The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. S. aureus Strains 6850 and JB1 Were Susceptibile to All Antibiotic Combinations during Planktonic Growth
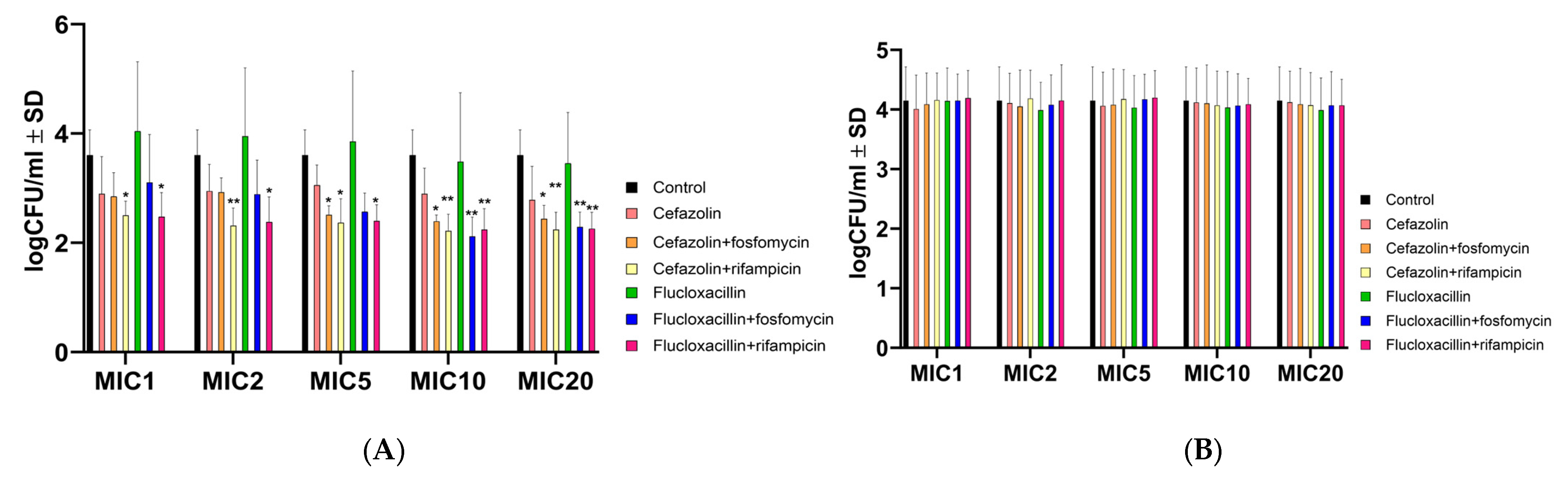
2.2. Antibiotic Combinations Containing Rifampicin Effectively Killed Intracellular Bacteria but Not the Intracellular SCV S. aureus JB1
2.3. Antibiotic Combinations with Rifampicin Promote the Selection of SCVs
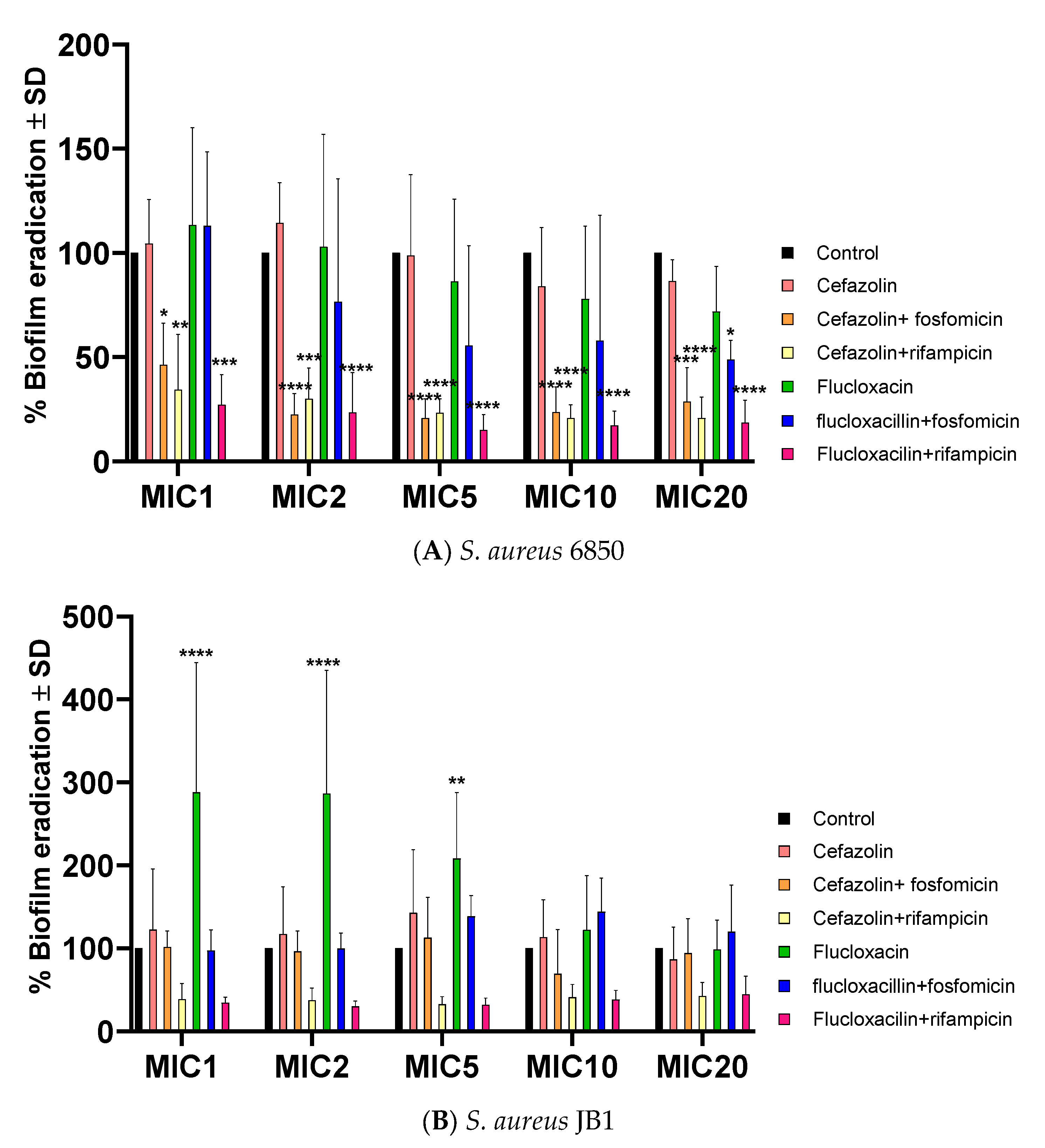
2.4. Antibiotics Successfully Halt Biofilm Formation in S. aureus WT and SCV
2.5. Successful Biofilm Eradication via Rifampicin and Fosfomycin Antibiotic Combinations, JB1 Biofilms Respond Solely to Rifampicin
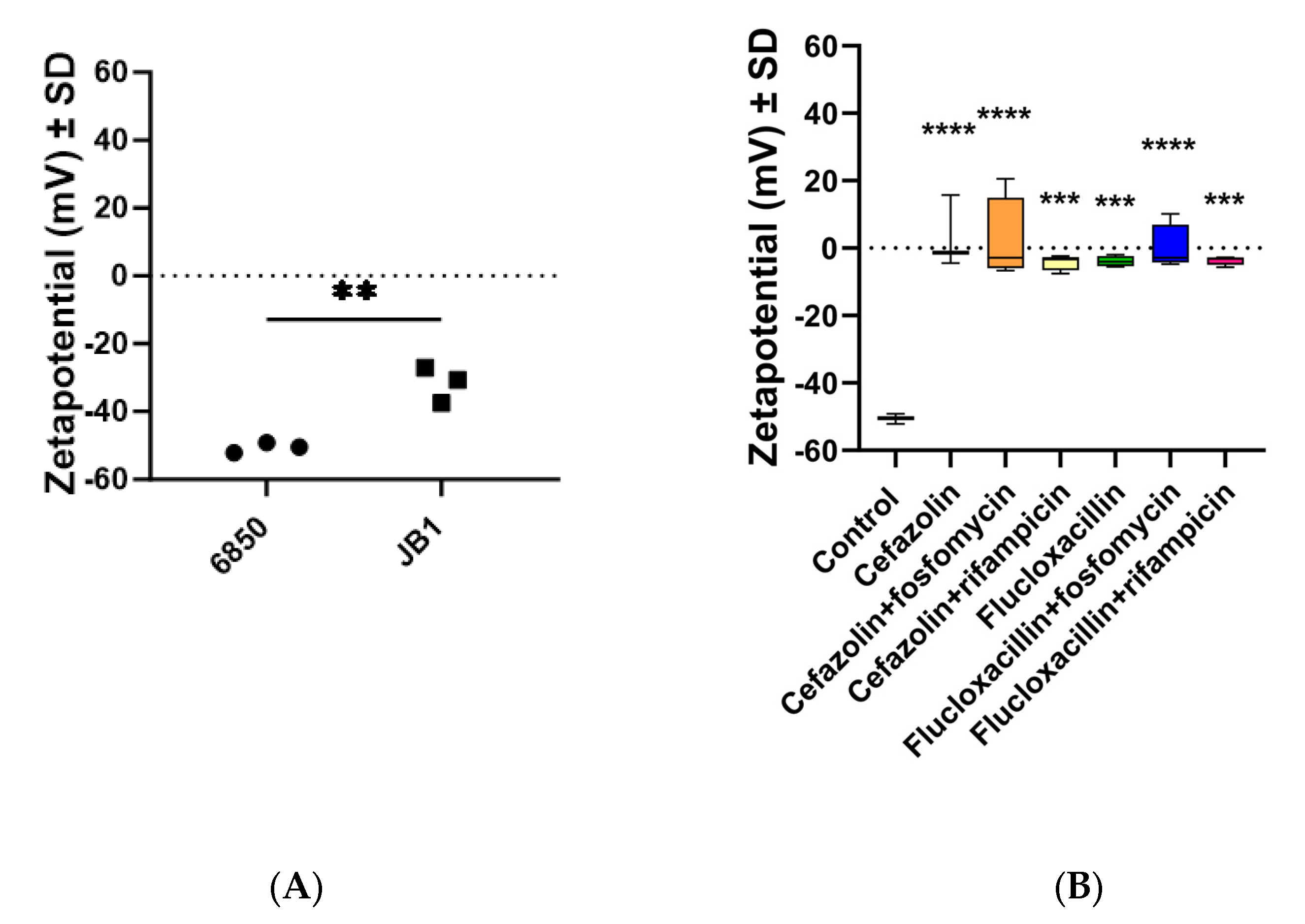
2.6. The Zeta Potential of All Strains Was Less Negative When Preincubated with Antibiotics
3. Discussion
4. Material and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Vaart, T.W.; Prins, J.M.; Soetekouw, R.; van Twillert, G.; Veenstra, J.; Herpers, B.L.; Rozemeijer, W.; Jansen, R.R.; Bonten, M.J.M.; van der Meer, J.T.M. All-Cause and Infection-Related Mortality in Staphylococcus aureus Bacteremia, a Multicenter Prospective Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac653. [Google Scholar] [CrossRef]
- Yarovoy, J.Y.; Monte, A.A.; Knepper, B.C.; Young, H.L. Epidemiology of Community-Onset Staphylococcus aureus Bacteremia. West J. Emerg. Med. 2019, 20, 438–442. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Bahrs, C.; Schumann, R.; Pletz, M.; Weis, S. Complicated and uncomplicated S. aureus bacteraemia: An international Delphi survey among infectious diseases experts on definitions and treatment. Clin. Microbiol. Infect. 2022, 28, 1026.e7–1026.e11. [Google Scholar] [CrossRef] [PubMed]
- Horino, T.; Hori, S. Metastatic infection during Staphylococcus aureus bacteremia. J. Infect. Chemother. 2020, 26, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ochomogo, M.; Lohans, C.T. β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med. Chem. 2021, 12, 1623–1639. [Google Scholar] [CrossRef]
