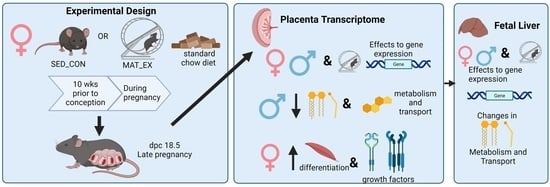
Maternal Exercise Prior to and during Gestation Induces Sex-Specific Alterations in the Mouse Placenta
Abstract
:1. Introduction
2. Results
2.1. Maternal Wheel Running Measurements and Phenotypic Characteristics
2.2. Placenta Characteristics and Fetal Weights
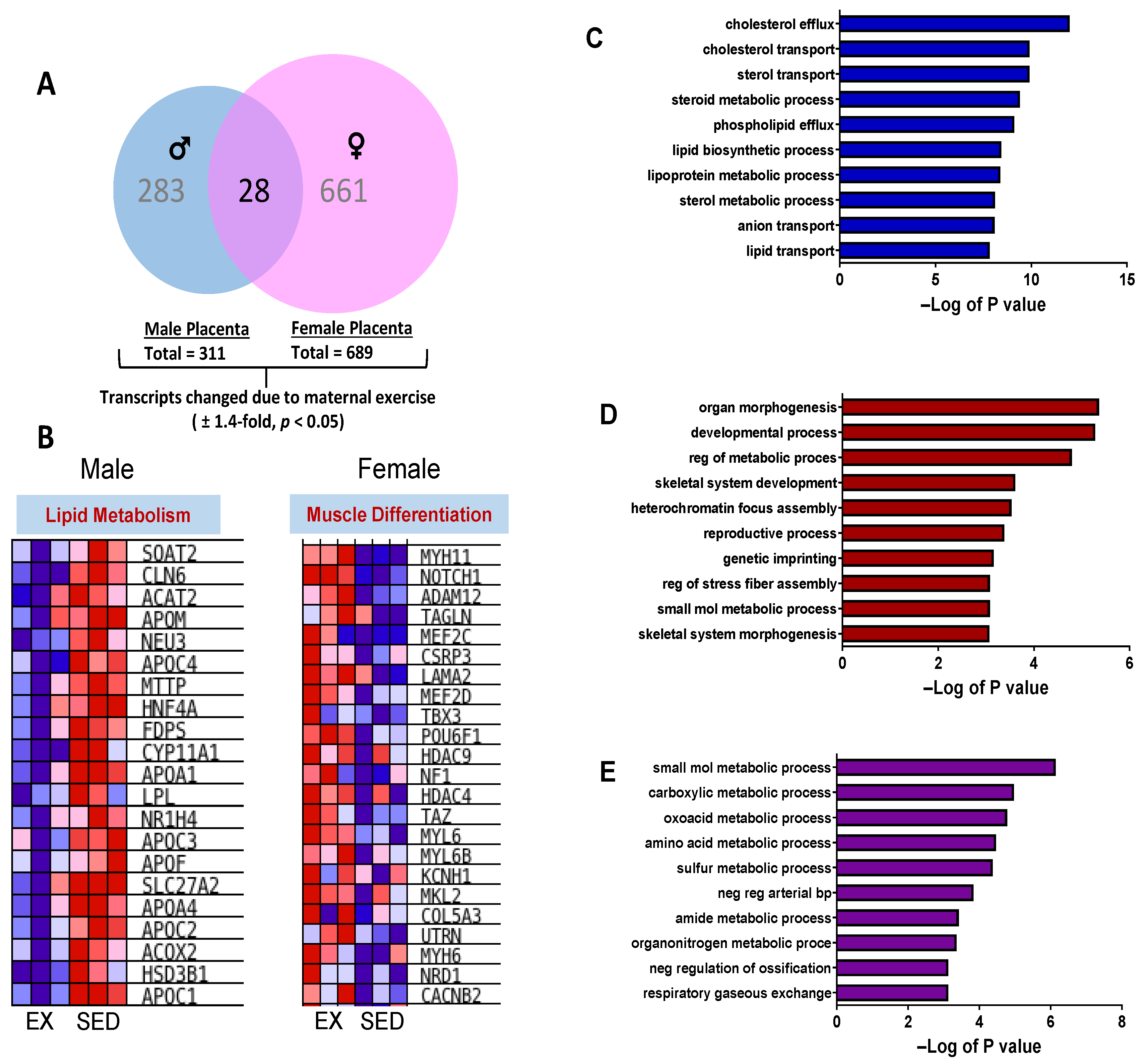
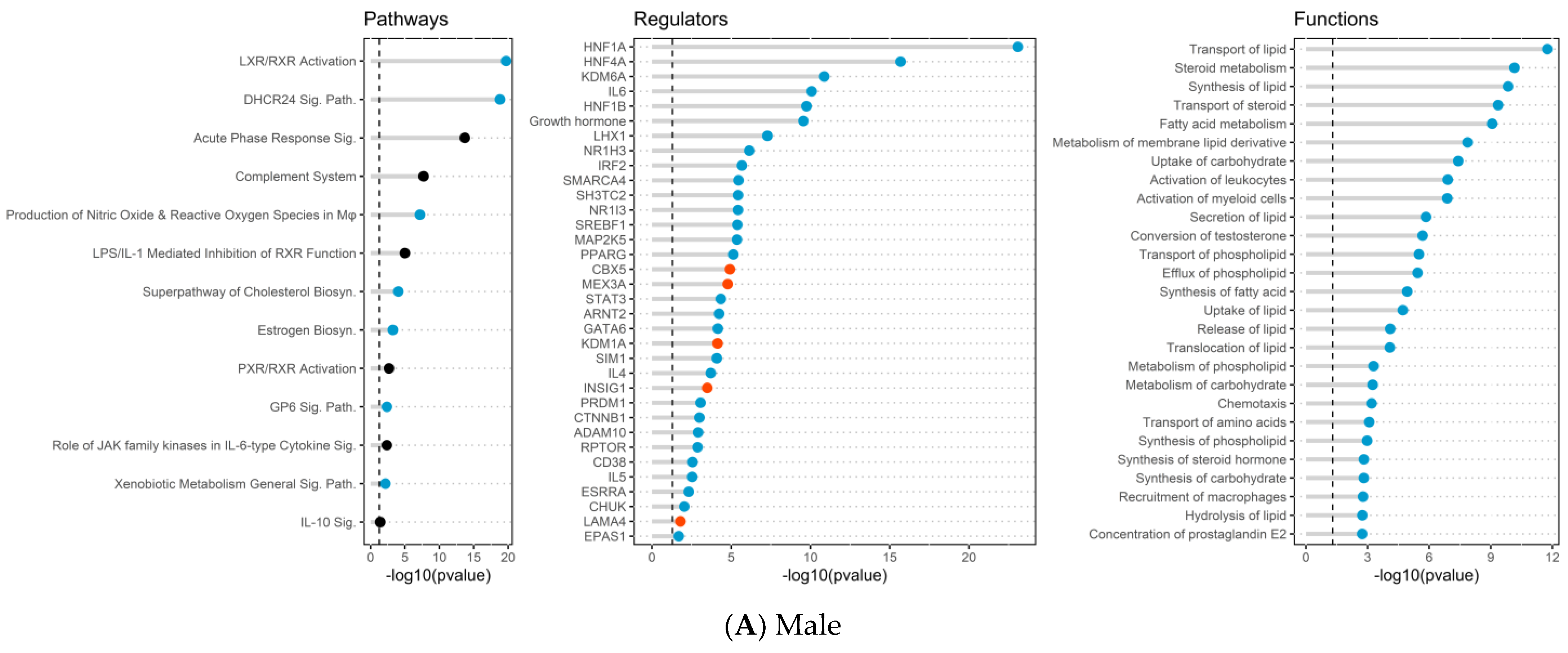
2.3. Placenta Microarray, GSEA, and IPA Results
2.4. Placental mRNA Expression of Lipid Transport and Developmental-Related Genes
2.5. Fetal Liver mRNA Expression of Lipid Metabolism and Transport Genes
3. Discussion
4. Materials and Methods
4.1. Animals and Chemicals
4.2. Experimental Protocol
4.3. Microarray Analysis
4.4. Real-Time Quantitative RT-PCR (qPCR)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholas, L.M.; Morrison, J.L.; Rattanatray, L.; Zhang, S.; Ozanne, S.E.; McMillen, I.C. The early origins of obesity and insulin resistance: Timing, programming and mechanisms. Int. J. Obes. 2016, 40, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lim, I.Y.; Wu, Y.; Teh, A.L.; Chen, L.; Aris, I.M.; Soh, S.E.; Tint, M.T.; MacIsaac, J.L.; Morin, A.M.; et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med. 2017, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Barisione, M.; Carlini, F.; Gradaschi, R.; Camerini, G.; Adami, G.F. Body weight at developmental age in siblings born to mothers before and after surgically induced weight loss. Surg. Obes. Relat. Dis. 2012, 8, 387–391. [Google Scholar] [CrossRef]
- Smith, J.; Cianflone, K.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G.; et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Kral, J.G.; Biron, S.; Simard, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Marceau, P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006, 118, e1644–e1649. [Google Scholar] [CrossRef]
- Clapp, M.A.; Bernstein, S.N. Preconception Counseling for Women With Cardiac Disease. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 67. [Google Scholar] [CrossRef]
- Vohr, B.R.; Poggi Davis, E.; Wanke, C.A.; Krebs, N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 2017, 139 (Suppl. S1), S38–S49. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Garces, A.; Goudar, S.S.; Kodkany, B.S.; Pasha, O.; Tshefu, A.; Bose, C.L.; Figueroa, L.; et al. Preconception maternal nutrition: A multi-site randomized controlled trial. BMC Pregnancy Childbirth 2014, 14, 111. [Google Scholar] [CrossRef]
- Huang, L.; Fan, L.; Ding, P.; He, Y.H.; Xie, C.; Niu, Z.; Tian, F.Y.; Yuan, S.X.; Jia, D.Q.; Chen, W.Q. Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J. Matern. Fetal Neonatal Med. 2017, 32, 109–116. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.A.; Romundstad, P.R.; Eggebo, T.M.; Carlsen, S.M.; Morkved, S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obs. Gynecol. 2012, 119, 29–36. [Google Scholar] [CrossRef]
