Plasmonic and Photothermal Effects of CuS Nanoparticles Biosynthesized from Acid Mine Drainage with Potential Drug Delivery Applications
Abstract
:1. Introduction
2. Results and Discussion
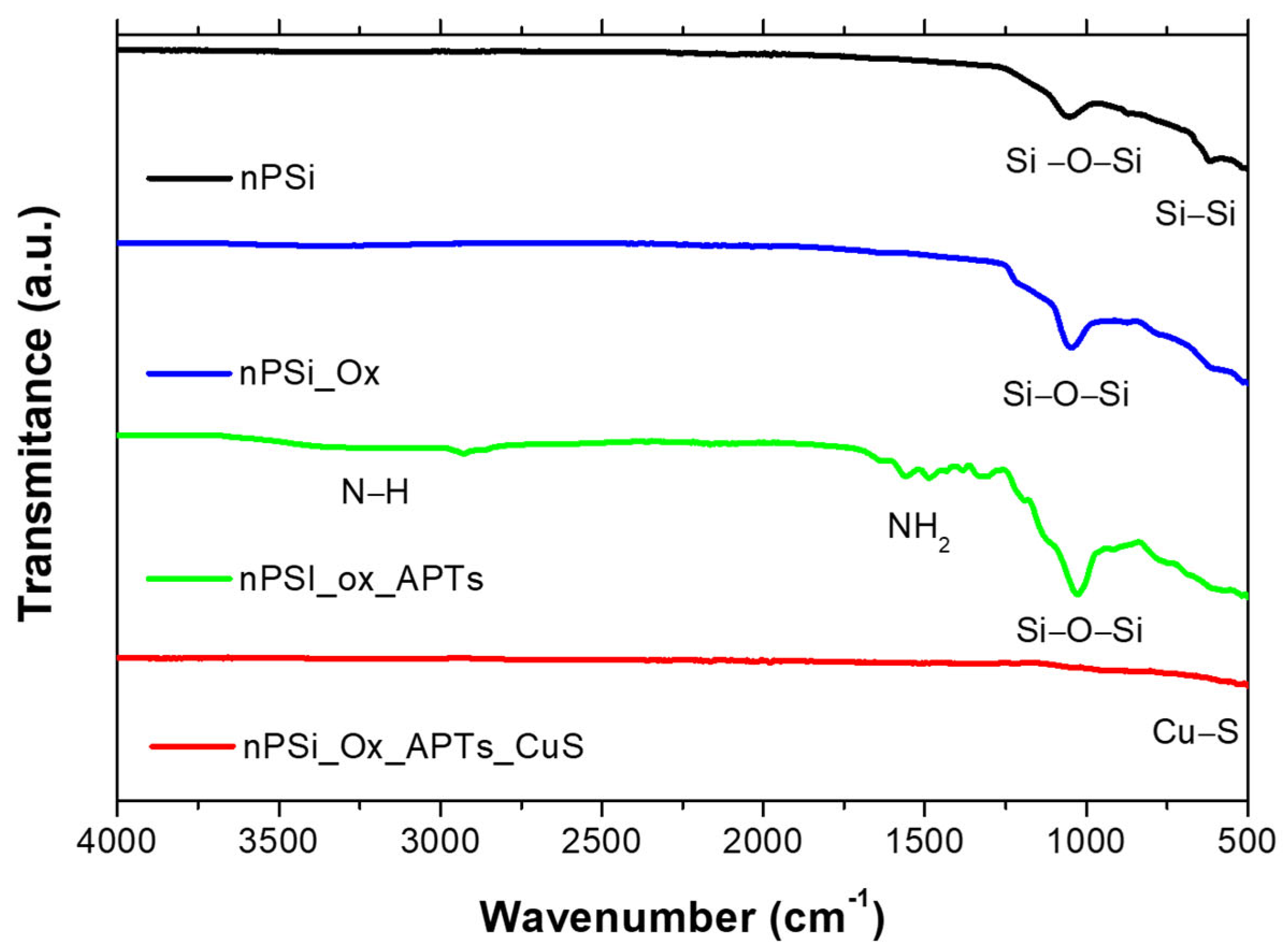
2.1. Physicochemical Characterization of CuS Nanoparticles
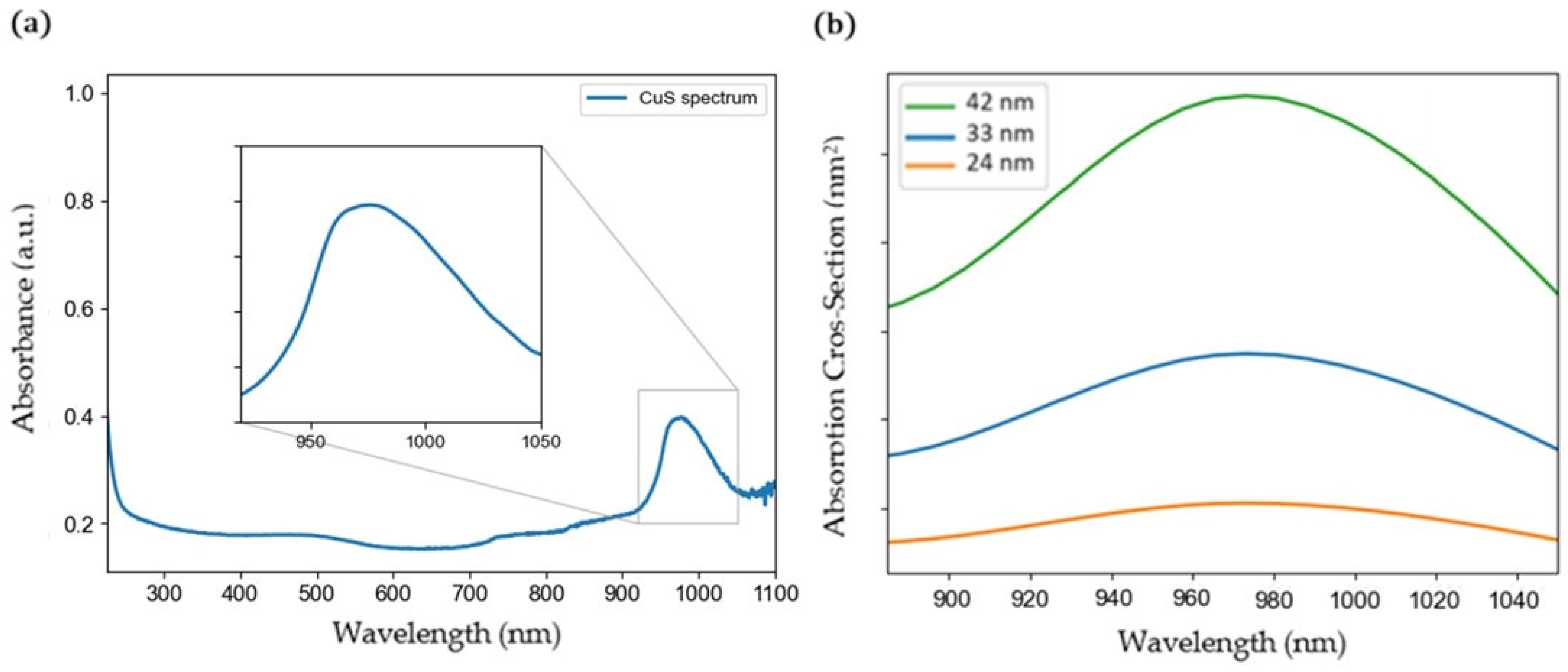
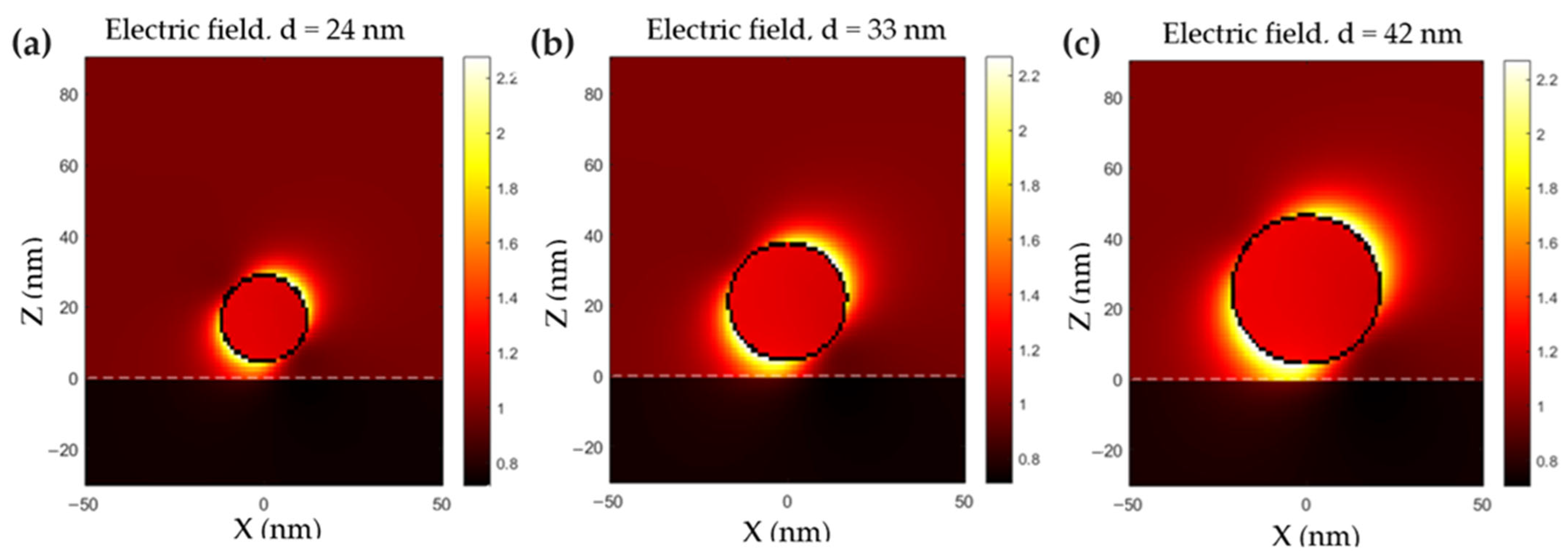
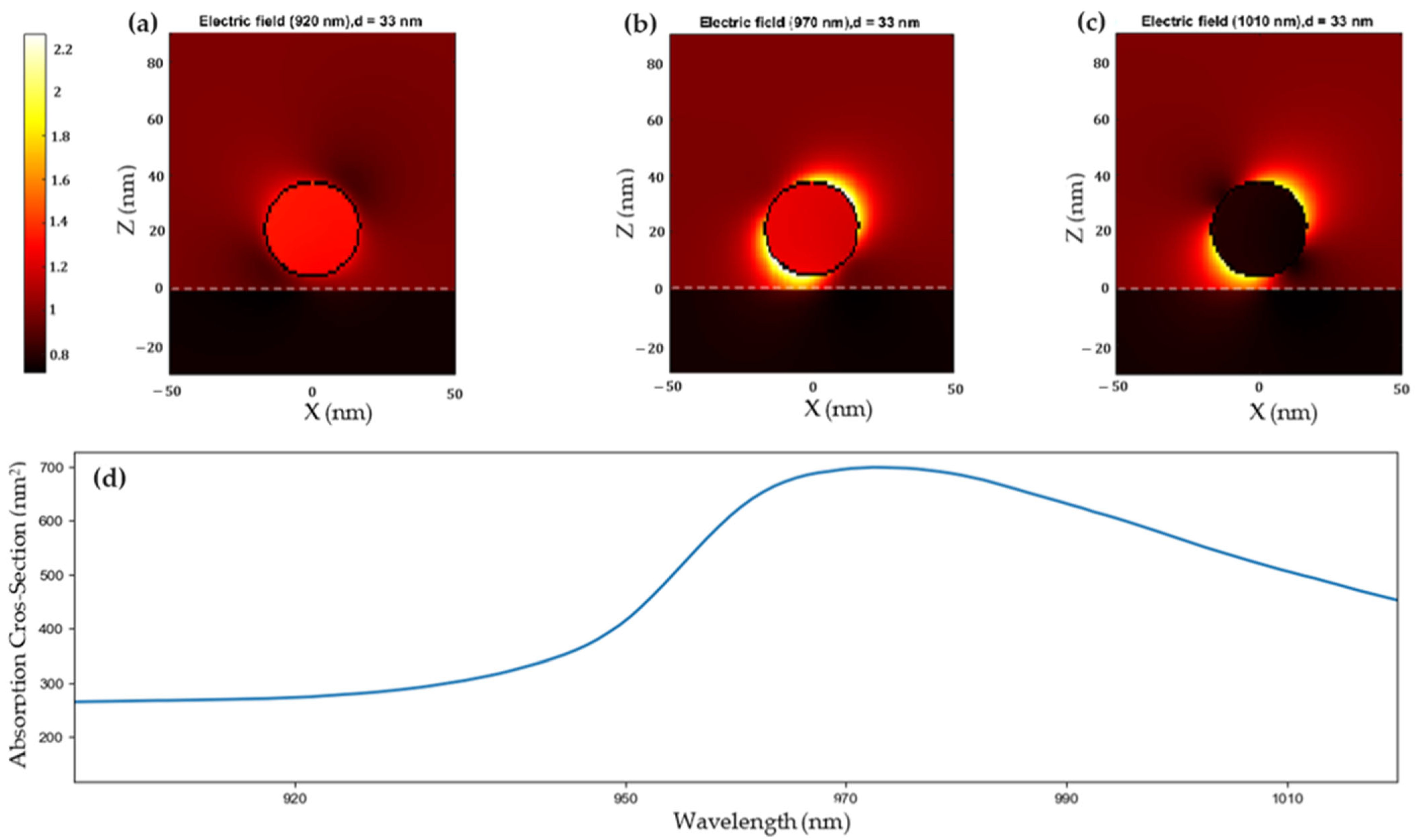
2.2. Plasmonic Properties
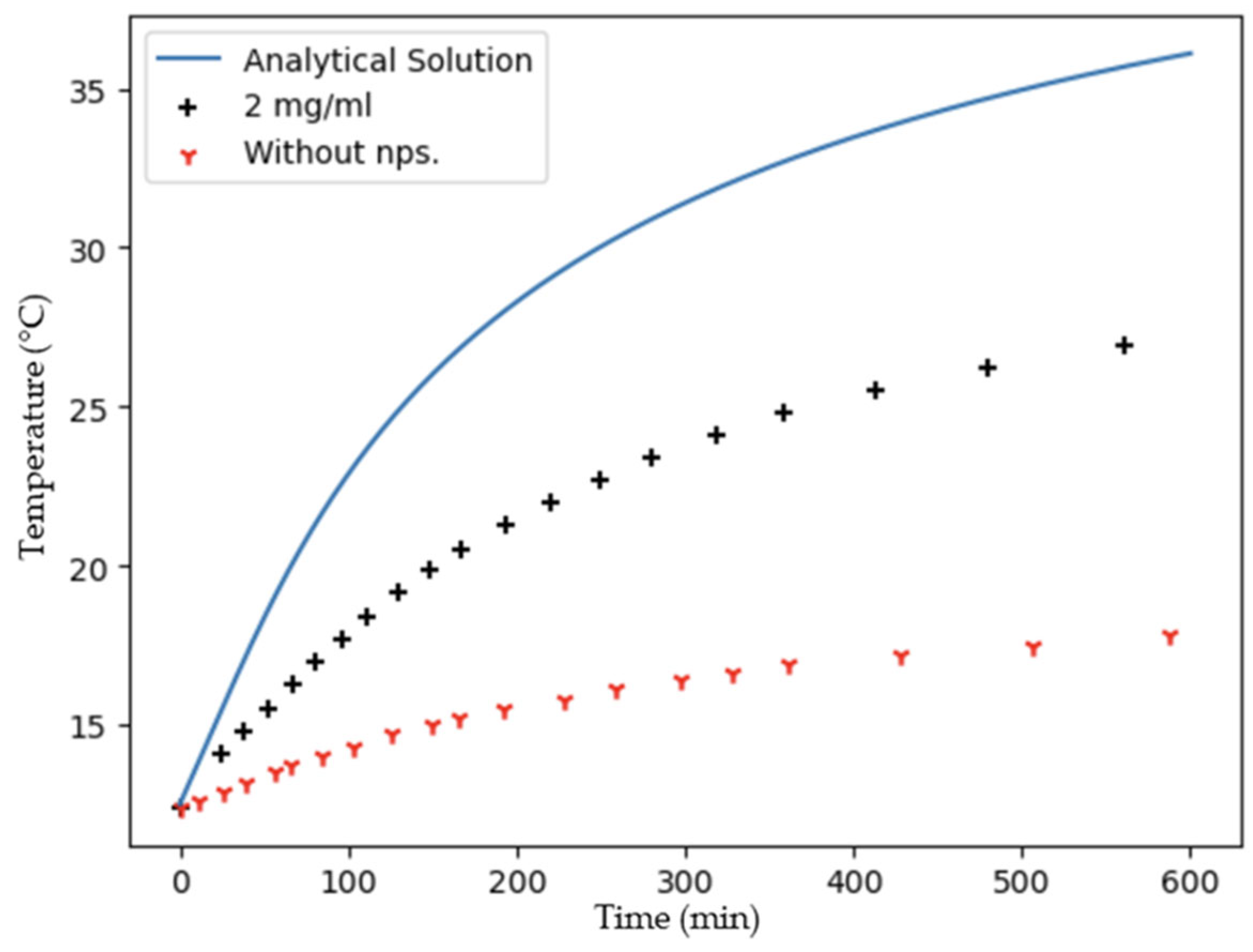
2.3. Photothermal Effect
2.4. Cytotoxic Assays
2.5. Drug Delivery Experiments
3. Materials and Methods
3.1. Materials
3.2. Biosynthesis of CuS Nanoparticles
3.3. Synthesis of Hybrid Platform nPSi–CuS
3.4. Characterization Techniques
3.5. Drug Delivery Test
3.6. Cell Culture and Cytotoxicity Assays
3.7. Simulations
3.7.1. Localized Surface Plasmon Resonance
3.7.2. Temperature Elevation of Nanoparticles via NIR Illumination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, R.; Lifante, J.; Besteiro, L.V.; Wang, Z.; Govorov, A.O.; Rivero, F.; Alfonso, F.; Sanz-Rodríguez, F.; Jaque, D. Plasmonic Copper Sulfide Nanoparticles Enable Dark Contrast in Optical Coherence Tomography. Adv. Healthc. Mater. 2020, 9, 1901627. [Google Scholar] [CrossRef] [PubMed]
- ul Ain, N.; Abdul Nasir, J.; Khan, Z.; Butler, I.S.; Rehman, Z. Copper Sulfide Nanostructures: Synthesis and Biological Applications. RSC Adv. 2022, 12, 7550–7567. [Google Scholar] [CrossRef] [PubMed]
- Mageshwari, K.; Mali, S.S.; Hemalatha, T.; Sathyamoorthy, R.; Patil, P.S. Low Temperature Growth of CuS Nanoparticles by Reflux Condensation Method. Prog. Solid State Chem. 2011, 39, 108–113. [Google Scholar] [CrossRef]
- Casaca, A.; Lopes, E.B.; Gonçalves, A.P.; Almeida, M. Electrical Transport Properties of CuS Single Crystals. J. Phys. Condens. Matter 2012, 24, 015701. [Google Scholar] [CrossRef] [PubMed]
- Gainov, R.R.; Dooglav, A.V.; Pen’kov, I.N.; Mukhamedshin, I.R.; Mozgova, N.N.; Evlampiev, I.A.; Bryzgalov, I.A. Phase Transition and Anomalous Electronic Behavior in the Layered Superconductor CuS Probed by NQR. Phys. Rev. B 2009, 79, 075115. [Google Scholar] [CrossRef]
- Morales-García, A.; Soares, A.L.; Dos Santos, E.C.; de Abreu, H.A.; Duarte, H.A. First-Principles Calculations and Electron Density Topological Analysis of Covellite (CuS). J. Phys. Chem. A 2014, 118, 5823–5831. [Google Scholar] [CrossRef]