- Kimmig, A.; Hagel, S.; Weis, S.; Bahrs, C.; Löffler, B.; Pletz, M.W. Management of Staphylococcus aureus Bloodstream Infections. Front. Med. 2020, 7, 616524. [Google Scholar] [CrossRef]
- Rieg, S.; Ernst, A.; Peyerl-Hoffmann, G.; Joost, I.; Camp, J.; Hellmich, M.; Kern, W.V.; Kaasch, A.J.; Seifert, H. Combination therapy with rifampicin or fosfomycin in patients with Staphylococcus aureus bloodstream infection at high risk for complications or relapse: Results of a large prospective observational cohort. J. Antimicrob. Chemother. 2020, 75, 2282–2290. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, P.-Y.; Wang, J.-T.; Chang, S.-C. Prevalence of fosfomycin resistance and gene mutations in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob. Resist. Infect. Control 2020, 9, 135. [Google Scholar] [CrossRef]
- Wehrli, W. Rifampin: Mechanisms of Action and Resistance. Rev. Infect. Dis. 1983, 5, S407–S411. [Google Scholar] [CrossRef]
- Grillo, S.; Pujol, M.; Miró, J.M.; López-Contreras, J.; Euba, G.; Gasch, O.; Boix-Palop, L.; Garcia-País, M.J.; Pérez-Rodríguez, M.T.; Gomez-Zorrilla, S.; et al. Cloxacillin plus fosfomycin versus cloxacillin alone for methicillin-susceptible Staphylococcus aureus bacteremia: A randomized trial. Nat. Med. 2023, 29, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Pujol, M.; Miró, J.-M.; Shaw, E.; Aguado, J.-M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.N.; Tamura, K. Rifampin Combination Therapy for Nonmycobacterial Infections. Clin. Microbiol. Rev. 2010, 23, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, M.; Meincke, C.; Vogt, K.; Ruhnke, M.; Lajous-Petter, A.M. Intracellular bactericidal activity of fosfomycin against staphylococci: A comparison with other antibiotics. Infection 1992, 20, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C. Small colony variants (SCVs) of Staphylococcus aureus—A bacterial survival strategy. Infect. Genet. Evol. 2014, 21, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.-P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial Activity against Intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef]