- Di Mascio, D.; Magro-Malosso, E.R.; Saccone, G.; Marhefka, G.D.; Berghella, V. Exercise during pregnancy in normal-weight women and risk of preterm birth: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obs. Gynecol. 2016, 215, 561–571. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obs. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef]
- Kusuyama, J.; Alves-Wagner, A.B.; Makarewicz, N.S.; Goodyear, L.J. Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab. 2020, 2, 858–872. [Google Scholar] [CrossRef]
- McPherson, N.O.; Lane, M.; Sandeman, L.; Owens, J.A.; Fullston, T. An Exercise-Only Intervention in Obese Fathers Restores Glucose and Insulin Regulation in Conjunction with the Rescue of Pancreatic Islet Cell Morphology and MicroRNA Expression in Male Offspring. Nutrients 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, M.N.M.; Simmons, D.; Devlieger, R.; van Assche, F.A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: The DALI randomised controlled trial. Diabetologia 2019, 62, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., 3rd; Lopez, B.; Harcar-Sevcik, R. Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. Obs. Gynecol. 1999, 180, 91–94. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lee, M.Y.; Getchell, K.M.; So, K.; Hirshman, M.F.; Goodyear, L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015, 64, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Takahashi, H.; So, K.; Alves-Wagner, A.B.; Prince, N.B.; Lehnig, A.C.; Getchell, K.M.; Lee, M.Y.; Hirshman, M.F.; Goodyear, L.J. Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes 2017, 66, 2124–2136. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Moore, M.P.; Meers, G.M.; Ruegsegger, G.N.; Booth, F.W.; Rector, R.S. Maternal Physical Activity and Sex Impact Markers of Hepatic Mitochondrial Health. Med. Sci. Sports Exerc. 2018, 50, 2040–2048. [Google Scholar] [CrossRef]
- Herring, A.; Donath, A.; Yarmolenko, M.; Uslar, E.; Conzen, C.; Kanakis, D.; Bosma, C.; Worm, K.; Paulus, W.; Keyvani, K. Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring. FASEB J. 2012, 26, 117–128. [Google Scholar] [CrossRef]
- Sheldon, R.D.; Nicole Blaize, A.; Fletcher, J.A.; Pearson, K.J.; Donkin, S.S.; Newcomer, S.C.; Rector, R.S. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J. Hepatol. 2016, 64, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Saavedra, D.; Markunas, C.; Takahashi, H.; Baer, L.A.; Harris, J.E.; Hirshman, M.F.; Ilkayeva, O.; Newgard, C.B.; Stanford, K.I.; Goodyear, L.J. Maternal Exercise and Paternal Exercise Induce Distinct Metabolite Signatures in Offspring Tissues. Diabetes 2022, 71, 2094–2105. [Google Scholar] [CrossRef]