- Tirado, J.; Roldán-Carmona, C.; Muñoz-Guerrero, F.A.; Bonilla-Arboleda, G.; Ralaiarisoa, M.; Grancini, G.; Queloz, V.I.E.; Koch, N.; Nazeeruddin, M.K.; Jaramillo, F. Copper Sulfide Nanoparticles as Hole-Transporting-Material in a Fully-Inorganic Blocking Layers n-i-p Perovskite Solar Cells: Application and Working Insights. Appl. Surf. Sci. 2019, 478, 607–614. [Google Scholar] [CrossRef]
- Singh, N.; Taunk, M. Structural, Optical, and Electrical Studies of Sonochemically Synthesized CuS Nanoparticles. Semiconductors 2020, 54, 1016–1022. [Google Scholar] [CrossRef]
- Shawky, A.; El-Sheikh, S.M.; Gaber, A.; El-Hout, S.I.; El-Sherbiny, I.M.; Ahmed, A.I. Urchin-like CuS Nanostructures: Simple Synthesis and Structural Optimization with Enhanced Photocatalytic Activity under Direct Sunlight. Appl. Nanosci. 2020, 10, 2153–2164. [Google Scholar] [CrossRef]
- Nabi, G.; Tanveer, M.; Tahir, M.B.; Kiran, M.; Rafique, M.; Khalid, N.R.; Alzaid, M.; Fatima, N.; Nawaz, T. Mixed Solvent Based Surface Modification of CuS Nanostructures for an Excellent Photocatalytic Application. Inorg. Chem. Commun. 2020, 121, 108205. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Jalil, A.T.; Saleh, M.M.; Wu, B. In Vivo and In Vitro Biocompatibility Study of CuS Nanoparticles: Photosensitizer for Glioblastoma Photothermal Therapy. Appl. Biochem. Biotechnol. 2023, 195, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, H.J.; Tian, X.M.; Tang, J.; Liu, L.F.; Liu, F.Y. Biocompatible Copper Sulfide–Based Nanocomposites for Artery Interventional Chemo-Photothermal Therapy of Orthotropic Hepatocellular Carcinoma. Mater. Today Bio. 2021, 12, 100128. [Google Scholar] [CrossRef]
- Saona, L.A.; Campo-Giraldo, J.L.; Anziani-Ostuni, G.; Órdenes-Aenishanslins, N.; Venegas, F.A.; Giordana, M.F.; Díaz, C.; Isaacs, M.; Bravo, D.; Pérez-Donoso, J.M. Cysteine-Mediated Green Synthesis of Copper Sulphide Nanoparticles: Biocompatibility Studies and Characterization as Counter Electrodes. Nanomaterials 2022, 12, 3194. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Prasad, R.; Pandey, P.K.; Khan, A.; Jain, N.K.; Jones, E.V.; Srivastava, R.; Misra, S.K. Doxorubicin Encapsulated Hollow Self-Assembled CuS Nanoparticles Clusters for Bio-Responsive Chemo-Photo Therapy. Mater. Lett. 2022, 327, 133017. [Google Scholar] [CrossRef]
