Poncirus trifoliata (L.) Raf. Seed Extract Induces Cell Cycle Arrest and Apoptosis in the Androgen Receptor Positive LNCaP Prostate Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Profile of P. trifoliata Seed Extract
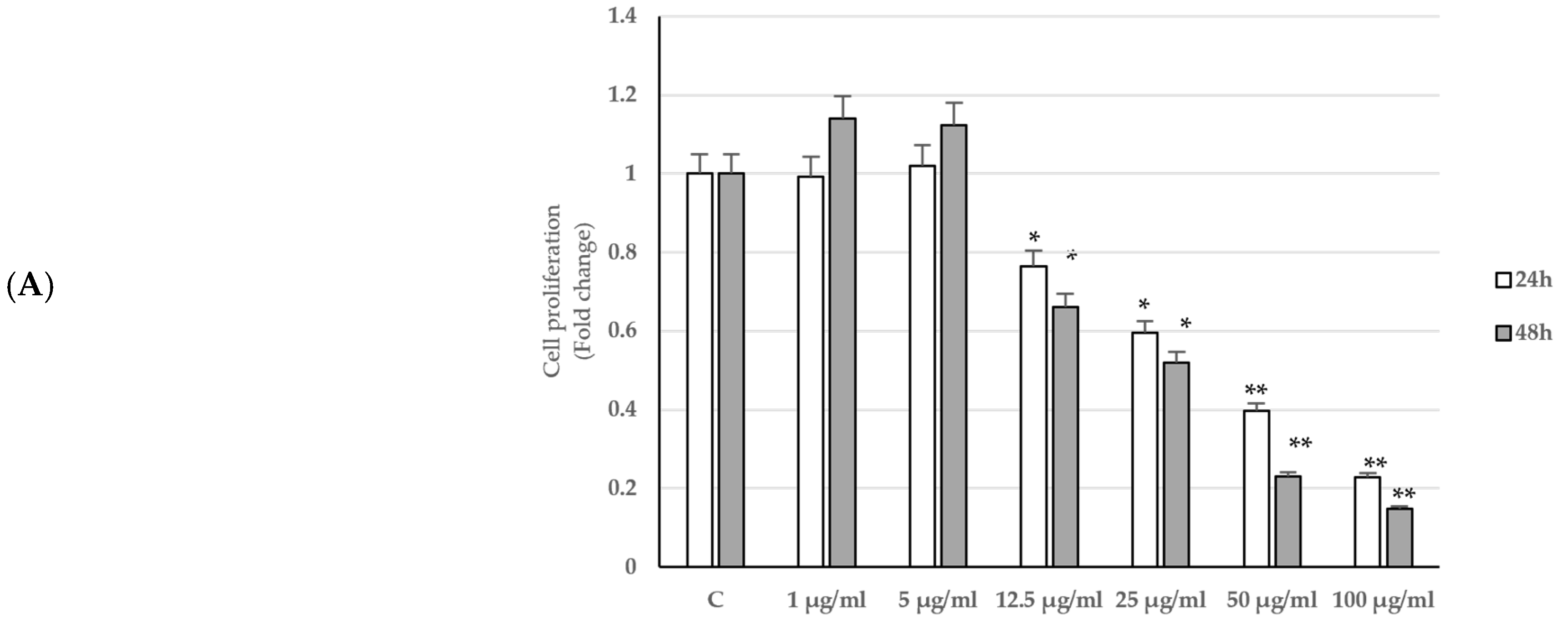
2.2. Effects of P. trifoliata on Proliferation Rate and Morphological Changes of LNCaP Cells
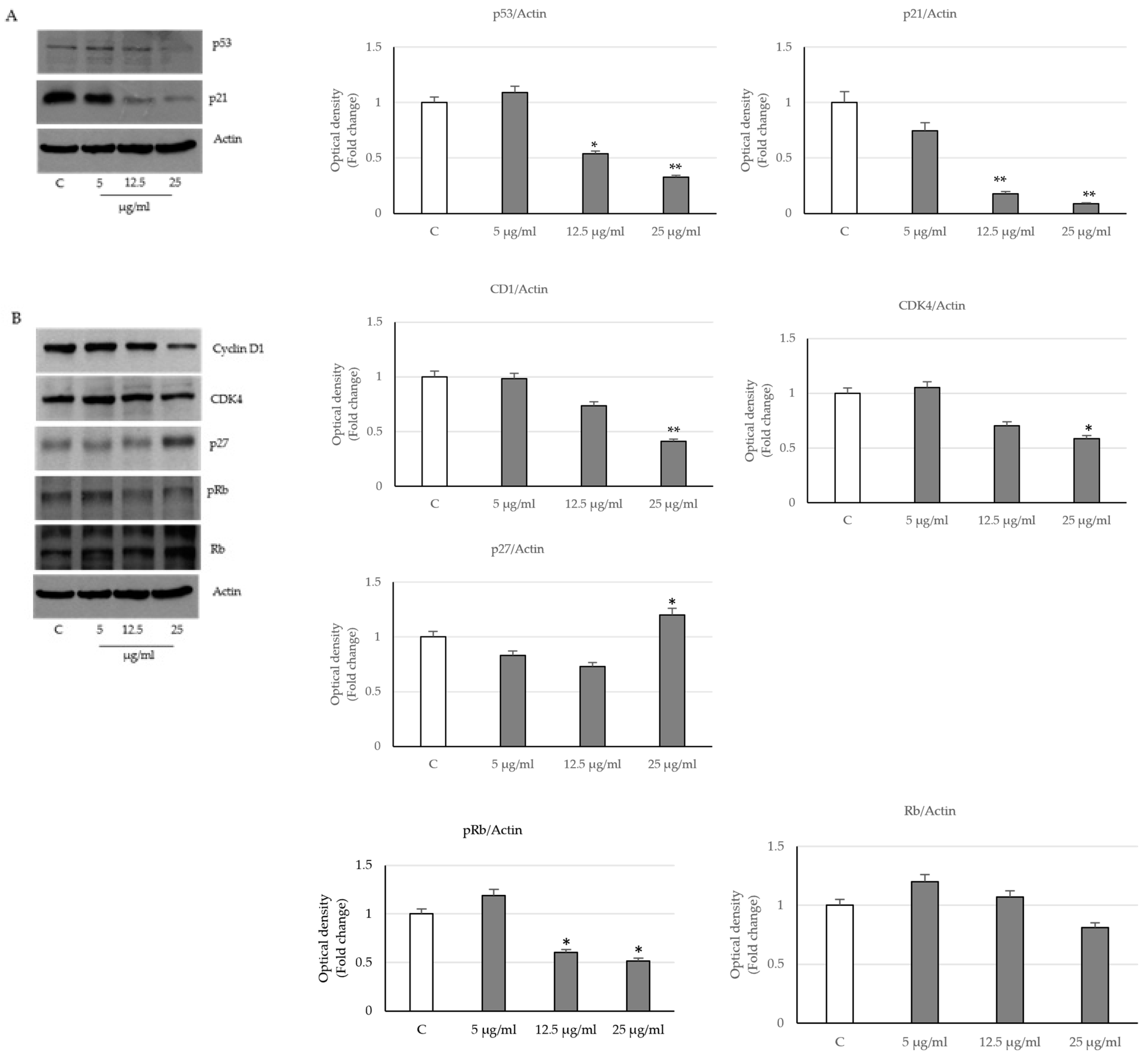
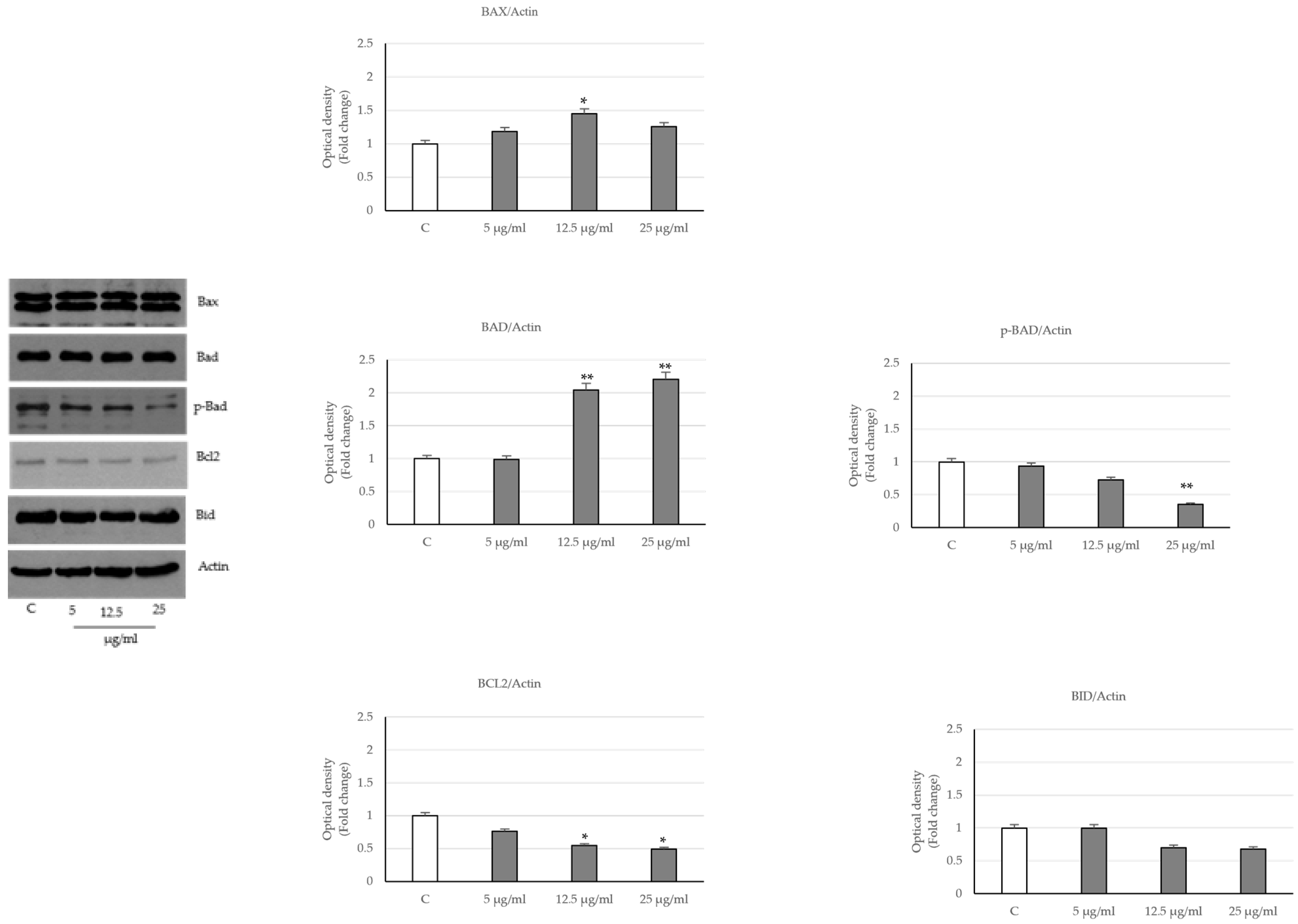
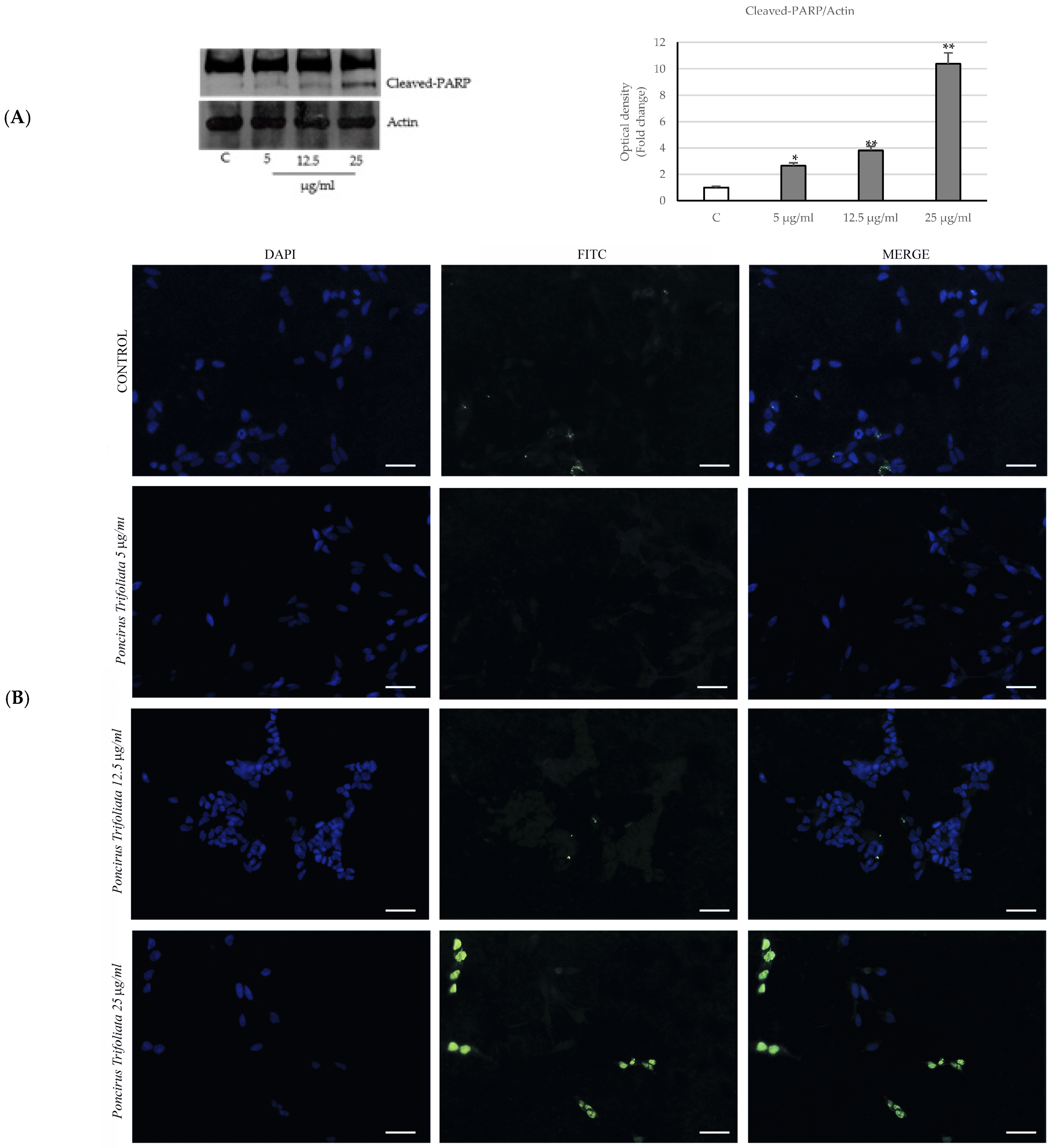
2.3. Activation of the Intrinsic Apoptosis Pathway following Seed Extract Treatment
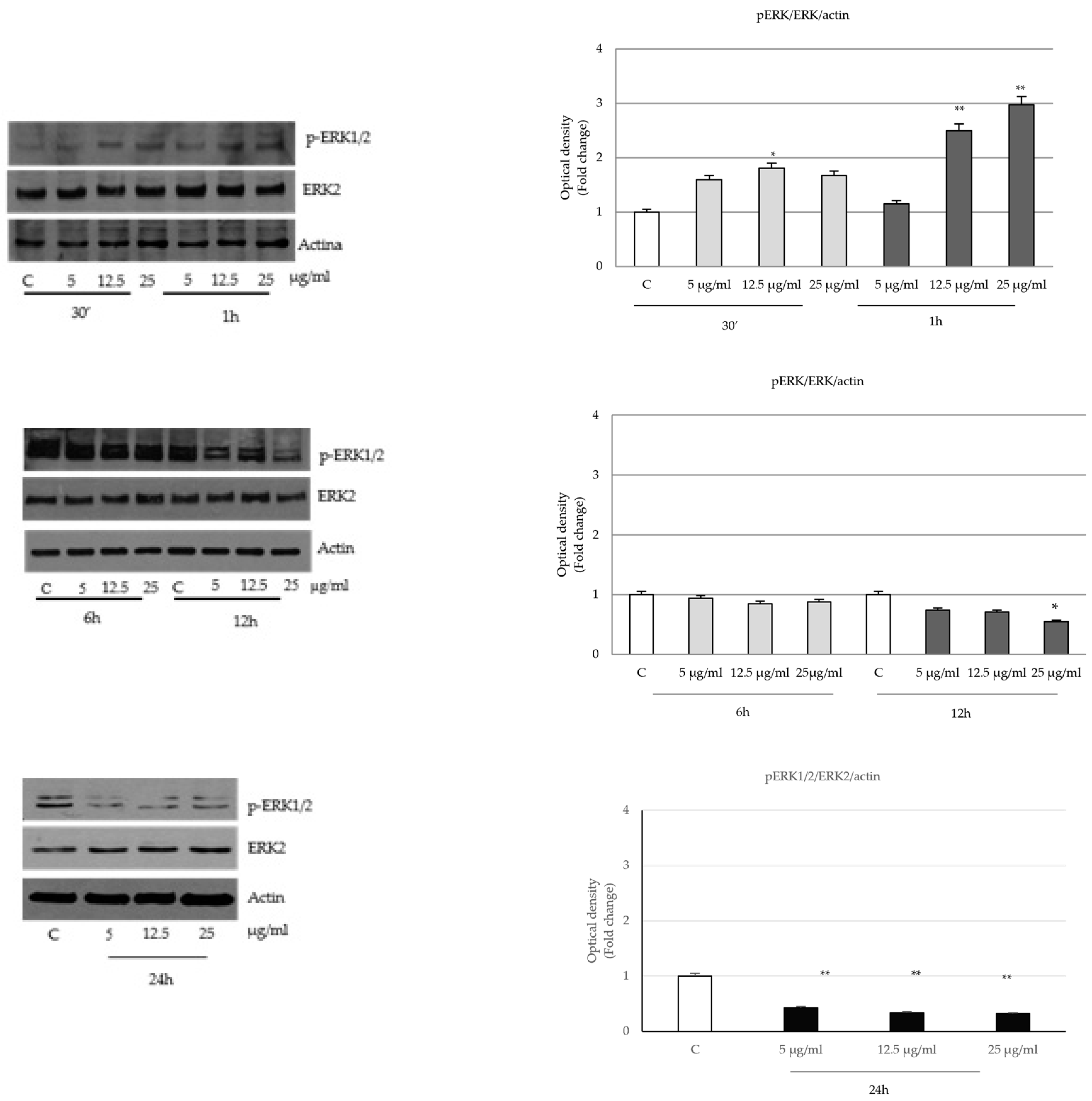
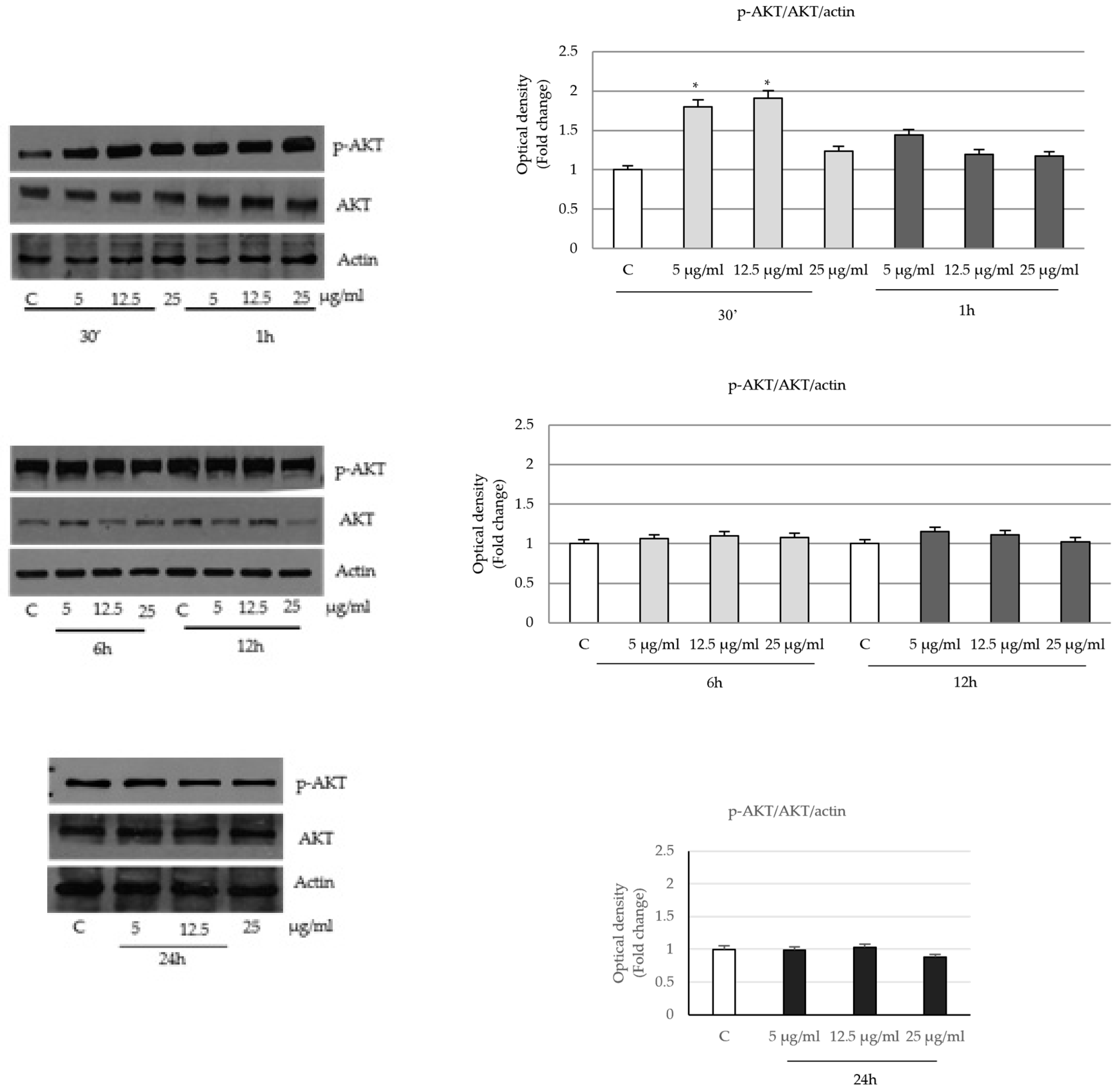
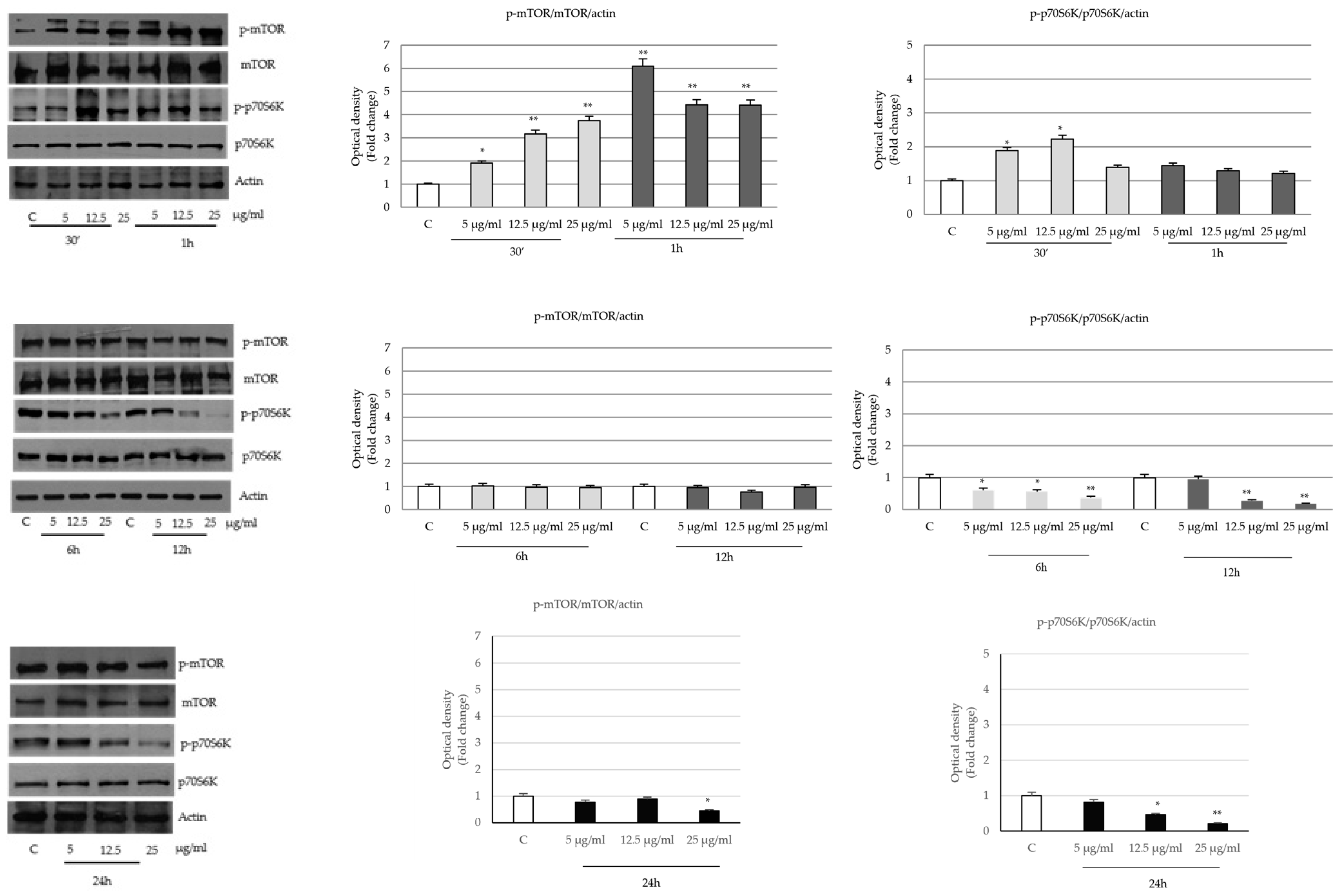
2.4. P. trifoliata Regulates MAPK and mTOR/p70S6K Signaling Pathways in Prostate Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction Procedure and Chemical Profile of P. trifoliata Seed Extract
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Flow Cytometer Analysis
4.6. Immunoblotting Analysis
4.7. Immunocytochemical Staining
4.8. The Lipid Oil Red O Staining
4.9. TUNEL Assay for Apoptosis Detection
4.10. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational bioinformatics for diagnostic and prognostic prediction of prostate cancer in the next-generation sequencing era. BioMed Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef] [PubMed]
- Hjelmberg, J.B.; Scheike, T.; Holst, K.; Skytthe, A.; Penney, K.L.; Graff, R.E.; Pukkala, E.; Christensen, K.; Adami, H.-O.; Holm, N.V.; et al. The heritability of prostate cancer in the nordic twin study of cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2303–2310. [Google Scholar] [CrossRef]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate cancer progression and mortality: A review ofdiet and lifestyle factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Kgatle, M.M.; Kalla, A.A.; Islam, M.M.; Sathekge, M.; Moorad, R. Prostate cancer: Epigenetic alterations, risk factors, and therapy. Prostate Cancer 2016, 2016, 5653862. [Google Scholar] [CrossRef]
