. 2014, 32, 308–315. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Delano, W.L. The PyMOL Molecular Graphics System. Proteins 2002, 30, 442–454. [Google Scholar]
- Siddiqui, K.S.; Ertan, H.; Poljak, A.; Bridge, W.J. Evaluating Enzymatic Productivity—The Missing Link to Enzyme Utility. Int. J. Mol. Sci. 2022, 23, 6908. [Google Scholar] [CrossRef]
- Miller, S.R. An appraisal of the enzyme stability-activity trade-off. Evolution 2017, 71, 1876–1887. [Google Scholar] [CrossRef]
- Feller, G. Protein stability and enzyme activity at extreme biological temperatures. J. Phys. Condens. Matter. 2010, 22, 323101. [Google Scholar] [CrossRef]
- Chen, G.; Khan, I.M.; He, W.; Li, Y.; Jin, P.; Campanella, O.H.; Zhang, H.; Huo, Y.; Chen, Y.; Yang, H.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef]
- Wells, J.A. Additivity of mutational effects in proteins. Biochemistry 1990, 29, 8509–8517. [Google Scholar] [CrossRef]
- Watanabe, K.; Suzuki, Y. Protein thermostabilization by proline substitutions. J. Mol. Catal. B Enzym. 1998, 4, 167–180. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Z.; Wang, X.; Guo, S.; Yang, B.; Wang, Y. Sequence-based proline incorporation improves the thermostability of Candida albicans lipase Lip5. Eur. J. Lipid Sci. Technol. 2015, 118, 821–826. [Google Scholar] [CrossRef]
- Lan, D.; Zhao, G.; Holzmann, N.; Yuan, S.; Wang, J.; Wang, Y. Structure-Guided Rational Design of a Mono- and Diacylglycerol Lipase from Aspergillus oryzae: A Single Residue Mutant Increases the Hydrolysis Ability. J. Agric. Food Chem. 2021, 69, 5344–5352. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef]
- Kumar, M.; Mukherjee, J.; Sinha, M.; Kaur, P.; Sharma, S.; Gupta, M.N.; Singh, T.P. Enhancement of stability of a lipase by subjecting to three phase partitioning (TPP): Structures of native and TPP-treated lipase from Thermomyces lanuginosa. Sustain. Chem. Process. 2015, 3, 14. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Savage, H.F.J.; Verma, C.S.; Turkenburg, J.P.; Lawson, D.M.; Svendsen, A.; Patkar, S.A. Structural origins of the interfacial activation in Thermomyces (Humicola) lanuginosa lipase. Biochemistry 2000, 39, 15071–15082. [Google Scholar] [CrossRef]
- Carugo, O.; Argos, P. Accessibility to internal cavities and ligand binding sites monitored by protein crystallographic thermal factors. Proteins Struct. Funct. Bioinform. 1998, 31, 201–213. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.A.; York, D.M.; Pedersen, L.G. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- van der Spoel, D.; van Maaren, P.J.; Larsson, P.; Tîmneanu, N. Thermodynamics of Hydrogen Bonding in Hydrophilic and Hydrophobic Media. J. Phys. Chem. B 2006, 110, 4393–4398. [Google Scholar] [CrossRef]

| Enzymes | TLL | K24S | D27N | D27R | P29S | A30P |
|---|---|---|---|---|---|---|
| Residual activity (%) | 41 ± 2 | 60.9 ± 0.5 | 46 ± 1 | 52 ± 2 | 54 ± 3 | 65 ± 3 |
| Tm (°C) | 93.2 ± 0.4 | 91.8 ± 0.1 | 91.4 ± 0.3 | 94.2 ± 0.2 | 95.5 ± 0.2 | 98.3 ± 0.4 |
| Enzymes | Vmax (µmol/min/mg) | Km (mM) | kcat (/s) | kcat/Km (/s/mM) |
|---|---|---|---|---|
| TLL | 402 ± 2 | 0.22 ± 0.05 | 594 ± 4 | 2722 ± 54 |
| K24S | 681 ± 11 | 0.31 ± 0.02 | 1379 ± 22 | 4485 ± 157 |
| D27N | 396 ± 2 | 0.22 ± 0.02 | 777 ± 5 | 3503 ± 31 |
| D27R | 828 ± 9 | 0.36 ± 0.01 | 1895 ± 21 | 5245 ± 118 |
| P29S | 668 ± 5 | 0.29 ± 0.03 | 1354 ± 9 | 4747 ± 28 |
| A30P | 503 ± 2 | 0.24 ± 0.02 | 1036 ± 4 | 4383 ± 42 |
| Primers | Primer Sequences |
|---|---|
| K24S-forward | CATACTGCGGATCAAACAATGATG |
| K24S-reverse | TTGATCCGCAGTATGCGGC |
| D27N-forward | TGCGGAAAAAACAATAATGCCCCAGCT |
| D27N-reverse | CATTATTGTTTTTTCCGCAGTATGCGGCTG |
| D27R-forward | TGCGGAAAAAACAATCGTGCCCCAGCT |
| D27R-reverse | CACGATTGTTTTTTCCGCAGTATGCGGCTG |
| P29S-forward | AAACAATGATGCCTCAGCTGGTA |
| P29S-reverse | CTGAGGCATCATTGTTTTTTCCGC |
| A30P-forward | AACAATGATGCCCCACCTGGTACAAACA |
| A30P-reverse | CAGGTGGGGCATCATTGTTTTTTCCGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.; Zhu, E.; Xiong, D.; Wen, Y.; Xing, Y.; Yue, L.; He, S.; Han, N.; Huang, Z. Improving the Thermostability of Thermomyces lanuginosus Lipase by Restricting the Flexibility of N-Terminus and C-Terminus Simultaneously via the 25-Loop Substitutions. Int. J. Mol. Sci. 2023, 24, 16562. https://doi.org/10.3390/ijms242316562
Xiang X, Zhu E, Xiong D, Wen Y, Xing Y, Yue L, He S, Han N, Huang Z. Improving the Thermostability of Thermomyces lanuginosus Lipase by Restricting the Flexibility of N-Terminus and C-Terminus Simultaneously via the 25-Loop Substitutions. International Journal of Molecular Sciences. 2023; 24(23):16562. https://doi.org/10.3390/ijms242316562
Chicago/Turabian StyleXiang, Xia, Enheng Zhu, Diao Xiong, Yin Wen, Yu Xing, Lirong Yue, Shuang He, Nanyu Han, and Zunxi Huang. 2023. "Improving the Thermostability of Thermomyces lanuginosus Lipase by Restricting the Flexibility of N-Terminus and C-Terminus Simultaneously via the 25-Loop Substitutions" International Journal of Molecular Sciences 24, no. 23: 16562. https://doi.org/10.3390/ijms242316562
APA StyleXiang, X., Zhu, E., Xiong, D., Wen, Y., Xing, Y., Yue, L., He, S., Han, N., & Huang, Z. (2023). Improving the Thermostability of Thermomyces lanuginosus Lipase by Restricting the Flexibility of N-Terminus and C-Terminus Simultaneously via the 25-Loop Substitutions. International Journal of Molecular Sciences, 24(23), 16562. https://doi.org/10.3390/ijms242316562