- Peng, S.; He, Y.; Er, M.; Sheng, Y.; Gu, Y.; Chen, H. Biocompatible CuS-Based Nanoplatforms for Efficient Photothermal Therapy and Chemotherapy in Vivo. Biomater. Sci. 2017, 5, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Lim, G.-D.; Kim, S.; Ryu, S.H.; Lim, H.-R.; Choa, Y.-H. Effect of Localized Surface Plasmon Resonance on Dispersion Stability of Copper Sulfide Nanoparticles. Appl. Surf. Sci. 2019, 477, 204–210. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In Situ Synthesis of CuS Nanoparticle with a Distinguishable SPR Peak in NIR Region. J. Mater. Sci. Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, G.; Lu, L.; Wang, Z.; Xu, H.; Liu, S.; Wang, D. High-Performance Pseudo-Capacitor Energy Storage Device Based on a Hollow-Structured Copper Sulfide Nanoflower and Carbon Quantum Dot Nanocomposite. Electrochim. Acta 2020, 353, 136606. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. Electrochemical Hydrogen-Storage Performance of Copper Sulfide Micro-Hexagons. Int. J. Hydrogen Energy 2021, 46, 5530–5536. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.; Wang, X.; Sun, R. Quaternized Chitosan-Stabilized Copper Sulfide Nanoparticles for Cancer Therapy. Mater. Sci. Eng. C 2019, 96, 129–137. [Google Scholar] [CrossRef]
- Wan, X.; Liu, M.; Ma, M.; Chen, D.; Wu, N.; Li, L.; Li, Z.; Lin, G.; Wang, X.; Xu, G. The Ultrasmall Biocompatible CuS@BSA Nanoparticle and Its Photothermal Effects. Front. Pharmacol. 2019, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, H.; Chen, Y.; Gao, J.; Li, G. Plasmonic Cu2−x S Nanoparticles: A Brief Introduction of Optical Properties and Applications. Mater. Adv. 2021, 2, 907–926. [Google Scholar] [CrossRef]
- Wu, X.; Liu, K.; Huang, Q.; Zhang, Q.; Yang, X.; Liu, X.; Wang, R. Photothermal Therapy Based on CuS Nanoparticles for Alleviating Arterial Restenosis Induced by Mechanical Injury of Endovascular Treatment. Front. Mater. 2021, 7, 591281. [Google Scholar] [CrossRef]
- Mou, J.; Li, P.; Liu, C.; Xu, H.; Song, L.; Wang, J.; Zhang, K.; Chen, Y.; Shi, J.; Chen, H. Ultrasmall Cu2−x S Nanodots for Highly Efficient Photoacoustic Imaging-Guided Photothermal Therapy. Small 2015, 11, 2275–2283. [Google Scholar] [CrossRef]
- Mathew, S.A.; Prakash, P.A.; Jaabir, M.S.M.; Dhanavel, S.; Manikandan, R.; Stephen, A. Dopamine-Conjugated CuS/Chitosan Nanocomposite for Targeted Photothermal Drug Delivery: In Vitro Cytotoxicity Study to Establish Bio-Compatibility. J. Drug Deliv. Sci. Technol. 2021, 61, 102193. [Google Scholar] [CrossRef]