- Lamb, D.J.; Wigel, N.L.; Marcelli, M. Androgen receptors and their biology. Vitam. Horm. 2001, 62, 199–230. [Google Scholar] [CrossRef]
- Sung, S.Y.; Chung, L.W.K. Prostate tumor-stroma interaction: Molecular mechanisms and opportunities for therapeutic targeting. Differentiation 2002, 70, 506–521. [Google Scholar] [CrossRef]
- Takayama, K.I. Splicing factors have an essential role in prostate cancer progression and androgen receptor signaling. Biomolecules 2019, 9, 131. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972, 22, 232–401. [Google Scholar] [CrossRef]
- Crawford, E.D.; Hou, A.H. The role of LHRH antagonists in the treatment of prostate cancer. Oncology 2009, 23, 626–630. [Google Scholar]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 162, 454. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.R.; Landry, F.; Aprikian, A.G.; Chevalier, S. Androgen ablation promotes neuroendocrine cell differentiation in dog and human prostate. Prostate 2002, 51, 117–125. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Choi, J.; Yang, C.; Lim, W.; Song, G.; Choi, H. Antioxidant and apoptotic activity of cocoa bean husk extract on prostate cancer cells. Mol. Cell. Toxicol. 2022, 18, 193–203. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Xiao, J.; Cornara, L.; Burlando, B.; Trombetta, D. New insights into Citrus genus: From ancient fruits to new hybrids. Food Front. 2020, 1, 305–328. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Tenuta, M.C.; Leporini, M.; Menichini, F.; Loizzo, M.R. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J. Funct. Foods 2016, 25, 477–485. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, E.K.; Won-Sik Shim, W.S. Phytotherapeutic effects of the fruits of Poncirus trifoliata (L.) Raf. on cancer, inflammation, and digestive dysfunction. Phytother Res. 2018, 32, 616–624. [Google Scholar] [CrossRef]
