Assaying ADAMTS13 Activity as a Potential Prognostic Biomarker for Sinusoidal Obstruction Syndrome in Mice
Abstract
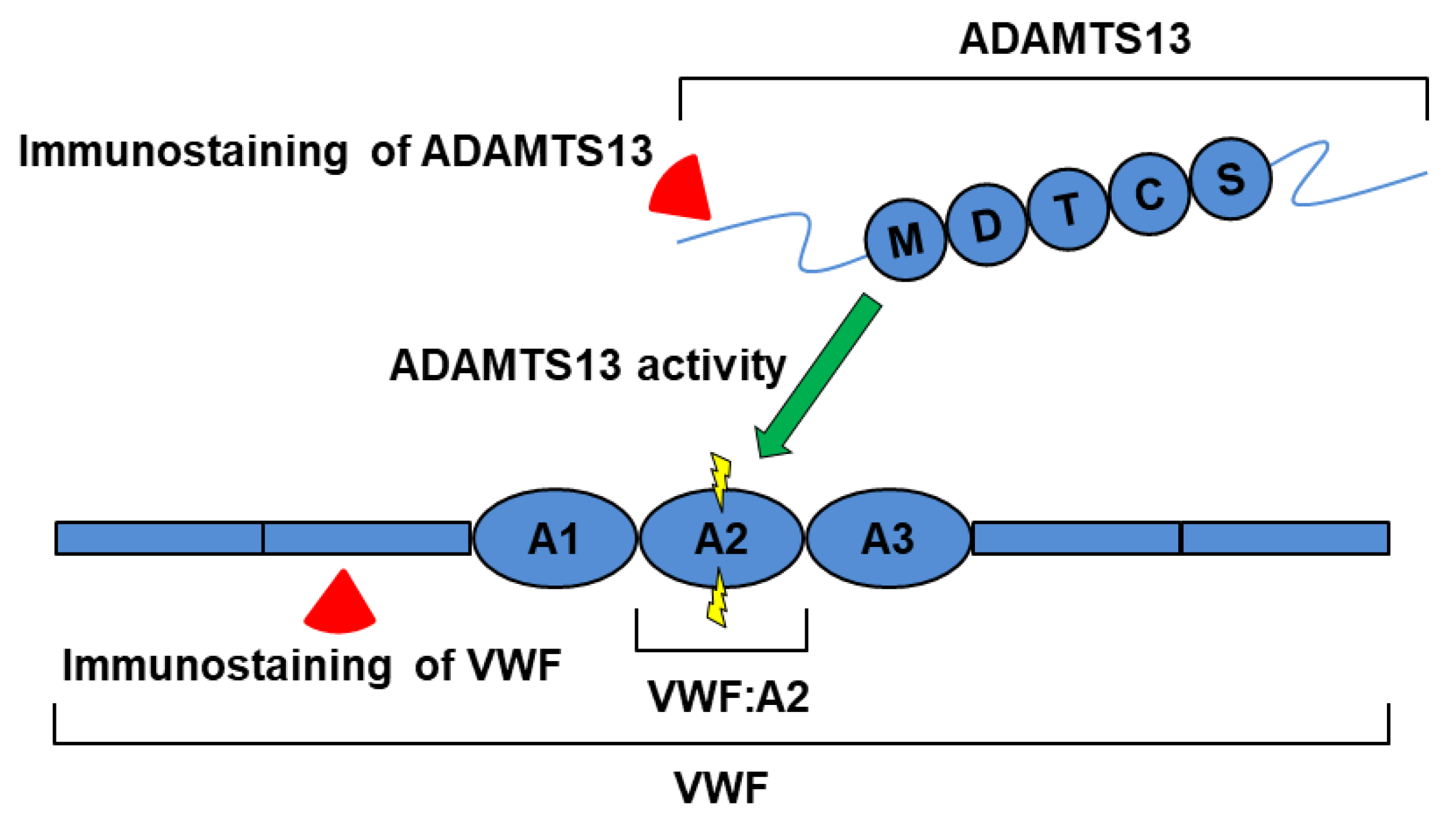
1. Introduction
2. Results
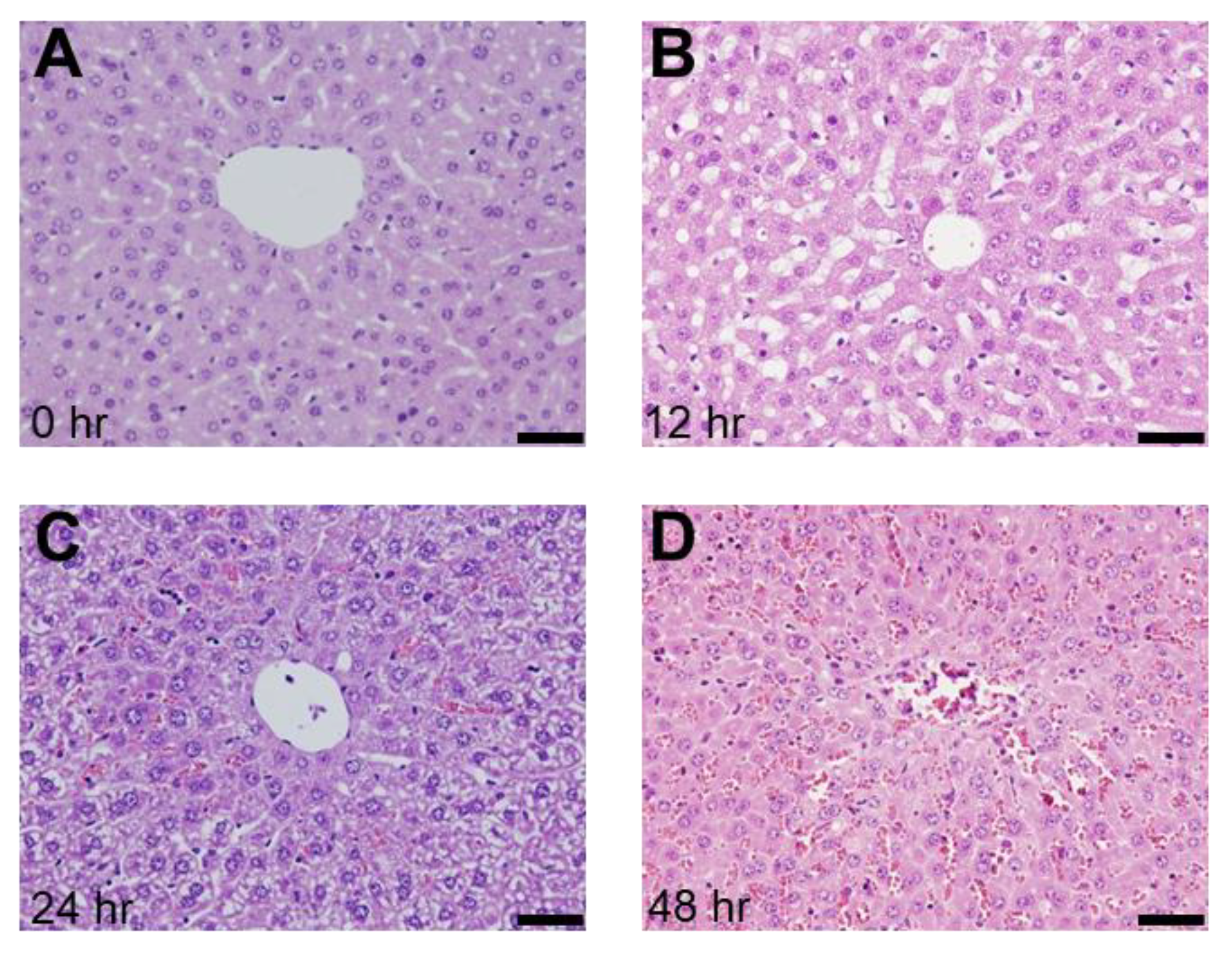
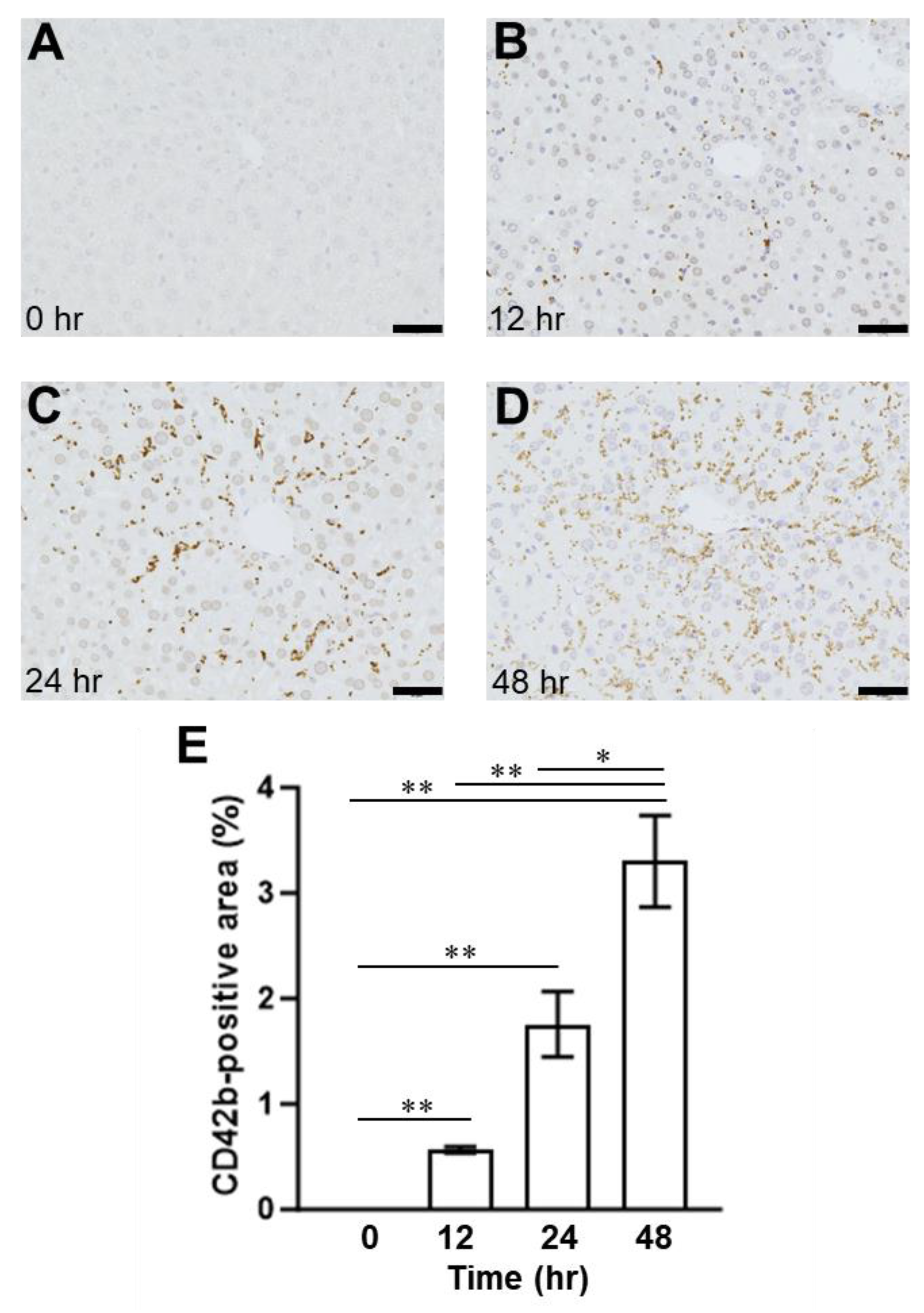
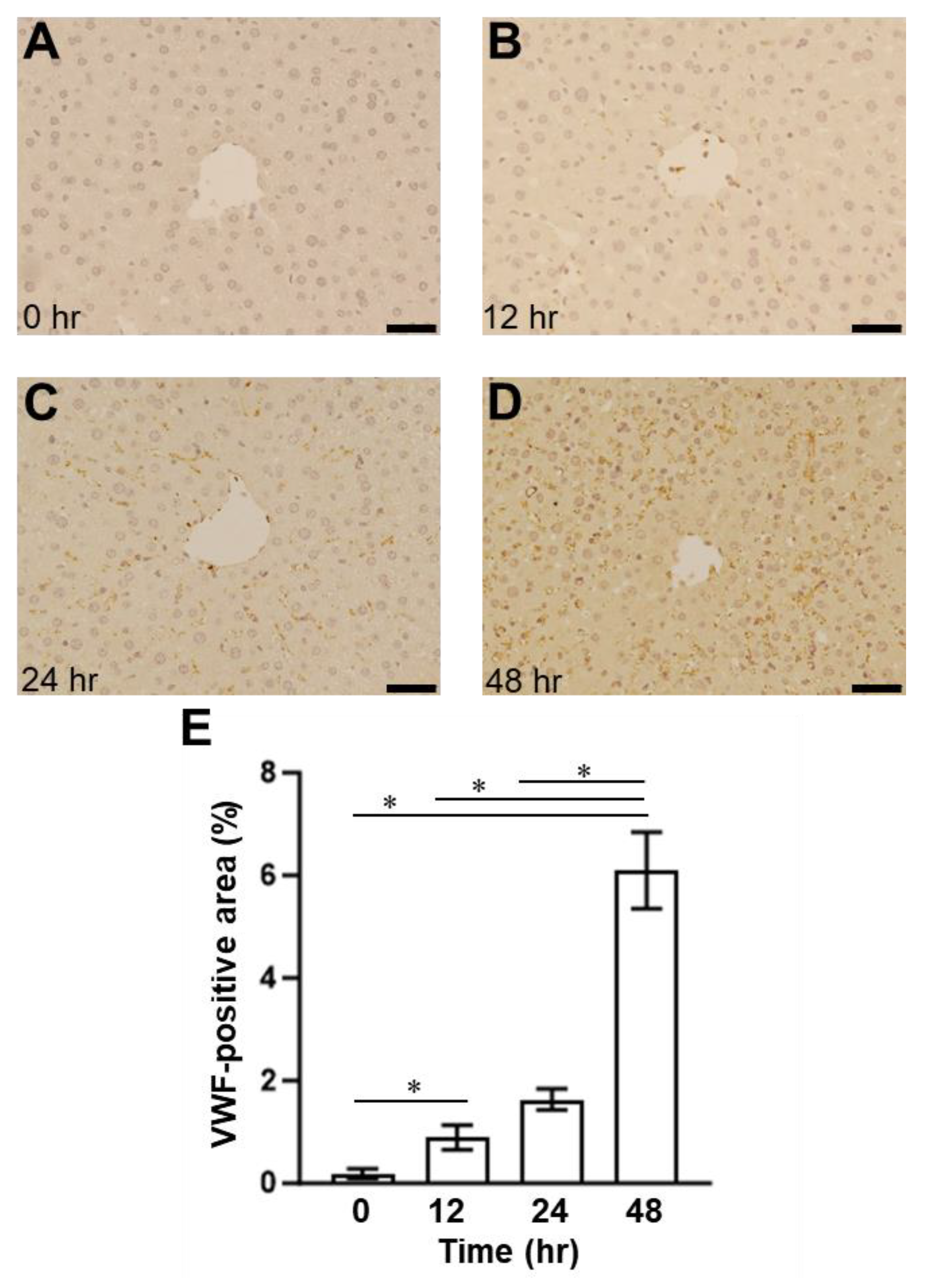
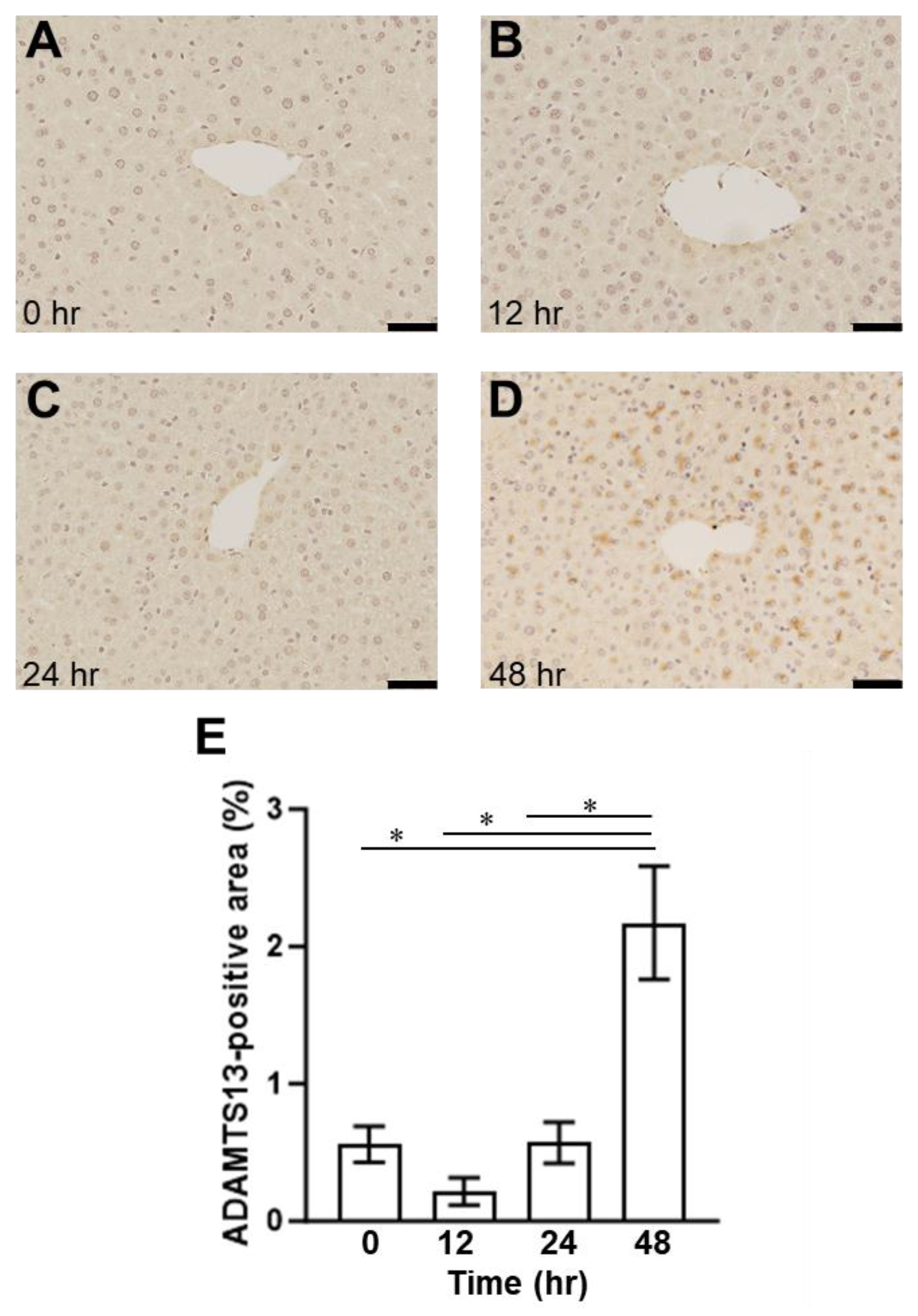
2.1. Presence of SOS and Time Course of VWF in H&E Staining and Immunohistological Staining
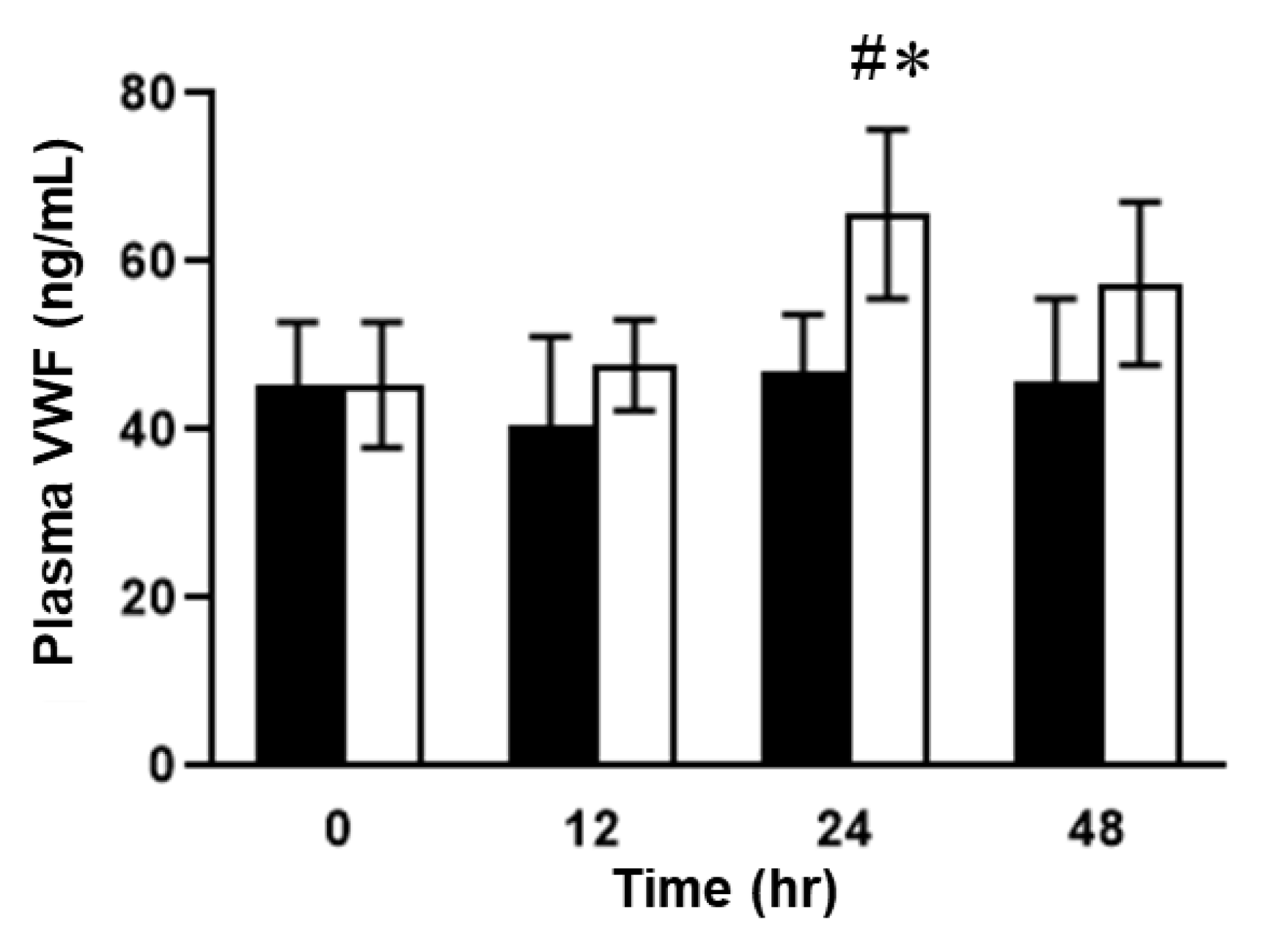
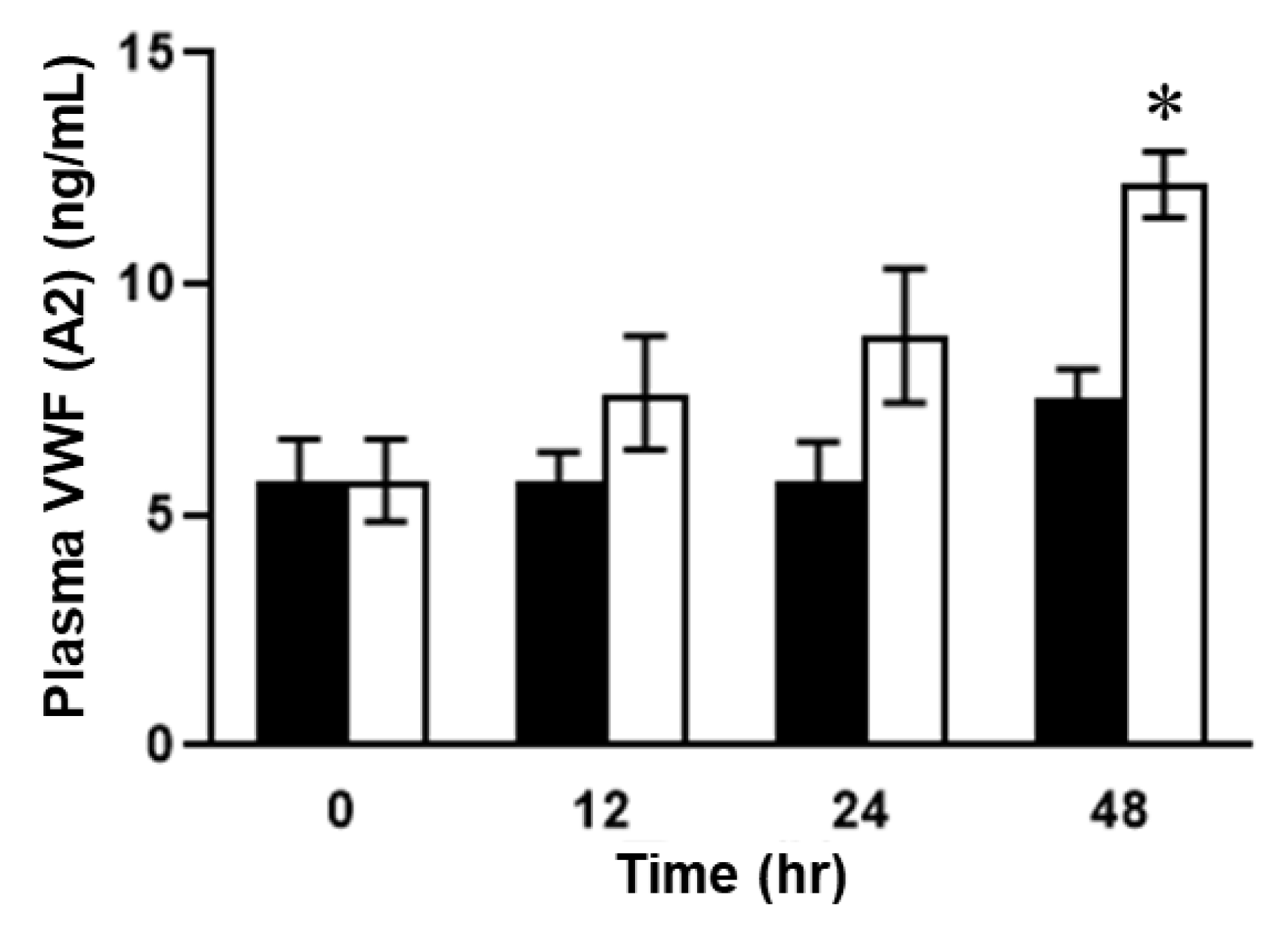
2.2. Plasma Concentrations of VWF in ELISA in SOS Model Mice Treated with MCT
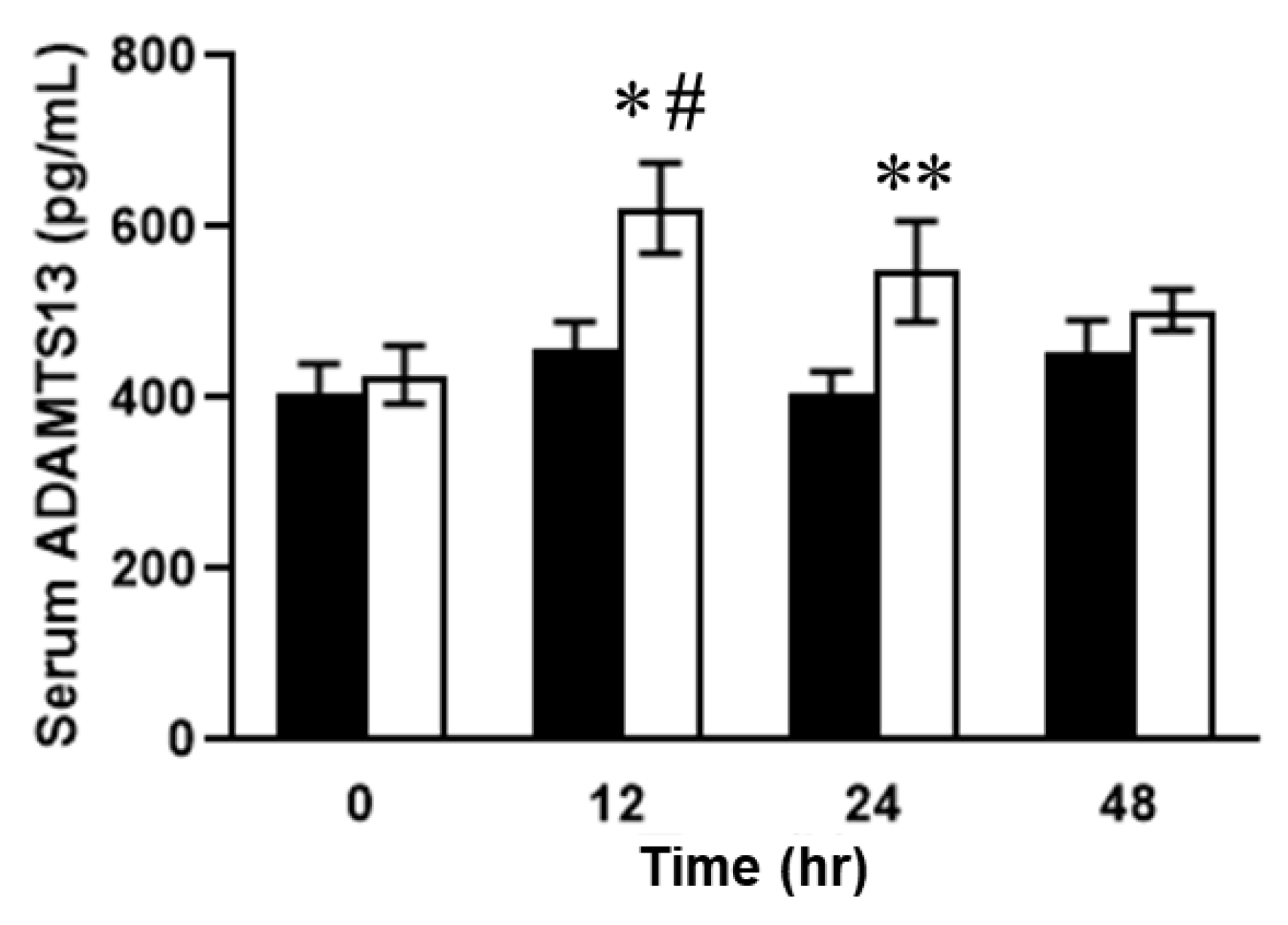
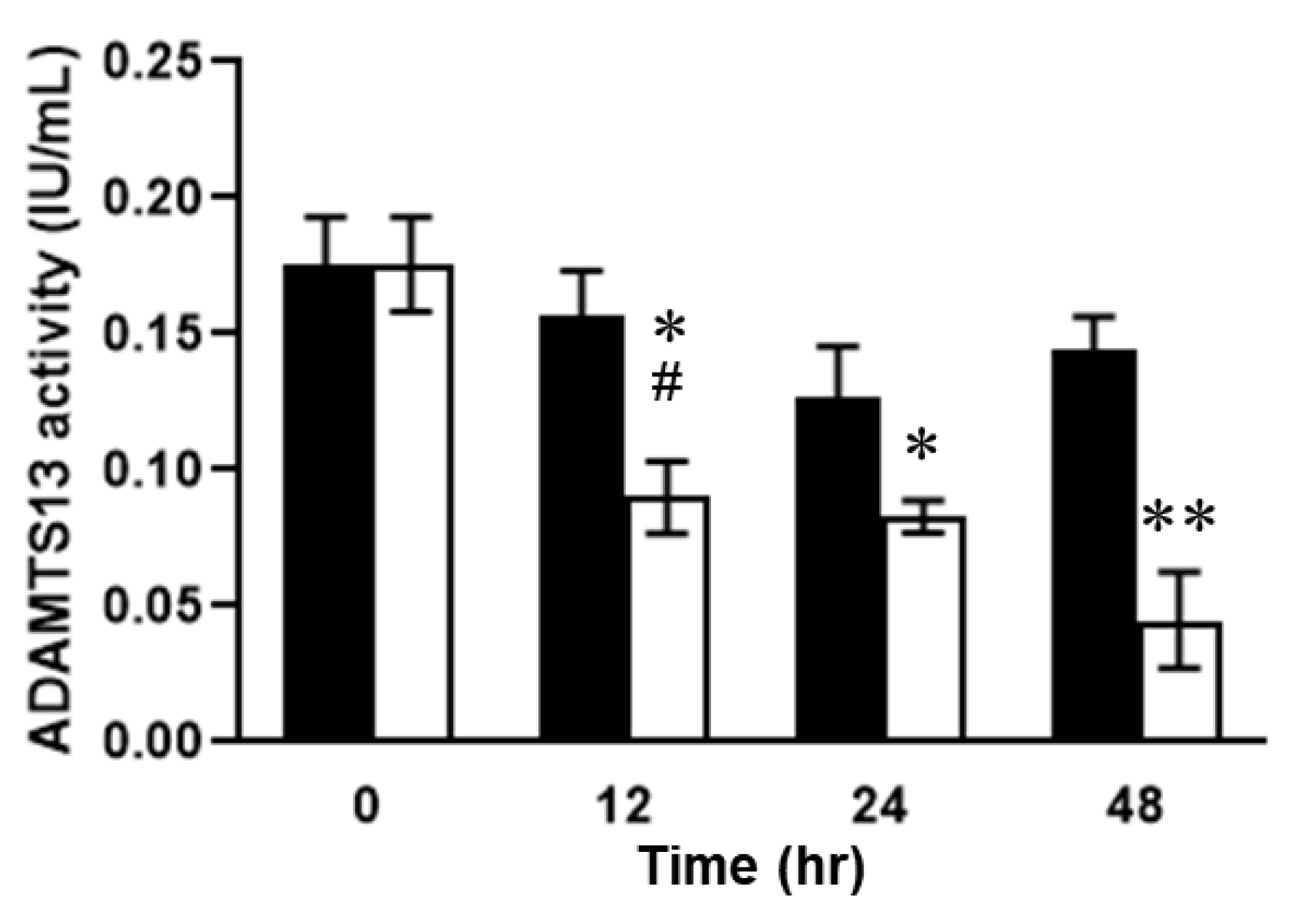
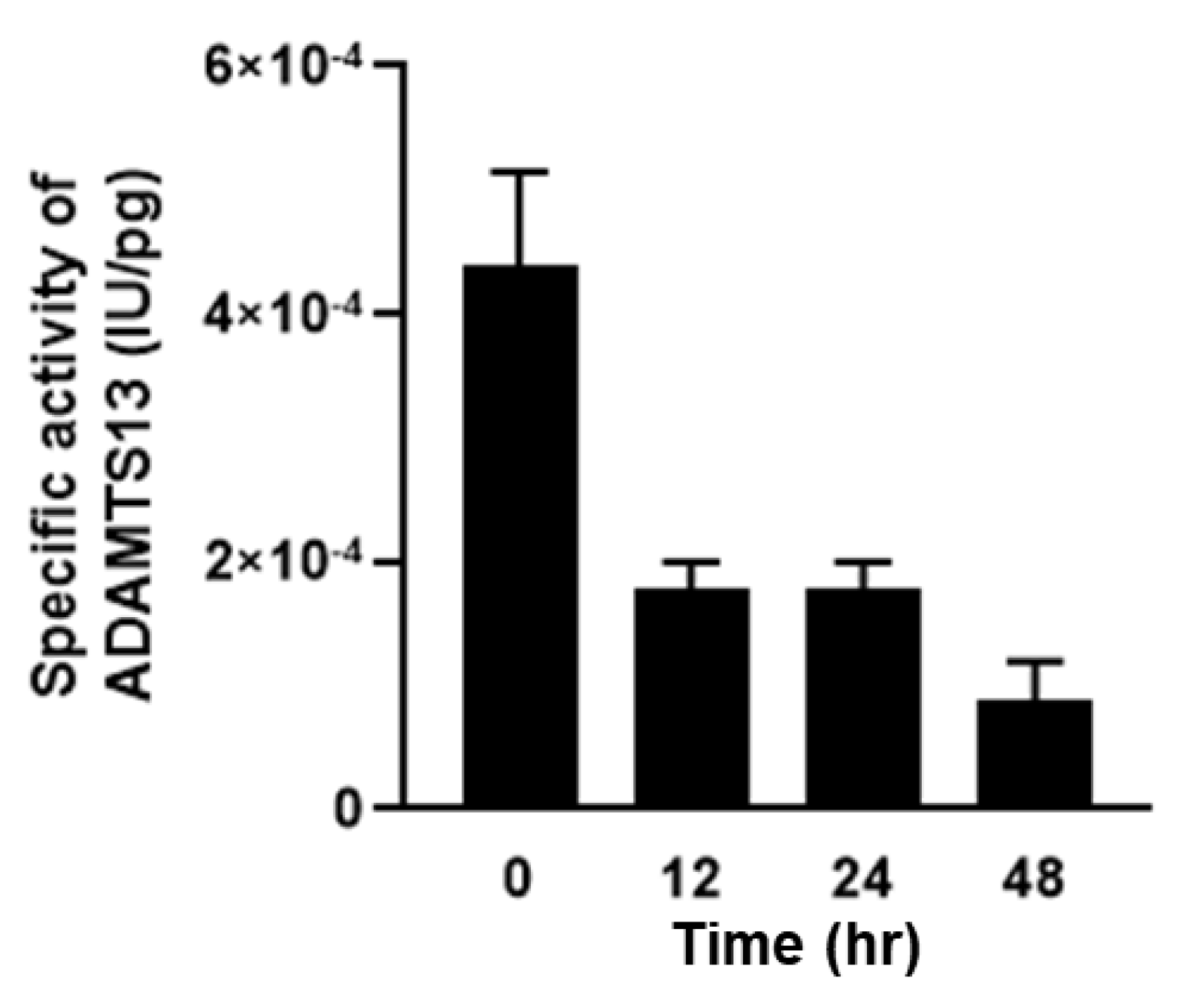
2.3. Relationship between Plasma ADAMTS13 Levels and Immunohistological Changes in a Mouse Model of SOS
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Experimental Protocols
4.4. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a major ingredient of ginger attenuates diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef] [PubMed]
- Faraci, M.; Bertaina, A.; Luksch, R.; Calore, E.; Lanino, E.; Saglio, F.; Prete, A.; Menconi, M.; De, S.G.; Tintori, V.; et al. Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease after Autologous or Allogeneic Hematopoietic Stem Cell Transplantation in Children: A retrospective study of the Italian Hematology-Oncology Association–Hematopoietic Stem Cell Transplantation Group. Biol. Blood Marrow Transpl. 2019, 25, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Q.; Crawford, J.M. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J. Clin. Exp. Hepatol. 2014, 4, 332–346. [Google Scholar] [CrossRef]
