Sex-Dependent Differences in Blood–Urine Barrier Are Subtle but Significant in Healthy and Chronically Inflamed Mouse Bladders
Abstract
1. Introduction
2. Results
2.1. Healthy Bladders of Control Mice
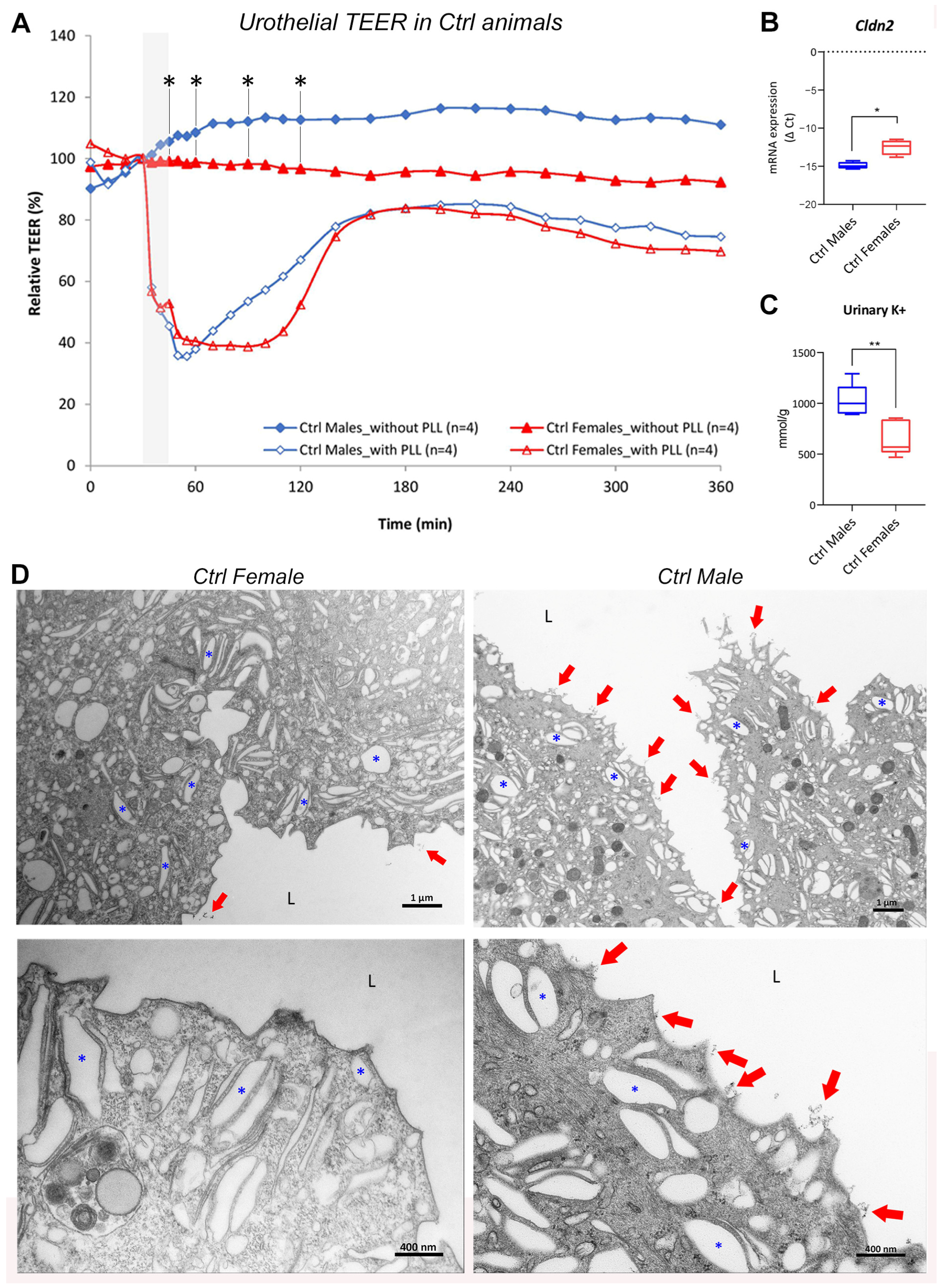
2.1.1. Urothelial Permeability Barrier Function Is Attenuated in Female Bladders Compared with Male Bladders
2.1.2. Molecular Structure and Function of Urothelial Permeability Barrier Differs between the Sexes
2.1.3. The Amount of Glycocalyx Covering the Urothelium Is Slightly Different in the Two Sexes
2.2. Chronically Inflamed Bladders of CYP-Treated Mice
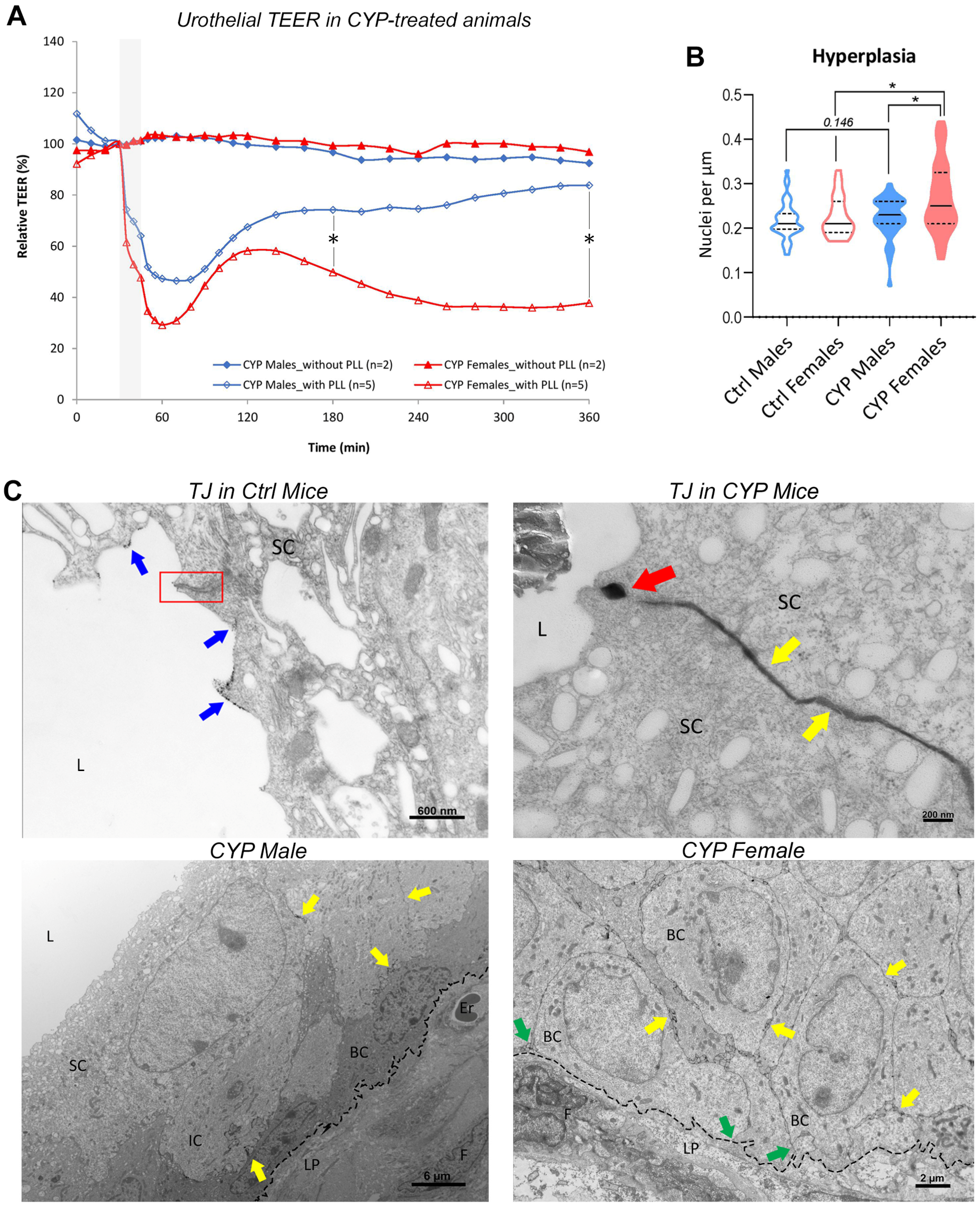
2.2.1. Restoration of Urothelial Permeability Barrier Function Is Impeded in Female Bladders
2.2.2. Urothelial Hyperplasia Is More Prominent in Female Bladders
2.2.3. Subtle Differences in Urothelial Cell Junction-Actin Cytoskeleton Network Are Present between the Sexes
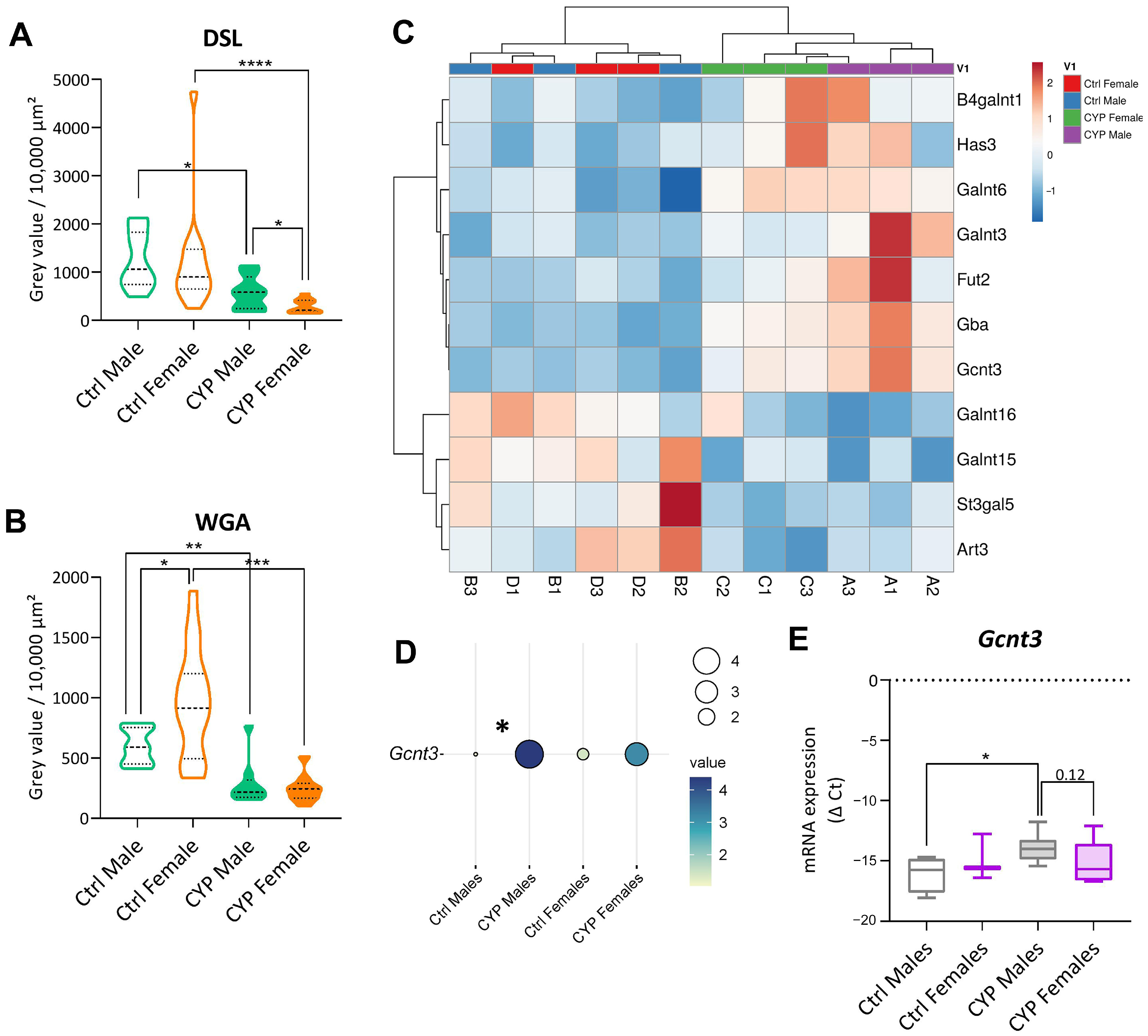
2.2.4. Urothelial Glycosylation Pattern Differs between the Sexes
3. Discussion
4. Materials and Methods
4.1. Animals and Induction of Chronic Bladder Inflammation
4.2. Ex Vivo Measurements of Urothelial Transepithelial Electrical Resistance
4.3. Determination of Potassium and Creatinine Concentration in Urine
4.4. Obtainment of Urothelial Tissue Samples, mRNA Extraction, and qPCR Analysis
4.5. RNA Sequencing Data
4.6. Immunofluorescence Labeling of Moesin and Quantitative Image Analysis
4.7. Lectin Histochemistry and Lectin Binding Evaluation
4.8. TEM Sample Preparation for the Glycocalyx Visualization
4.9. TEM Sample Preparation for the Permeability Study
4.10. Estimation of Urothelial Hyperplasia by Nuclear Density
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Manabe, M.; Wu, X.R.; Xu, C.; Surya, B.; Sun, T.T. Uroplakin I: A 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J. Cell Biol. 1990, 111, 1207–1216. [Google Scholar] [CrossRef]
- Parsons, C.L.; Greenspan, C.; Mulholland, S.G. The primary antibacterial defense mechanism of the bladder. Investig. Urol. 1975, 13, 72–78. [Google Scholar]