- Shin, T.Y.; Oh, J.M.; Choi, B.J.; Park, W.H.; Kim, C.H.; Jun, C.D.; Kim, S.H. Anti-inflammatory effect of Poncirus trifoliata fruit through inhibition of NF-κB activation in mast cells. Toxicol. In Vitro 2006, 20, 1071–1076. [Google Scholar] [CrossRef]
- Kim, D.H.; Bae, E.A.; Han, M.J. Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria. Biol. Pharm. Bull. 1999, 22, 422–424. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, D.K.; Kim, S.H.; Shin, T.Y.; Kim, H.M. Antianaphylactic activity of Poncirus trifoliata fruit extract. J. Ethnopharmacol. 1996, 54, 77–84. [Google Scholar] [CrossRef]
- Park, S.H.; Park, E.K.; Kim, D.H. Passive cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus unshiu and Poncirus trifoliata. Planta Med. 2005, 71, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Min, H.Y.; Xu, G.H.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Kang, S.S.; Lee, S.K. Growth inhibition and G1 cell cycle arrest mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated from the fruit of Poncirus trifoliata in human hepatocellular carcinoma cells. Planta Med. 2008, 74, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.M.; Kim, M.S.; Koo, H.N.; Song, B.K.; Yoo, Y.H.; Kim, H.M. Poncirus trifoliata fruit induces apoptosis in human promyelocytic leukemia cells. Clin. Chim. Acta 2004, 340, 179–185. [Google Scholar] [CrossRef]
- Rahman, A.; Siddiqui, S.A.; Jakhar, R.; Kang, S.C. Growth inhibition of various human cancer cell lines by imperatorin and limonin from Poncirus trifoliata Rafin. seeds. Anticancer Agents Med. Chem. 2015, 15, 236–241. [Google Scholar] [CrossRef]