- McDonald, G.B.; Hinds, M.S.; Fisher, L.D.; Schoch, H.G.; Wolford, J.L.; Banaji, M.; Hardin, B.J.; Shulman, H.M.; Clift, R.A. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann. Intern. Med. 1993, 118, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, S.; Miyashita, T.; Hayashi, H.; Tajima, H.; Takamura, H.; Tsukada, T.; Okamoto, K.; Sakai, S.; Makino, I.; Kinoshita, J.; et al. Extravasated platelet aggregation in liver zone 3 may correlate with the progression of sinusoidal obstruction syndrome following living donor liver transplantation: A case report. Exp. Ther. Med. 2015, 9, 1119–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanuma, S.; Miyashita, T.; Hayashi, H.; Tajima, H.; Takamura, H.; Makino, I.; Oyama, K.; Nakagawara, H.; Fushida, S.; Ohta, T. Extravasated Platelet Aggregation in Liver Zone 3 Is Associated with Thrombocytopenia and Deterioration of Graft Function After Living-Donor Liver Transplant. Exp. Clin. Transpl. 2015, 13, 556–562. [Google Scholar]
- Nakanuma, S.; Miyashita, T.; Hayashi, H.; Ohbatake, Y.; Takamura, H.; Okazaki, M.; Yamaguchi, T.; Sakai, S.; Makino, I.; Oyama, K.; et al. von Willebrand Factor Deposition and ADAMTS-13 Consumption in Allograft Tissue of Thrombotic Microangiopathy-like Disorder After Living Donor Liver Transplantation: A Case Report. Transpl. Proc. 2017, 49, 1596–1603. [Google Scholar] [CrossRef]