- Zheng, J.; Alves-Wagner, A.B.; Stanford, K.I.; Prince, N.B.; So, K.; Mul, J.D.; Dirice, E.; Hirshman, M.F.; Kulkarni, R.N.; Goodyear, L.J. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res. Care 2020, 8, e000890. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; Qi, N.R.; De Cabo, R.; Pearson, K.J. Maternal exercise improves insulin sensitivity in mature rat offspring. Med. Sci. Sports Exerc. 2013, 45, 832–840. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Gascoin, G.; Musial, B.; Carr, S.; Duque-Guimaraes, D.; Blackmore, H.L.; Alfaradhi, M.Z.; Loche, E.; Sferruzzi-Perri, A.N.; Fowden, A.L.; et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 2017, 7, 44650. [Google Scholar] [CrossRef]
- Vega, C.C.; Reyes-Castro, L.A.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int. J. Obes. 2015, 39, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Kaur, G.; Falcao-Tebas, F.; Wadley, G.D.; Wlodek, M.E.; Laker, R.C.; Ebeling, P.R.; McConell, G.K. Exercise as an intervention to improve metabolic outcomes after intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E999–E1012. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obs. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Kolahi, K.; Pierce, M.; Valent, A.; Drake, R.; Louey, S. Biological features of placental programming. Placenta 2016, 48 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Dumolt, J.H.; Powell, T.L.; Jansson, T. Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction. Obs. Gynecol. Clin. North. Am. 2021, 48, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Velez, R.; Bustamante, J.; Czerniczyniec, A.; Aguilar de Plata, A.C.; Lores-Arnaiz, S. Effect of exercise training on eNOS expression, NO production and oxygen metabolism in human placenta. PLoS ONE 2013, 8, e80225. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef]
- Kusuyama, J.; Alves-Wagner, A.B.; Conlin, R.H.; Makarewicz, N.S.; Albertson, B.G.; Prince, N.B.; Kobayashi, S.; Kozuka, C.; Moller, M.; Bjerre, M.; et al. Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab. 2021, 33, 939–956.e938. [Google Scholar] [CrossRef]
- Diaz, P.; Powell, T.L.; Jansson, T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol. Reprod. 2014, 91, 82. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Beetch, M.; Alejandro, E.U. Placental mTOR Signaling and Sexual Dimorphism in Metabolic Health across the Lifespan of Offspring. Children 2021, 8, 970. [Google Scholar] [CrossRef]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef]
- Braun, A.E.; Mitchel, O.R.; Gonzalez, T.L.; Sun, T.; Flowers, A.E.; Pisarska, M.D.; Winn, V.D. Sex at the interface: The origin and impact of sex differences in the developing human placenta. Biol. Sex. Differ. 2022, 13, 50. [Google Scholar] [CrossRef]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Breuer, S.; Hoffmann, T.; Vohlen, C.; Janoschek, R.; Schmitz, L.; Appel, S.; Fink, G.; Hunseler, C.; Quaas, A.; et al. Maternal Exercise Mediates Hepatic Metabolic Programming via Activation of AMPK-PGC1alpha Axis in the Offspring of Obese Mothers. Cells 2021, 10, 1247. [Google Scholar] [CrossRef] [PubMed]