- Kim, B.Y.; Yoon, H.Y.; Yun, S.I.; Woo, E.R.; Song, N.K.; Kim, H.G.; Jeong, S.Y.; Chung, Y.S. In vitro and in vivo inhibition of glucocorticoid-induced osteoporosis by the hexane extract of Poncirus trifoliata. Phytother. Res. 2011, 25, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Park, K.H.; Kim, M.H.; Oh, M.H.; Kim, S.R.; Park, K.J.; Heo, J.H.; Lee, M.W. Antiproliferative effects of native plants on prostate cancer cells. Nat. Prod. Sci. 2013, 19, 192–200. [Google Scholar]
- She, Q.B.; Bode, A.M.; Ma, W.Y.; Chen, N.Y.; Dong, Z. Resveratrol-induced Activation of p53 and Apoptosis Is Mediated by Extracellular Signal-regulated Protein Kinases and p38 Kinase1. Cancer Res. 2001, 61, 1604–1610. [Google Scholar]
- Platanias, L.C. Map kinase signaling pathways and hematologic malignancies. Blood 2003, 101, 4667–4679. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Özturan, D.; Morova, T.; Lack, N.A. Androgen receptor-mediated transcription in prostate cancer. Cells 2022, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Zhong, W.; Oberley, T.D. Increasing discordant antioxidant protein levels and enzymatic activities contribute to increasing redox imbalance observed during human prostate cancer progression. Free Radic. Biol. Med. 2014, 67, 342–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary factors and prostate cancer development, progression, and reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef]
- Heo, Y.; Cho, Y.; Ju, K.S.; Cho, H.; Park, K.H.; Choi, H.; Yoon, J.K.; Moon, C.; Kim, Y.B. Antiviral activity of Poncirus trifoliata seed extract against oseltamivir-resistant influenza virus. J. Microbiol. 2018, 56, 586–592. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids regulate inflammation and oxidative stress in cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Mitra, S.; Lami, M.S.; Uddin, T.M.; Das, R.; Islam, F.; Anjum, J.; Hossain, J.; Emran, T.B. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed. Pharmacother. 2022, 150, 112932. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zang, J.; Chen, D.; Zhang, T.; Zhan, H.; Lu, M.; Zhuge, H. Neohesperidin induces cellular apoptosis in human breast adenocarcinoma MDA-MB-231 cells via activating the bcl-2/bax-mediated signaling pathway. Nat. Prod. Commun. 2012, 7, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Jeong, S.A.; Yang, C.; Song, J.; Song, G.; Jeong, W.; Lim, W. Hesperidin suppresses the proliferation of prostate cancer cells by inducing oxidative stress and disrupting Ca2+ homeostasis. Antioxidants 2022, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Chang, W.C.; Hsieh, C.H.; Hsiao, M.W.; Lin, W.C.; Hung, Y.C.; Ye, J.C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J. Obs. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Venkata Reddy, B. Inhibitory effect of caffeic acid on cancer cell prolifera-tion by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Medema, R.H.; Macurek, L. Checkpoint control and cancer. Oncogene 2012, 31, 2601–2613. [Google Scholar] [CrossRef]
- Jaiswal, A.S.; Narayan, S. SN2 DNA-alkylating agent-induced phosphorylationof p53 and activation of p21 gene expression. Mutat. Res. 2002, 500, 17–30. [Google Scholar] [CrossRef]
- Kops, G.J.P.L.; de Ruiter, N.D.; De Vries-Smits, A.M.M.; Powell, D.R.; Bos, J.L.; Burgering, B.M.T. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 1999, 398, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.D.; Nunez, G.; Barri, F.G.; Guan, K.-L. Negative Regulation of the Forkhead Transcription Factor FKHR by Akt. J. Biol. Chem. 1999, 274, 16741–16746. [Google Scholar] [CrossRef] [PubMed]