- Simon, F.D.M.; Hans, D.; Karen, V. von Willebrand factor to the rescue. Blood 2009, 113, 5049–5057. [Google Scholar]
- Mazzucato, M.; Spessotto, P.; Masotti, A.; De, A.L.; Cozzi, M.R.; Yoshioka, A.; Perris, R.; Colombatti, A.; De, M.L. Identification of domains responsible for von Willebrand factor type VI collagen interaction mediating platelet adhesion under high flow. J. Biol. Chem. 1999, 274, 3033–3041. [Google Scholar] [CrossRef]
- Springer, T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014, 124, 1412–1425. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Wagner, D.D. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc. Natl. Acad. Sci. USA 1992, 89, 3531–3535. [Google Scholar] [CrossRef] [PubMed]
- Eveline, A.M.B.; Marjon, J.M.; Maartje, V.D.B.; Jeroen, C.J.E.; Jan, V.; Karine, M.V.; Koen, M. Factor VIII alters tubular organization and functional properties of von Willebrand factor stored in Weibel-Palade bodies. Blood 2011, 118, 5947–5956. [Google Scholar]
- Rondaij, M.G.; Sellink, E.; Gijzen, K.A.; Klooster, J.P.t.; Hordijk, P.L.; Mourik, J.A.v.; Voorberg, J. Small GTP-binding protein Ral is involved in cAMP-mediated release of von Willebrand factor from endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M. Localization of ADAMTS13 to the stellate cells of human liver. Blood 2005, 106, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J. Biol. Chem. 2001, 276, 41059–41063. [Google Scholar] [CrossRef]