- Ding, H.; Yong, J.; Zhang, J.; Chen, B.; Ding, B.; Wang, X. Anchoring Pd Nanoparticles on Hollow CuS Nanoparticles for Enhanced NIR Induced Photothermal Effects for Chemotherapeutic Drug Delivery and Gastric Cancer Treatment. Ceram. Int. 2022, 48, 16085–16090. [Google Scholar] [CrossRef]
- Glover, T.G.; Melde, B.J.; Johnson, B.J. Nanoporous Silicas and Their Composites. In Nanoporous Materials for Molecule Separation and Conversion; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–140. [Google Scholar]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering Mesoporous Silica Nanoparticles for Drug Delivery: Where Are We after Two Decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montelongo, J.; Fernández-Fierro, C.; Benito-Gómez, N.; Romero-Sáez, M.; Parodi, J.; Carmona, E.R.; Recio-Sánchez, G. Hybrid Porous Silicon/Green Synthetized Ag Microparticles as Potential Carries for Ag Nanoparticles and Drug Delivery. Mater. Sci. Eng. C 2020, 116, 111183. [Google Scholar] [CrossRef]
- Yadav, S.; Shrivas, K.; Bajpai, P.K. Role of Precursors in Controlling the Size, Shape and Morphology in the Synthesis of Copper Sulfide Nanoparticles and Their Application for Fluorescence Detection. J. Alloys Compd. 2019, 772, 579–592. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, L.; Wu, H.; Hu, H.; Lin, H.; Qin, L.; Li, Q. Shape-Controlled Synthesis of CuS as a Fenton-like Photocatalyst with High Catalytic Performance and Stability. J. Alloys Compd. 2022, 896, 163045. [Google Scholar] [CrossRef]
- Arshad, M.; Wang, Z.; Nasir, J.A.; Amador, E.; Jin, M.; Li, H.; Chen, Z.; Rehman, Z.u.; Chen, W. Single Source Precursor Synthesized CuS Nanoparticles for NIR Phototherapy of Cancer and Photodegradation of Organic Carcinogen. J. Photochem. Photobiol. B 2021, 214, 112084. [Google Scholar] [CrossRef]
- Motaung, M.P.; Onwudiwe, D.C.; Wei, L.; Lou, C. CuS, In2S3 and CuInS2 Nanoparticles by Microwave-Assisted Solvothermal Route and Their Electrochemical Studies. J. Phys. Chem. Solids 2022, 160, 110319. [Google Scholar] [CrossRef]