- Medema, R.H.; Kops, G.J.P.L.; Bos, J.L.; Burgering, B.M.T. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 2000, 404, 782–787. [Google Scholar] [CrossRef]
- Chao, D.T.; Korsmeyer, S.J. Bcl-2 family: Regulators of cell death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Eskes, R.; Desangher, S.; Antonsson, B.; Martinou, J.M. Bid induces oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000, 102, 1–4. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, H.K.; Yun, B.S.; Lee, D.Y.; Hwang, P.H.; Park, H.R.; Kim, M.S. The extract of the immature fruit of Poncirus trifoliata induces apoptosis in colorectal cancer cells via mitochondrial autophagy. Food Sci. Hum. Well. 2020, 9, 237–244. [Google Scholar] [CrossRef]
- Han, H.Y.; Ryu, M.H.; Son, Y.; Lee, G.; Jeong, S.H.; Kim, H. Poncirus trifoliata Rafin. induces the apoptosis of triple-negative breast cancer cells via activation of the c-Jun NH(2)-terminal kinase and extracellular signal-regulated kinase pathways. Pharma-Cognosy Mag. 2015, 11, S237–S243. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Hussein, U.K.; Yun, B.S.; Park, H.R.; Jeong, Y.J. Anticancer effects of Poncirus fructus on hepatocellular carcinoma through regulation of apoptosis, migration, and invasion. Oncol. Rep. 2020, 44, 2537–2546. [Google Scholar] [CrossRef]
- Cross, T.G.; Scheel-Toellner, D.; Henriquez, N.V.; Deacon, E.; Salmon, M.; Lord, J.M. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000, 256, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Hallahan, A.R. p38 MAP Kinase: A convergence point in cancer therapy. Trends Mol. Med. 2004, 10, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Perez, I.; Murguía, J.R.; Perona, R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 1998, 16, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tan, T.H. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol. 2000, 16, 651–662. [Google Scholar] [CrossRef]