- South, K.; Luken, B.M.; Crawley, J.T.B.; Phillips, R.; Thomas, M.; Ccollins, R.F.; Deforche, L.; Vanhoorelbeke, K.; Lane, D.A. Conformational activation of ADAMTS13. Proc. Natl. Acad. Sci. USA 2014, 111, 18578–18583. [Google Scholar] [CrossRef]
- Muia, J.; Zhu, J.; Gupta, G.; Haberichter, S.L.; Friedman, K.D.; Feys, H.B.; Deforche, L.; Vanhoorelbeke, K.; Westfield, L.A.; Roth, R.; et al. Allosteric activation of ADAMTS13 by von Willebrand factor. Proc. Natl. Acad. Sci. USA. 2014, 30, 18584–18589. [Google Scholar] [CrossRef]
- Zheng, X.L. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu. Rev. Med. 2015, 66, 211–225. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, D.; Cao, W.; Song, W.C.; Zheng, X.L. Synergistic effects of ADAMTS13 deficiency and complement activation in pathogenesis of thrombotic microangiopathy. Blood 2019, 134, 1095–1105. [Google Scholar] [CrossRef]
- Ernest, T.P.; Pete, L. Conformation of the von Willebrand factor/factor VIII complex in quasi-static flow. J. Biol. Chem. 2021, 296, 100420. [Google Scholar]
- McCabe, D.J.; Murphy, S.J.; Starke, R.; Harrison, P.; Brown, M.M.; Sidhu, P.S.; Mackie, I.J.; Scully, M.; Machin, S.J. Relationship between ADAMTS13 activity, von Willebrand factor antigen levels and platelet function in the early and late phases after TIA or ischaemic stroke. J. Neurol. Sci. 2015, 348, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Tajima, H.; Miyashita, T.; Miyata, T.; Nakanuma, S.; Makino, I.; Hayashi, H.; Oyama, K.; Takamura, H.; Ninomiya, I.; et