- McCoin, C.S.; Von Schulze, A.; Allen, J.; Fuller, K.N.Z.; Xia, Q.; Koestler, D.C.; Houchen, C.J.; Maurer, A.; Dorn, G.W., 2nd; Shankar, K.; et al. Sex modulates hepatic mitochondrial adaptations to high-fat diet and physical activity. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E298–E311. [Google Scholar] [CrossRef] [PubMed]
- Platt, K.M.; Przybylowski, J.; Charnigo, R.J.; Ngo Tenlep, S.Y.; Reynolds, L.J.; Pearson, K.J. Effects of maternal controlled exercise on offspring adiposity and glucose tolerance. J. Dev. Orig. Health Dis. 2022, 13, 455–462. [Google Scholar] [CrossRef]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-induced maternal obesity impacts feto-placental growth and induces sex-specific alterations in placental morphology, mitochondrial bioenergetics, dynamics, lipid metabolism and oxidative stress in mice. Acta. Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef]
- Holcomb, L.E.; Rowe, P.; O’Neill, C.C.; DeWitt, E.A.; Kolwicz, S.C., Jr. Sex differences in endurance exercise capacity and skeletal muscle lipid metabolism in mice. Physiol. Rep. 2022, 10, e15174. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E898–E907. [Google Scholar] [CrossRef]
- Quadro, L.; Giordano, E.; Costabile, B.K.; Nargis, T.; Iqbal, J.; Kim, Y.; Wassef, L.; Hussain, M.M. Interplay between beta-carotene and lipoprotein metabolism at the maternal-fetal barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158591. [Google Scholar] [CrossRef]
- Costabile, B.K.; Kim, Y.K.; Iqbal, J.; Zuccaro, M.V.; Wassef, L.; Narayanasamy, S.; Curley, R.W., Jr.; Harrison, E.H.; Hussain, M.M.; Quadro, L. beta-Apo-10’-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact beta-Carotene to the Embryo. J. Biol. Chem. 2016, 291, 18525–18535. [Google Scholar] [CrossRef]
- Nargis, T.; Lin, X.; Giordano, E.; Ijaz, L.; Suhail, S.; Gurzenda, E.M.; Kiefer, D.; Quadro, L.; Hanna, N.; Hussain, M.M. Characterization of lipoproteins in human placenta and fetal circulation as well as gestational changes in lipoprotein assembly and secretion in human and mouse placentas. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159357. [Google Scholar] [CrossRef]
- Brass, E.; Hanson, E.; O’Tierney-Ginn, P.F. Placental oleic acid uptake is lower in male offspring of obese women. Placenta 2013, 34, 503–509. [Google Scholar] [CrossRef]
- Sedlmeier, E.M.; Brunner, S.; Much, D.; Pagel, P.; Ulbrich, S.E.; Meyer, H.H.; Amann-Gassner, U.; Hauner, H.; Bader, B.L. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genom. 2014, 15, 941. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Maliqueo, M.; Benrick, A.; Johansson, J.; Shao, R.; Hou, L.; Jansson, T.; Wu, X.; Stener-Victorin, E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1373–E1385. [Google Scholar] [CrossRef]