- Parsons, C.L.; Boychuk, D.; Jones, S.; Hurst, R.; Callahan, H. Bladder surface glycosaminoglycans: An epithelial permeability barrier. J. Urol. 1990, 143, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Xin, P.; Buckley, M.S.; Erickson, D.R.; Bhavanandan, V.P. Characterization of the rabbit homolog of human MUC1 glycoprotein isolated from bladder by affinity chromatography on immobilized jacalin. Glycobiology 2000, 10, 659–667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, S.A.; Diamond, J.M. Na+ transport by rabbit urinary bladder, a tight epithelium. J. Membr. Biol. 1976, 28, 1–40. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Frömter, E.; Diamond, J. Route of passive ion permeation in epithelia. Nat. New Biol. 1972, 235, 9–13. [Google Scholar] [CrossRef]
- Parsons, C.L. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007, 69, 9–16. [Google Scholar] [CrossRef]
- Lavelle, J.P.; Meyers, S.A.; Ruiz, W.G.; Buffington, C.A.; Zeidel, M.L.; Apodaca, G. Urothelial pathophysiological changes in feline interstitial cystitis: A human model. Am. J. Physiol. Renal Physiol. 2000, 278, F540–F553. [Google Scholar] [CrossRef]
- Veranic, P.; Erman, A.; Kerec-Kos, M.; Bogataj, M.; Mrhar, A.; Jezernik, K. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem. Cell Biol. 2009, 131, 129–139. [Google Scholar] [CrossRef]
- Shin, K.; Lee, J.; Guo, N.; Kim, J.; Lim, A.; Qu, L.; Mysorekar, I.U.; Beachy, P.A. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011, 472, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Batourina, E.; Schneider, K.; Souza, S.; Swayne, T.; Liu, C.; George, C.D.; Tate, T.; Dan, H.; Wiessner, G.; et al. Polyploid Superficial Cells that Maintain the Urothelial Barrier Are Produced via Incomplete Cytokinesis and Endoreplication. Cell Rep. 2018, 25, 464–477.e4. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.L.; Lilly, J.D.; Stein, P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J. Urol. 1991, 145, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Slobodov, G.; Feloney, M.; Gran, C.; Kyker, K.D.; Hurst, R.E.; Culkin, D.J. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J. Urol. 2004, 171, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.A.; de Groat, W.C. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 2007, 4, 46–54. [Google Scholar] [CrossRef]