al. Extravasated platelet aggregation in the livers of rats with drug-induced hepatic sinusoidal obstruction syndrome. Mol. Med. Rep. 2017, 15, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Tajima, H.; Hirata, M.; Nakanuma, S.I.; Makino, I.; Hayashi, H.; Oyama, K.; Miyashita, T.; Takamura, H.; Ninomiya, I.; et al. Phosphodiesterase III inhibitor attenuates rat sinusoidal obstruction syndrome through inhibition of platelet aggregation in Disse’s space. J. Gastroenterol. Hepatol. 2018, 33, 950–957. [Google Scholar] [CrossRef]
- Takada, S.; Yamamoto, Y.; Tajima, H.; Ohta, T. Soluble Thrombomodulin Attenuates Endothelial Cell Damage in Hepatic Sinusoidal Obstruction Syndrome. In Vivo 2018, 32, 1409–1417. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tajima, H.; Yamamoto, Y.; Munesue, S.; Okazaki, M.; Ohbatake, Y.; Nakanuma, S.; Makino, I.; Miyashita, T.; Takamura, H.; et al. Thrombopoietin accumulation in hepatocytes induces a decrease in its serum levels in a sinusoidal obstruction syndrome model. Mol. Med. Rep. 2022, 25, 201. [Google Scholar] [CrossRef] [PubMed]
- Tajima, H.; Ohta, T. Oxaliplatin-based chemotherapy induces extravasated platelet aggregation in the liver. Mol. Clin. Oncol. 2015, 3, 555–558. [Google Scholar] [CrossRef]
- Choi, J.-H.; Won, Y.-W.; Kim, H.S.; Oh, Y.-H.; Lim, S.; Kim, H.-J. Oxaliplatin-induced sinusoidal obstruction syndrome mimicking metastatic colon cancer in the liver. Oncol. Lett. 2016, 11, 2861–2864. [Google Scholar] [CrossRef]