- Lakisic, G.; Lebreton, A.; Pourpre, R.; Wendling, O.; Libertini, E.; Radford, E.J.; Le Guillou, M.; Champy, M.F.; Wattenhofer-Donze, M.; Soubigou, G.; et al. Role of the BAHD1 Chromatin-Repressive Complex in Placental Development and Regulation of Steroid Metabolism. PLoS Genet. 2016, 12, e1005898. [Google Scholar] [CrossRef]
- Rinn, J.L.; Snyder, M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Selva, D.M.; Hogeveen, K.N.; Innis, S.M.; Hammond, G.L. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J. Clin. Investig. 2007, 117, 3979–3987. [Google Scholar] [CrossRef]
- Wang, S.H.; Yeh, S.H.; Lin, W.H.; Yeh, K.H.; Yuan, Q.; Xia, N.S.; Chen, D.S.; Chen, P.J. Estrogen receptor alpha represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4alpha. Gastroenterology 2012, 142, 989–998.e984. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, Q.; Zhou, S.; Wilbur, R.R.; Li, X. Roles of apolipoprotein E (ApoE) and inducible nitric oxide synthase (iNOS) in inflammation and apoptosis in preeclampsia pathogenesis and progression. PLoS ONE 2013, 8, e58168. [Google Scholar] [CrossRef] [PubMed]
- Anelli, G.M.; Mando, C.; Letizia, T.; Mazzocco, M.I.; Novielli, C.; Lisso, F.; Personeni, C.; Vago, T.; Cetin, I. Placental ESRRG-CYP19A1 Expressions and Circulating 17-Beta Estradiol in IUGR Pregnancies. Front. Pediatr. 2019, 7, 154. [Google Scholar] [CrossRef]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; de Avila, J.M.; Zhu, M.J.; Du, M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef]
- Oliveira, A.O.; Fileto, C.; Melis, M.S. Effect of strenuous maternal exercise before and during pregnancy on rat progeny renal function. Braz. J. Med. Biol. Res. 2004, 37, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.E.; Lean, S.; Sibley, C.P.; Jones, R.L.; Wareing, M.; Greenwood, S.L.; Dilworth, M.R. Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio? Front. Physiol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.A.; Son, J.S.; Du, M. Prenatal exercise in fetal development: A placental perspective. FEBS J. 2022, 289, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Chuva de Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFbeta Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules 2020, 10, 453. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Zhou, Z.; Ying, C.; Zhou, X.; Shi, Y.; Xu, J.; Zhu, Y.; Wang, M.; Li, Y.; Li, X.; Xiang, J. Aerobic exercise training alleviates renal injury in db/db mice through inhibiting Nox4-mediated NLRP3 inflammasome activation. Exp. Gerontol. 2022, 168, 111934. [Google Scholar] [CrossRef]