- Montalbetti, N.; Rued, A.C.; Clayton, D.R.; Ruiz, W.G.; Bastacky, S.I.; Prakasam, H.S.; Eaton, A.F.; Kullmann, F.A.; Apodaca, G.; Carattino, M.D. Increased urothelial paracellular transport promotes cystitis. Am. J. Physiol. Renal Physiol. 2015, 309, F1070–F1081. [Google Scholar] [CrossRef]
- Montalbetti, N.; Rued, A.C.; Taiclet, S.N.; Birder, L.A.; Kullmann, F.A.; Carattino, M.D. Urothelial Tight Junction Barrier Dysfunction Sensitizes Bladder Afferents. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Meenan, R.T.; O’Keeffe Rosetti, M.C.; Brown, S.O.; Gao, S.Y.; Calhoun, E.A. Prevalence of interstitial cystitis symptoms in a managed care population. J. Urol. 2005, 174, 576–580. [Google Scholar] [CrossRef]
- Anger, J.T.; Dallas, K.B.; Bresee, C.; De Hoedt, A.M.; Barbour, K.E.; Hoggatt, K.J.; Goodman, M.T.; Kim, J.; Freedland, S.J. National prevalence of IC/BPS in women and men utilizing veterans health administration data. Front. Pain Res. 2022, 3, 925834. [Google Scholar] [CrossRef]
- Abelson, B.; Sun, D.; Que, L.; Nebel, R.A.; Baker, D.; Popiel, P.; Amundsen, C.L.; Chai, T.; Close, C.; DiSanto, M.; et al. Sex differences in lower urinary tract biology and physiology. Biol. Sex. Differ. 2018, 9, 45. [Google Scholar] [CrossRef]
- Peskar, D.; Kuret, T.; Lakota, K.; Erman, A. Molecular Profiling of Inflammatory Processes in a Mouse Model of IC/BPS: From the Complete Transcriptome to Major Sex-Related Histological Features of the Urinary Bladder. Int. J. Mol. Sci. 2023, 24, 5758. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, J.; Meyers, S.; Ramage, R.; Bastacky, S.; Doty, D.; Apodaca, G.; Zeidel, M.L. Bladder permeability barrier: Recovery from selective injury of surface epithelial cells. Am. J. Physiol. Renal Physiol. 2002, 283, F242–F253. [Google Scholar] [CrossRef]