- Tetyana, P.; Mphuthi, N.; Jijana, A.N.; Moloto, N.; Shumbula, P.M.; Skepu, A.; Vilakazi, L.S.; Sikhwivhilu, L. Synthesis, Characterization, and Electrochemical Evaluation of Copper Sulfide Nanoparticles and Their Application for Non-Enzymatic Glucose Detection in Blood Samples. Nanomaterials 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Pejjai, B.; Reddivari, M.; Kotte, T.R.R. Phase Controllable Synthesis of CuS Nanoparticles by Chemical Co-Precipitation Method: Effect of Copper Precursors on the Properties of CuS. Mater. Chem. Phys. 2020, 239, 122030. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef]
- Nancucheo, I.; Segura, A.; Hernández, P.; Hernández-Montelongo, J.; Pesenti, H.; Arranz, A.; Benito, N.; Romero-Sáez, M.; Contreras, B.; Díaz, V.; et al. Covellite Nanoparticles with High Photocatalytic Activity Bioproduced by Using H2S Generated from a Sulfidogenic Bioreactor. J. Environ. Chem. Eng. 2022, 10, 107409. [Google Scholar] [CrossRef]
- Singovszka, E.; Balintova, M.; Junakova, N. The Impact of Heavy Metals in Water from Abandoned Mine on Human Health. SN Appl. Sci. 2020, 2, 934. [Google Scholar] [CrossRef]
- Yan, S.U.; Cheng, K.Y.; Morris, C.; Douglas, G.; Ginige, M.P.; Zheng, G.; Zhou, L.; Kaksonen, A.H. Sequential Hydrotalcite Precipitation and Biological Sulfate Reduction for Acid Mine Drainage Treatment. Chemosphere 2020, 252, 126570. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Lima, R.M.F.; Leão, V.A. Mine Water Treatment with Limestone for Sulfate Removal. J. Hazard. Mater. 2012, 221, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Suárez, J.I.; Aybar, M.; Nancucheo, I.; Maríinez, P.; Rittmann, B.E. A Membrane-Biofilm System for Sulfate Conversion to Elemental Sulfur in Mining-Influenced Waters. Sci. Total Environ. 2020, 740, 140088. [Google Scholar] [CrossRef]
- Virpiranta, H.; Sotaniemi, V.-H.; Leiviskä, T.; Taskila, S.; Rämö, J.; Johnson, D.B.; Tanskanen, J. Continuous Removal of Sulfate and Metals from Acidic Mining-Impacted Waters at Low Temperature Using a Sulfate-Reducing Bacterial Consortium. Chem. Eng. J. 2022, 427, 132050. [Google Scholar]
- Silva, P.M.P.; Lucheta, A.R.; Bitencourt, J.A.P.; Carmo, A.L.V.d.; Cuevas, I.P.Ñ.; Siqueira, J.O.; de Oliveira, G.C.; Alves, J.O. Covellite (CuS) Production from a Real Acid Mine Drainage Treated with Biogenic H2S. Metals 2019, 9, 206. [Google Scholar] [CrossRef]