- Dreher, S.I.; Hockele, S.; Huypens, P.; Irmler, M.; Hoffmann, C.; Jeske, T.; Hastreiter, M.; Moller, A.; Birkenfeld, A.L.; Haring, H.U.; et al. TGF-beta Induction of miR-143/145 Is Associated to Exercise Response by Influencing Differentiation and Insulin Signaling Molecules in Human Skeletal Muscle. Cells 2021, 10, 3443. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Mohammad, S.; Goudreau, A.D.; Adamo, K.B. Physical activity differentially regulates VEGF, PlGF, and their receptors in the human placenta. Physiol. Rep. 2021, 9, e14710. [Google Scholar] [CrossRef]
- Wits, M.; Becher, C.; de Man, F.; Sanchez-Duffhues, G.; Goumans, M.J. Sex-biased TGFbeta signaling in pulmonary arterial hypertension. Cardiovasc. Res. 2023, 119, 2262–2277. [Google Scholar] [CrossRef]
- Connors, L.T.; Zhu, H.L.; Gill, M.; Walsh, E.; Singh, R.D.; Easson, S.; Ahmed, S.B.; Habibi, H.R.; Cole, W.C.; Thompson, J.A. Prenatal exposure to a low dose of BPS causes sex-dependent alterations to vascular endothelial function in adult offspring. Front. Toxicol. 2022, 4, 933572. [Google Scholar] [CrossRef]
- Boonpattrawong, N.P.; Golbidi, S.; Tai, D.C.; Aleliunas, R.E.; Bernatchez, P.; Miller, J.W.; Laher, I.; Devlin, A.M. Exercise during pregnancy mitigates the adverse effects of maternal obesity on adult male offspring vascular function and alters one-carbon metabolism. Physiol. Rep. 2020, 8, e14582. [Google Scholar] [CrossRef] [PubMed]
- Hozer, R.M.; Dos Santos, B.G.; August, P.M.; Rodrigues, K.S.; Maurmann, R.M.; Flores, E.B.; Matte, C. Maternal exercise during pregnancy modulates mitochondrial function and redox status in a sex-dependent way in adult offspring’s skeletal muscle. J. Dev. Orig. Health Dis. 2022, 13, 204–211. [Google Scholar] [CrossRef]
- Ma, W.; Fang, H.; Pease, N.; Filippova, G.N.; Disteche, C.M.; Berletch, J.B. Sex-biased and parental allele-specific gene regulation by KDM6A. Biol. Sex. Differ. 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Davegardh, C.; Hall Wedin, E.; Broholm, C.; Henriksen, T.I.; Pedersen, M.; Pedersen, B.K.; Scheele, C.; Ling, C. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Res. Ther. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Alvarez, P.; Rizos, D.; Rath, D.; Lonergan, P.; Gutierrez-Adan, A. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol. Genom. 2008, 32, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Galetzka, D.; Weis, E.; Tralau, T.; Seidmann, L.; Haaf, T. Sex-specific windows for high mRNA expression of DNA methyltransferases 1 and 3A and methyl-CpG-binding domain proteins 2 and 4 in human fetal gonads. Mol. Reprod. Dev. 2007, 74, 233–241. [Google Scholar] [CrossRef]