- Erman, A.; Kerec Kos, M.; Žakelj, S.; Resnik, N.; Romih, R.; Veranič, P. Correlative study of functional and structural regeneration of urothelium after chitosan-induced injury. Histochem. Cell Biol. 2013, 140, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Tzan, C.J.; Berg, J.R.; Lewis, S.A. Modification of epithelial permeability by cationic polypeptides. Am. Physiol. Soc. 1993, 265, C1637–C1647. [Google Scholar] [CrossRef] [PubMed]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef]
- Amasheh, S.; Milatz, S.; Krug, S.M.; Markov, A.G.; Günzel, D.; Amasheh, M.; Fromm, M. Tight junction proteins as channel formers and barrier builders. Ann. N. Y. Acad. Sci. 2009, 1165, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef]
- Montalbetti, N.; Stocker, S.D.; Apodaca, G.; Bastacky, S.I.; Carattino, M.D. Urinary K+ promotes irritative voiding symptoms and pain in the face of urothelial barrier dysfunction. Sci. Rep. 2019, 9, 5509. [Google Scholar] [CrossRef]
- Goldfischer, S.; Kress, Y.; Coltoff-Schiller, B.; Berman, J. Primary fixation in osmium-potassium ferrocyanide: The staining of glycogen, glycoproteins, elastin, an intranuclear reticular structure, and intercisternal trabeculae. J. Histochem. Cytochem. 1981, 29, 1105–1111. [Google Scholar] [CrossRef]
- Coltoff-Schiller, B.; Goldfischer, S. Glycosaminoglycans in the rat aorta. Ultrastructural localization with toluidine blue O and osmium--ferrocyanide procedure. Am. J. Pathol. 1981, 105, 232–240. [Google Scholar] [PubMed]
- Jost, S.P. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 57, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R.; Kong, X.P.; Pellicer, A.; Kreibich, G.; Sun, T.T. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009, 75, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Speck, O.; Hughes, S.C.; Noren, N.K.; Kulikauskas, R.M.; Fehon, R.G. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature 2003, 421, 83–87. [Google Scholar] [CrossRef]
- Peskar, D.; Kuret, T.; Jeruc, J.; Erman, A. Lectins as Biomarkers of IC/BPS Disease: A Comparative Study of Glycosylation Patterns in Human Pathologic Urothelium and IC/BPS Experimental Models. Diagnostics 2022, 12, 1078. [Google Scholar] [CrossRef]