- Hu, R.; Kong, A.N. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition 2004, 20, 83–88. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.Y.; Hwang, H.J.; Kim, W.J.; Choi, Y.H. Diallyl trisulfide-induced apoptosis of bladder cancer cells is caspase-dependent and regulated by PI3K/Akt and JNK pathways. Environ. Toxicol. Pharmacol. 2014, 37, 74–83. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef]
- Changa, L.; Graham, P.H.; Ni, J.; Hao, J.; Bucci, J.; Cozzi, P.J.; Li, Y. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit. Rev. Oncol./Hematol. 2015, 96, 507–517. [Google Scholar] [CrossRef]
- Berven, L.A.; Willard, F.S.; Crouch, M.F. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp. Cell Res. 2004, 296, 183–195. [Google Scholar] [CrossRef]
- Chen, J.S.; Wang, Q.; Fu, X.H.; Huang, X.H.; Chen, X.L.; Cao, L.Q.; Chen, L.Z.; Tan, H.X.; Li, W.; Bi, J.; et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol. Res. 2009, 39, 177–186. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Cardelli, J.A.; Martin, K.A.; Blenis, J.; Huang, S. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 2006, 25, 7029–7040. [Google Scholar] [CrossRef] [PubMed]
- Olshavsky, N.A.; Groh, E.M.; Comstock, C.E.S.; Morey, L.M.; Wang, Y.; Revelo, M.P.; Burd, C.; J Meller, J.; Knudsen, K.E. Cyclin D3 action in androgen receptor regulation and prostate cancer. Oncogene 2008, 27, 3111–3121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giordano, F.; Paolì, A.; Forastiero, M.; Marsico, S.; De Amicis, F.; Marrelli, M.; Naimo, G.D.; Mauro, L.; Panno, M.L. Valproic acid inhibits cell growth in both MCF-7 and MDA-MB231 cells by triggering different responses in a cell type-specifc manner. J. Transl. Med. 2023, 21, 165. [Google Scholar] [CrossRef] [PubMed]