- Chung, P.Y. Immunotherapies for the prevention and treatment of Staphylococcus aureus infections: Updates and challenges. Pathog. Dis. 2023, 81, ftad016. [Google Scholar] [CrossRef]
- Conlon, B.P. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 2014, 36, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Mashayamombe, M.; Carda-Diéguez, M.; Mira, A.; Fitridge, R.; Zilm, P.S.; Kidd, S.P. Subpopulations in Strains of Staphylococcus aureus Provide Antibiotic Tolerance. Antibiotics 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Löffler, B.; Proctor, R.A. Persistence of Staphylococcus aureus: Multiple Metabolic Pathways Impact the Expression of Virulence Factors in Small-Colony Variants (SCVs). Front. Microbiol. 2020, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C. Staphylococcus aureus small colony variants: A challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents 2008, 31, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Vesga, O.; Groeschel, M.C.; Otten, M.F.; Brar, D.W.; Vann, J.M.; Proctor, R.A. Staphylococcus aureus Small Colony Variants Are Induced by the Endothelial Cell Intracellular Milieu. J. Infect. Dis. 1996, 173, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Onyango, L.A.; Dunstan, R.H.; Roberts, T.K.; Macdonald, M.M.; Gottfries, J. Phenotypic Variants of Staphylococci and Their Underlying Population Distributions Following Exposure to Stress. PLoS ONE 2013, 8, e77614. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Heitmann, V.; Hussain, M.; Viemann, D.; Roth, J.; von Eiff, C.; Peters, G.; Becker, K.; Löffler, B. Staphylococcus aureus Small-Colony Variants Are Adapted Phenotypes for Intracellular Persistence. J. Infect. Dis. 2010, 202, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Quiblier, C.; Hernandez, D.; Herzog, K.; Bodler, P.; Senn, M.M.; Gizard, Y.; Schrenzel, J.; François, P. Characterization of Streptococcus tigurinus Small-Colony Variants Causing Prosthetic Joint Infection by Comparative Whole-Genome Analyses. J. Clin. Microbiol. 2014, 52, 467–474. [Google Scholar] [CrossRef]