- Nancucheo, I.; Segura, A.; Hernández, P.; Canales, C.; Benito, N.; Arranz, A.; Romero-Sáez, M.; Recio-Sánchez, G. Bio-Recovery of CuS Nanoparticles from the Treatment of Acid Mine Drainage with Potential Photocatalytic and Antibacterial Applications. Sci. Total Environ. 2023, 902, 166194. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.d.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.B.; Abdel-Kader, M.H.; Almarashi, J.Q.M. Role of Cu/S Ratio and Mg Doping on Modification of Structural and Optical Characteristics of Nano CuS. Int. J. Appl. Ceram. Technol. 2020, 17, 832–840. [Google Scholar] [CrossRef]
- Deb, S.; Kalita, P.K. Green Synthesis of Copper Sulfide (CuS) Nanostructures for Heterojunction Diode Applications. J. Mater. Sci. Mater. Electron. 2021, 32, 24125–24137. [Google Scholar] [CrossRef]
- Hussein, O.N.; AL-Jawad, S.M.H.; Imran, N.J. Efficient Antibacterial Activity Enhancement in Fe/Mn Co-Doped CuS Nanoflowers and Nanosponges. Bull. Mater. Sci. 2023, 46, 139. [Google Scholar] [CrossRef]
- Al-Jawad, S.M.H.; Taha, A.A.; Muhsen, M.M. Preparation and Characterization of CuS Nanoparticles Prepared by Two-Phase Colloidal Method. J. Phys. Conf. Ser. 2021, 1795, 012053. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Sanchez-Mora, E.; Pal, U.; Paraguay-Delgado, F.; Mathew, X. Synthesis of CuS Nanoparticles by a Wet Chemical Route and Their Photocatalytic Activity. J. Nanoparticle Res. 2015, 17, 301. [Google Scholar] [CrossRef]
- Riedinger, A.; Avellini, T.; Curcio, A.; Asti, M.; Xie, Y.; Tu, R.; Marras, S.; Lorenzoni, A.; Rubagotti, S.; Iori, M.; et al. Post-Synthesis Incorporation of 64Cu in CuS Nanocrystals to Radiolabel Photothermal Probes: A Feasible Approach for Clinics. J. Am. Chem. Soc. 2015, 137, 15145–15151. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Nam, J. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shen, L.; Tian, L.; Zhao, F.; Yin, J.; Shao, Y. Excitation of Quantum-Sized (CuS)n Clusters for NIR-II Photothermal Application: Insights from Experiment and DFT Calculation. J. Alloys Compd. 2023, 941, 169005. [Google Scholar] [CrossRef]
- Feng, W.; Nie, W.; Cheng, Y.; Zhou, X.; Chen, L.; Qiu, K.; Chen, Z.; Zhu, M.; He, C. In Vitro and in Vivo Toxicity Studies of Copper Sulfide Nanoplates for Potential Photothermal Applications. Nanomedicine 2015, 11, 901–912. [Google Scholar] [CrossRef]