- Chen, S.; Osborn, J.M.; Chen, X.; Boyer, M.M.; McDonald, G.B.; Hildebrandt, G.C. Subacute hepatic necrosis mimicking veno-occlusive disease in a patient with HFE H63D homozygosity after allogeneic hematopoietic cell transplantation with busulfan conditioning. Int. J. Hematol. 2015, 102, 729–731. [Google Scholar] [CrossRef]
- Nakura, M.; Miyashita, T.; Yamamoto, Y.; Takada, S.; Kanou, S.; Tajima, H.; Takamura, H.; Ohta, T. Inhibitory Effects of Beraprost Sodium in Murine Hepatic Sinusoidal Obstruction Syndrome. Anticancer Res. 2020, 40, 5171–5180. [Google Scholar] [CrossRef]
- Miyashita, T.; Nakanuma, S.; Ahmed, A.K.; Makino, I.; Hayashi, H.; Oyama, K.; Nakagawara, H.; Tajima, H.; Takamura, H.; Ninomiya, I.; et al. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur. Surg. 2016, 48, 92–98. [Google Scholar] [CrossRef]
- Lisman, T.; Luyendyk, J.P. Platelets as modulators of liver diseases. Semin. Thromb. Hemost. 2018, 44, 114–125. [Google Scholar] [PubMed]
- Uemura, M.; Fujimura, Y.; Ko, S.; Matsumoto, M.; Nakajima, Y.; Fukui, H. Pivotal role of ADAMTS13 function in liver diseases. Int. J. Hematol. 2010, 91, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kume, Y.; Ikeda, H.; Inoue, M.; Tejima, K.; Tomiya, T.; Nishikawa, T.; Watanabe, N.; Ichikawa, T.; Kaneko, M.; Okubo, S.; et al. Hepatic stellate cell damage may lead to decreased plasma ADAMTS13 activity in rats. FEBS Lett. 2007, 581, 