- Proctor, R.A.; Peters, G. Small Colony Variants in Staphylococcal Infections: Diagnostic and Therapeutic Implications. Clin. Infect. Dis. 1998, 27, 419–422. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.J.; Hartson, S.D.; Rogers, J.; Gustafson, J.E. Proteomic and Metabolomic Analyses of a Tea-Tree Oil-Selected Staphylococcus aureus Small Colony Variant. Antibiotics 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Husain, F.M.; Al-Kheraif, A.A.; Ali, A.; Haq, Q.M. Colistin Interaction and Surface Changes Associated with mcr-1 Conferred Plasmid Mediated Resistance in E. coli and A. veronii Strains. Pharmaceutics 2022, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.P.F.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta potential beyond materials science: Applications to bacterial systems and to the development of novel antimicrobials. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Weis, S.; Kesselmeier, M.; Davis, J.S.; Morris, A.M.; Lee, S.; Scherag, A.; Hagel, S.; Pletz, M.W. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2019, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.M.; Conlon, B.P.; Kidd, S.P. Antibiotic tolerance and the alternative lifestyles of Staphylococcus aureus. Essays Biochem. 2017, 61, 71–79. [Google Scholar] [CrossRef]
- Tran, N.N.; Morrisette, T.; Jorgensen, S.C.J.; Orench-Benvenutti, J.M.; Kebriaei, R. Current therapies and challenges for the treatment of Staphylococcus aureus biofilm-related infections. Pharmacotherapy 2023, 43, 816–832. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Kreis, C.A.; Hoerr, V.; Flint, L.; Hachmeister, M.; Geraci, J.; Bremer-Streck, S.; Kiehntopf, M.; Medina, E.; Kribus, M.; et al. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J. Antimicrob. Chemother. 2015, 71, 438–448. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Martins, K.B.; Ferreira, A.M.; Pereira, V.C.; Pinheiro, L.; de Oliveira, A.; Cunha, M.d.L.R.d.S.d. In vitro Effects of Antimicrobial Agents on Planktonic and Biofilm Forms of Staphylococcus saprophyticus Isolated from Patients with Urinary Tract Infections. Front. Microbiol. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Mandell, G.L. The Antimicrobial Activity of Rifampin: Emphasis on the Relation to Phagocytes. Rev. Infect. Dis. 1983, 5, S463–S467. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ting, Y.-P. Effect of sub-inhibitory antibacterial stress on bacterial surface properties and biofilm formation. Colloids Surf. B Biointerfaces 2013, 111, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, C.P.; Daniels, M.; Zhao, F.; Alegre, M.-L.; Chong, A.S.; Daum, R.S. Protective Immunity against Recurrent Staphylococcus aureus Skin Infection Requires Antibody and Interleukin-17A. Infect. Immun. 2014, 82, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential Roles of Interleukin-17A and -17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity 2009, 30, 108–119. [Google Scholar] [CrossRef]
- Kleinhenz, M.; Beesetty, P.; Yang, C.; Li, Z.; Montgomery, C.P. Antibiotic Treatment of Staphylococcus aureus Infection Inhibits the Development of Protective Immunity. Antimicrob. Agents Chemother. 2022, 66, e0227021. [Google Scholar] [CrossRef]
- Kriegeskorte, A.; Grubmüller, S.; Huber, C.; Kahl, B.C.; von Eiff, C.; Proctor, R.A.; Peters, G.; Eisenreich, W.; Becker, K. Staphylococcus aureus small colony variants show common metabolic features in central metabolism irrespective of the underlying auxotrophism. Front. Cell. Infect. Microbiol. 2014, 4, 141. [Google Scholar] [CrossRef]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef]
- Becker, K. Detection, Identification and Diagnostic Characterization of the Staphylococcal Small Colony-Variant (SCV) Phenotype. Antibiotics 2023, 12, 1446. [Google Scholar] [CrossRef]
- Loss, G.; Simões, P.M.; Valour, F.; Cortês, M.F.; Gonzaga, L.; Bergot, M.; Trouillet-Assant, S.; Josse, J.; Diot, A.; Ricci, E.; et al. Staphylococcus aureus Small Colony Variants (SCVs): News from a Chronic Prosthetic Joint Infection. Front. Cell. Infect. Microbiol. 2019, 9, 363. [Google Scholar] [CrossRef]