- Jia, Q.; Li, D.; Zhang, Q.; Ye, S.; Xi, Z.; Wang, X.; Shan, W.; Ren, L. Biomineralization Synthesis of HBc-CuS Nanoparticles for near-Infrared Light-Guided Photothermal Therapy. J. Mater. Sci. 2019, 54, 13255–13264. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, W.; Gao, C.; Feng, L.; Wu, J.; Zhao, L. Chiral CuS Nanoparticles and Their Photothermal Properties. CrystEngComm 2022, 24, 4955–4961. [Google Scholar] [CrossRef]
- Pérez-San Martín, A.; Uribe, K.; Hernández-Montelongo, J.; Naveas, N.; Manso-Silván, M.; Oyarzún, P.; Díaz-García, V.; Contreras, B.; Recio-Sánchez, G. Antibacterial Activity and Kinetic Release of Laureliopsis Philippiana (Looser) Essential Oil from Nanostructured Porous Silicon with Surface-Functionalization Alternatives. Appl. Sci. 2022, 12, 8258. [Google Scholar] [CrossRef]
- Rodriguez, C.; Muñoz Noval, A.; Torres-Costa, V.; Ceccone, G.; Manso Silván, M. Visible Light Assisted Organosilane Assembly on Mesoporous Silicon Films and Particles. Materials 2019, 12, 131. [Google Scholar] [CrossRef]
- Azarshin, S.; Moghadasi, J.; A Aboosadi, Z. Surface Functionalization of Silica Nanoparticles to Improve the Performance of Water Flooding in Oil Wet Reservoirs. Energy Explor. Exploit. 2017, 35, 685–697. [Google Scholar] [CrossRef]
- Naveas, N.; Pulido, R.; Torres-Costa, V.; Agulló-Rueda, F.; Santibáñez, M.; Malano, F.; Recio-Sánchez, G.; Garrido-Miranda, K.A.; Manso-Silván, M.; Hernández-Montelongo, J. Antibacterial Films of Silver Nanoparticles Embedded into Carboxymethylcellulose/Chitosan Multilayers on Nanoporous Silicon: A Layer-by-Layer Assembly Approach Comparing Dip and Spin Coating. Int. J. Mol. Sci. 2023, 24, 10595. [Google Scholar] [CrossRef]
- Belay, A.; Kim, H.K.; Hwang, Y. Probing the Interaction of Caffeic Acid with ZnO Nanoparticles. Luminescence 2016, 31, 654–659. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Salehpour, F.; Cassano, P.; Rouhi, N.; Hamblin, M.R.; De Taboada, L.; Farajdokht, F.; Mahmoudi, J. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul Photomed. Laser Surg. 2019, 37, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Oyarzo, D.; Plaza, T.; Recio-Sánchez, G.; Abdalla, D.S.P.; Salazar, L.A.; Hernández-Montelongo, J. Use of NPSi-ΒCD Composite Microparticles for the Controlled Release of Caffeic Acid and Pinocembrin, Two Main Polyphenolic Compounds Found in a Chilean Propolis. Pharmaceutics 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Plane Waves and Planar Interfaces. In Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 537–572.