- He, B.; Yin, C.; Gong, Y.; Liu, J.; Guo, H.; Zhao, R. Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J. Cell Physiol. 2018, 233, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Krueger, C.; Mellado-Lopez, M.; Hemberger, M.; Dean, W.; Perez-Garcia, V.; Hanna, C.W. Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B. Nat. Commun. 2023, 14, 371. [Google Scholar] [CrossRef]
- Yamagata, Y.; Asada, H.; Tamura, I.; Lee, L.; Maekawa, R.; Taniguchi, K.; Taketani, T.; Matsuoka, A.; Tamura, H.; Sugino, N. DNA methyltransferase expression in the human endometrium: Down-regulation by progesterone and estrogen. Hum. Reprod. 2009, 24, 1126–1132. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, L.; Frick, K.M. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. USA 2010, 107, 5605–5610. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127 (Suppl. S5), 838S–841S. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Shankar, K.; Zhong, Y.; Kang, P.; Lau, F.; Blackburn, M.L.; Chen, J.R.; Borengasser, S.J.; Ronis, M.J.; Badger, T.M. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 2011, 152, 4158–4170. [Google Scholar] [CrossRef]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]






| SED | EX | |
|---|---|---|
| Body wt (g) | 24.2 ± 1.1 | 24.9 ± 0.4 |
| Liver wt (g) | 1.7 ± 0.09 | 1.9 ± 0.04 |
| % Liver wt | 7.3 ± 0.04 | 7.7 ± 0.01 |
| Fat pad wt (g) | 0.7 ± 0.05 | 0.9 ± 0.12 |
| % Fat wt | 2.8 ± 0.02 | 3.0 ± 0.01 |
| Total Litters | 8 | 7 |
| Total Pups/Litter | 7.1 ± 0.3 | 8.3 ± 0.5 |
| Male:Female Ratio | 2.6 ± 0.7 | 1.8 ± 0.7 |
| Insulin (ng/mL) | 1.5 ± 0.2 | 1.7 ± 0.2 |
| Glucose (mmol/L) | 13.8 ± 0.8 | 13.7 ± 0.4 |
| Triglycerides (mg/dL) | 50.7 ± 2.2 | * 61.1 ± 3.3 |
| NEFA (meq/L) | 0.39 ± 0.09 | 0.42 ± 0.09 |
| Cholesterol (mg/dL) | 56.2 ± 4.5 | 70.2 ± 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruebel, M.L.; Borengasser, S.J.; Zhong, Y.; Kang, P.; Faske, J.; Shankar, K. Maternal Exercise Prior to and during Gestation Induces Sex-Specific Alterations in the Mouse Placenta. Int. J. Mol. Sci. 2023, 24, 16441. https://doi.org/10.3390/ijms242216441
Ruebel ML, Borengasser SJ, Zhong Y, Kang P, Faske J, Shankar K. Maternal Exercise Prior to and during Gestation Induces Sex-Specific Alterations in the Mouse Placenta. International Journal of Molecular Sciences. 2023; 24(22):16441. https://doi.org/10.3390/ijms242216441
Chicago/Turabian StyleRuebel, Meghan L., Sarah J. Borengasser, Ying Zhong, Ping Kang, Jennifer Faske, and Kartik Shankar. 2023. "Maternal Exercise Prior to and during Gestation Induces Sex-Specific Alterations in the Mouse Placenta" International Journal of Molecular Sciences 24, no. 22: 16441. https://doi.org/10.3390/ijms242216441
APA StyleRuebel, M. L., Borengasser, S. J., Zhong, Y., Kang, P., Faske, J., & Shankar, K. (2023). Maternal Exercise Prior to and during Gestation Induces Sex-Specific Alterations in the Mouse Placenta. International Journal of Molecular Sciences, 24(22), 16441. https://doi.org/10.3390/ijms242216441