- Yeh, J.C.; Ong, E.; Fukuda, M. Molecular cloning and expression of a novel beta-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J. Biol. Chem. 1999, 274, 3215–3221. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Lacerda Mariano, L.; Ingersoll, M.A. The immune response to infection in the bladder. Nat. Rev. Urol. 2020, 17, 439–458. [Google Scholar] [CrossRef]
- Raju, P.; Shashikanth, N.; Tsai, P.Y.; Pongkorpsakol, P.; Chanez-Paredes, S.; Steinhagen, P.R.; Kuo, W.T.; Singh, G.; Tsukita, S.; Turner, J.R. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J. Clin. Investig. 2020, 130, 5197–5208. [Google Scholar] [CrossRef]
- Torres-Pinzon, D.L.; Ralph, D.L.; Veiras, L.C.; McDonough, A.A. Sex-specific adaptations to high-salt diet preserve electrolyte homeostasis with distinct sodium transporter profiles. Am. J. Physiol. Cell Physiol. 2021, 321, C897–C909. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, M.J.; Rosen, S.I.; Knight, L.C.; Maurer, A.H.; Hanno, P.M.; Ruggieri, M.R. Bladder permeability in interstitial cystitis is similar to that of normal volunteers: Direct measurement by transvesical absorption of 99mtechnetium-diethylenetriaminepentaacetic acid. J. Urol. 1994, 151, 346–349. [Google Scholar] [CrossRef]
- Boudes, M.; Uvin, P.; Kerselaers, S.; Vennekens, R.; Voets, T.; De Ridder, D. Functional characterization of a chronic cyclophosphamide-induced overactive bladder model in mice. Neurourol. Urodyn. 2011, 30, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, A.V.; Zhdanov, A.V.; Mallel, G.; Dinan, T.G.; Cryan, J.F. The mouse cyclophosphamide model of bladder pain syndrome: Tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol. Rep. 2014, 2, e00260. [Google Scholar] [CrossRef] [PubMed]
- Romih, R.; Veranic, P.; Jezernik, K. Actin filaments during terminal differentiation of urothelial cells in the rat urinary bladder. Histochem. Cell Biol. 1999, 112, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef]