1631–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanou, S.; Miyashita, T.; Yamamoto, Y.; Takada, S.; Nakura, M.; Okazaki, M.; Ohbatake, Y.; Nakanuma, S.; Makino, I.; Tajima, H.; et al. Prophylactic Effect of Recombinant Human Soluble Thrombomodulin for Hepatic Sinusoidal Obstruction Syndrome Model Mice. In Vivo 2020, 34, 1037–1045. [Google Scholar] [CrossRef]
- Ferro, D.; Quintarelli, C.; Lattuada, A.; Leo, R.; Alessandroni, M.; Mannucci, P.M.; Violi, F. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: Relationship to endotoxemia. Hepatology 1996, 23, 1377–1383. [Google Scholar] [CrossRef]
- Reuken, A.P.; Kussmann, A.; Kiehntopf, M.; Budde, U.; Stallmach, A.; Ralf, A.C.; Bruns, T. Imbalance of von Willebrand factor and its cleaving protease ADAMTS13 during systemic inflammation superimposed on advanced cirrhosis. Liver Int. 2015, 35, 37–45. [Google Scholar] [CrossRef]
- Cao, W.J.; Niiya, M.; Zheng, X.W.; Shang, D.Z.; Zheng, X.L. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J. Thromb. Haemost. 2008, 6, 1233–1235. [Google Scholar] [CrossRef]
- Yagita, M.; Uemura, M.; Nakamura, T.; Kunitomi, A.; Matsumoto, M.; Fujimura, Y. Development of ADAMTS13 inhibitor in a patient with hepatitis C virus-related liver cirrhosis causes thrombotic thrombocytopenic purpura. J. Hepatol. 2005, 42, 420–421. [Google Scholar] [CrossRef]