| Compound | μg/g Extract |
|---|---|
| Flavanone-O-glycosides | |
| Narirutin | 37.62 ± 0.53 |
| Naringin | 156.42 ± 0.35 |
| Neohesperidin | 80.12 ± 0.25 |
| Phenolic acid | |
| Caffeic acid | 32.85 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, F.; Comità, S.; Venneri, G.; Rago, V.; Naimo, G.D.; De Amicis, F.; De Bartolo, A.; Tundis, R.; Mauro, L.; Panno, M.L. Poncirus trifoliata (L.) Raf. Seed Extract Induces Cell Cycle Arrest and Apoptosis in the Androgen Receptor Positive LNCaP Prostate Cancer Cells. Int. J. Mol. Sci. 2023, 24, 16351. https://doi.org/10.3390/ijms242216351
Giordano F, Comità S, Venneri G, Rago V, Naimo GD, De Amicis F, De Bartolo A, Tundis R, Mauro L, Panno ML. Poncirus trifoliata (L.) Raf. Seed Extract Induces Cell Cycle Arrest and Apoptosis in the Androgen Receptor Positive LNCaP Prostate Cancer Cells. International Journal of Molecular Sciences. 2023; 24(22):16351. https://doi.org/10.3390/ijms242216351
Chicago/Turabian StyleGiordano, Francesca, Stefano Comità, Giulia Venneri, Vittoria Rago, Giuseppina Daniela Naimo, Francesca De Amicis, Anna De Bartolo, Rosa Tundis, Loredana Mauro, and Maria Luisa Panno. 2023. "Poncirus trifoliata (L.) Raf. Seed Extract Induces Cell Cycle Arrest and Apoptosis in the Androgen Receptor Positive LNCaP Prostate Cancer Cells" International Journal of Molecular Sciences 24, no. 22: 16351. https://doi.org/10.3390/ijms242216351
APA StyleGiordano, F., Comità, S., Venneri, G., Rago, V., Naimo, G. D., De Amicis, F., De Bartolo, A., Tundis, R., Mauro, L., & Panno, M. L. (2023). Poncirus trifoliata (L.) Raf. Seed Extract Induces Cell Cycle Arrest and Apoptosis in the Androgen Receptor Positive LNCaP Prostate Cancer Cells. International Journal of Molecular Sciences, 24(22), 16351. https://doi.org/10.3390/ijms242216351