- De Abajo, F.J.G.; Howie, A. Retarded Field Calculation of Electron Energy Loss in Inhomogeneous Dielectrics. Phys. Rev. B 2002, 65, 115418. [Google Scholar] [CrossRef]
- Jackson, J.D. Electrodynamics, Classical. In Digital Encyclopedia of Applied Physics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Hutchings, D.C.; Sheik-Bahae, M.; Hagan, D.J.; Van Stryland, E.W. Kramers-Krönig Relations in Nonlinear Optics. Opt. Quantum Electron. 1992, 24, 1–30. [Google Scholar] [CrossRef]
- Norton, S.J.; Vo-Dinh, T. Photothermal Effects of Plasmonic Metal Nanoparticles in a Fluid. J. Appl. Phys. 2016, 119, 83105. [Google Scholar] [CrossRef]
- Norton, S.J.; Vo-Dinh, T. Nanoparticle-Mediated Heating: A Theoretical Study for Photothermal Treatment and Photo Immunotherapy. In Nanoparticle-Mediated Immunotherapy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 89–114. [Google Scholar]
- Świsłocka, R. Spectroscopic (FT-IR, FT-Raman, UV Absorption, 1H and 13C NMR) and Theoretical (in B3LYP/6-311++G** Level) Studies on Alkali Metal Salts of Caffeic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 100, 21–30. [Google Scholar] [CrossRef]
- Tosovic, J. Spectroscopic Features of Caffeic Acid: Theoretical Study. Kragujev. J. Sci. 2017, 99–108. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/Caffeic Acid Hybrid Materials: Synthesis, Spectroscopic Characterization, and Bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]




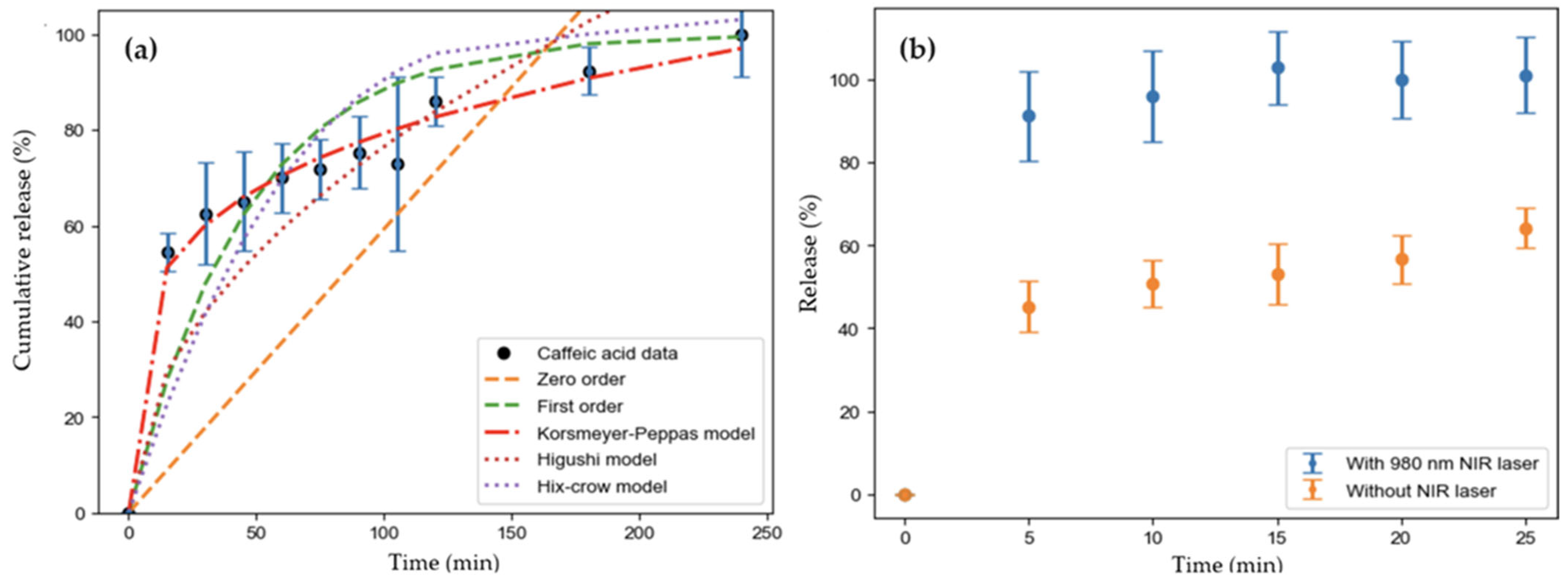
| Model | Parameters | R2adj. | |
|---|---|---|---|
| K | n | ||
| Zero order | 0.59 ± 0.02 | 0.493 | |
| First order | 0.021 ± 0.003 | 0.728 | |
| Korsmeyer–Peppas | 27 ± 2 | 0.23 ± 0.01 | 0.985 |
| Higuchi | 7.6 ± 0.2 | 0.728 | |
| Hixson–Crowell | 0.0054 ± 0.0006 | 0.673 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Sánchez, H.; Carril Pardo, C.; Benito, N.; Hernández-Montelongo, J.; Nancucheo, I.; Recio-Sánchez, G. Plasmonic and Photothermal Effects of CuS Nanoparticles Biosynthesized from Acid Mine Drainage with Potential Drug Delivery Applications. Int. J. Mol. Sci. 2023, 24, 16489. https://doi.org/10.3390/ijms242216489
Escobar-Sánchez H, Carril Pardo C, Benito N, Hernández-Montelongo J, Nancucheo I, Recio-Sánchez G. Plasmonic and Photothermal Effects of CuS Nanoparticles Biosynthesized from Acid Mine Drainage with Potential Drug Delivery Applications. International Journal of Molecular Sciences. 2023; 24(22):16489. https://doi.org/10.3390/ijms242216489
Chicago/Turabian StyleEscobar-Sánchez, Hernán, Claudio Carril Pardo, Noelia Benito, Jacobo Hernández-Montelongo, Iván Nancucheo, and Gonzalo Recio-Sánchez. 2023. "Plasmonic and Photothermal Effects of CuS Nanoparticles Biosynthesized from Acid Mine Drainage with Potential Drug Delivery Applications" International Journal of Molecular Sciences 24, no. 22: 16489. https://doi.org/10.3390/ijms242216489
APA StyleEscobar-Sánchez, H., Carril Pardo, C., Benito, N., Hernández-Montelongo, J., Nancucheo, I., & Recio-Sánchez, G. (2023). Plasmonic and Photothermal Effects of CuS Nanoparticles Biosynthesized from Acid Mine Drainage with Potential Drug Delivery Applications. International Journal of Molecular Sciences, 24(22), 16489. https://doi.org/10.3390/ijms242216489