- Merritt, J.; Kadouri, D.; O’Toole, G.; Coico, R.; Kowalik, T.; Quarles, J.M.; Stevenson, B.; Taylor, R.K. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 22, 1B–1. [Google Scholar]


| (A) | ||||
| Antibiotics | 6850 MIC | JB1 MIC | ||
| Cefazolin | 0.5 | 0.08 ± 0.05 | ||
| Flucloxacillin | 0.17 ± 0.09 | 0.09 ± 0.04 | ||
| Fosfomycin | 0.85 ± 0.69 | 0.4 ± 0.2 | ||
| Rifampicin | 0.015 | 0.015 | ||
| (B) | ||||
| Antibiotic Combinations | 6850 MIC | 6850 FICI | JB1 MIC | JB1 FICI |
| Cefazolin + fosfomycin | 0.5 ± 0.01/ 1 ± 0.26 | indifferent | 0.16 ± 0.1/ 0.12 ± 0.11 | indifferent |
| Cefazolin + rifampicin | 0.08 ± 0.08/ 0.03 ± 0.02 | indifferent | 0.1 ± 0.5/ 0.002 ± 0.001 | synergistic |
| Flucloxacillin + fosfomycin | 0.375 ± 0.323/ 1 ± 1.3 | indifferent | 0.17 ± 0.1/ 0.35 ± 0.36 | indifferent |
| Flucloxacillin + rifampicin | 0.05 ± 0.02/ 0.015 ± 0.008 | synergistic | 0.15 ± 0.09/ 0.008 ± 0.006 | indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackemann, V.C.J.; Hagel, S.; Jandt, K.D.; Rödel, J.; Löffler, B.; Tuchscherr, L. The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study. Int. J. Mol. Sci. 2023, 24, 16308. https://doi.org/10.3390/ijms242216308
Hackemann VCJ, Hagel S, Jandt KD, Rödel J, Löffler B, Tuchscherr L. The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study. International Journal of Molecular Sciences. 2023; 24(22):16308. https://doi.org/10.3390/ijms242216308
Chicago/Turabian StyleHackemann, Valeria C. J., Stefan Hagel, Klaus D. Jandt, Jürgen Rödel, Bettina Löffler, and Lorena Tuchscherr. 2023. "The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study" International Journal of Molecular Sciences 24, no. 22: 16308. https://doi.org/10.3390/ijms242216308
APA StyleHackemann, V. C. J., Hagel, S., Jandt, K. D., Rödel, J., Löffler, B., & Tuchscherr, L. (2023). The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study. International Journal of Molecular Sciences, 24(22), 16308. https://doi.org/10.3390/ijms242216308