- Takaya, H.; Namisaki, T.; Asada, S.; Iwai, S.; Kubo, T.; Suzuki, J.; Enomoto, M.; Tsuji, Y.; Fujinaga, Y.; Nishimura, N.; et al. ADAMTS13, VWF, and endotoxin are interrelated and associated with the severity of liver cirrhosis via hypercoagulability. J. Clin. Med. 2022, 11, 1835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, M.; Munesue, S.; Higashi, Y.; Harashima, A.; Takei, R.; Takada, S.; Nakanuma, S.; Ohta, T.; Yagi, S.; Tajima, H.; et al. Assaying ADAMTS13 Activity as a Potential Prognostic Biomarker for Sinusoidal Obstruction Syndrome in Mice. Int. J. Mol. Sci. 2023, 24, 16328. https://doi.org/10.3390/ijms242216328
Saeki M, Munesue S, Higashi Y, Harashima A, Takei R, Takada S, Nakanuma S, Ohta T, Yagi S, Tajima H, et al. Assaying ADAMTS13 Activity as a Potential Prognostic Biomarker for Sinusoidal Obstruction Syndrome in Mice. International Journal of Molecular Sciences. 2023; 24(22):16328. https://doi.org/10.3390/ijms242216328
Chicago/Turabian StyleSaeki, Masakazu, Seiichi Munesue, Yuri Higashi, Ai Harashima, Ryohei Takei, Satoshi Takada, Shinichi Nakanuma, Tetsuo Ohta, Shintaro Yagi, Hidehiro Tajima, and et al. 2023. "Assaying ADAMTS13 Activity as a Potential Prognostic Biomarker for Sinusoidal Obstruction Syndrome in Mice" International Journal of Molecular Sciences 24, no. 22: 16328. https://doi.org/10.3390/ijms242216328
APA StyleSaeki, M., Munesue, S., Higashi, Y., Harashima, A., Takei, R., Takada, S., Nakanuma, S., Ohta, T., Yagi, S., Tajima, H., & Yamamoto, Y. (2023). Assaying ADAMTS13 Activity as a Potential Prognostic Biomarker for Sinusoidal Obstruction Syndrome in Mice. International Journal of Molecular Sciences, 24(22), 16328. https://doi.org/10.3390/ijms242216328