- Sato, N.; Funayama, N.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J. Cell Sci. 1992, 103 Pt 1, 131–143. [Google Scholar] [CrossRef]
- Wyndaele, J.J.J.; Riedl, C.; Taneja, R.; Lovász, S.; Ueda, T.; Cervigni, M. GAG replenishment therapy for bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 2019, 38, 535–544. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef]
- Xi, X.; Wang, J.; Qin, Y.; Huang, W.; You, Y.; Zhan, J. Glycosylated modification of MUC1 maybe a new target to promote drug sensitivity and efficacy for breast cancer chemotherapy. Cell Death Dis. 2022, 13, 708. [Google Scholar] [CrossRef]
- Erzar, E.; Kerec Kos, M.; Lakota, K.; Veranič, P.; Erman, A. Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice? Int. J. Mol. Sci. 2020, 21, 3502. [Google Scholar] [CrossRef]
- Yu, Z.; Liao, J.; Chen, Y.; Zou, C.; Zhang, H.; Cheng, J.; Liu, D.; Li, T.; Zhang, Q.; Li, J.; et al. Single-Cell Transcriptomic Map of the Human and Mouse Bladders. J. Am. Soc. Nephrol. 2019, 30, 2159–2176. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peskar, D.; Kerec Kos, M.; Cerkvenik, U.; Nemec Svete, A.; Erman, A. Sex-Dependent Differences in Blood–Urine Barrier Are Subtle but Significant in Healthy and Chronically Inflamed Mouse Bladders. Int. J. Mol. Sci. 2023, 24, 16296. https://doi.org/10.3390/ijms242216296
Peskar D, Kerec Kos M, Cerkvenik U, Nemec Svete A, Erman A. Sex-Dependent Differences in Blood–Urine Barrier Are Subtle but Significant in Healthy and Chronically Inflamed Mouse Bladders. International Journal of Molecular Sciences. 2023; 24(22):16296. https://doi.org/10.3390/ijms242216296
Chicago/Turabian StylePeskar, Dominika, Mojca Kerec Kos, Uroš Cerkvenik, Alenka Nemec Svete, and Andreja Erman. 2023. "Sex-Dependent Differences in Blood–Urine Barrier Are Subtle but Significant in Healthy and Chronically Inflamed Mouse Bladders" International Journal of Molecular Sciences 24, no. 22: 16296. https://doi.org/10.3390/ijms242216296
APA StylePeskar, D., Kerec Kos, M., Cerkvenik, U., Nemec Svete, A., & Erman, A. (2023). Sex-Dependent Differences in Blood–Urine Barrier Are Subtle but Significant in Healthy and Chronically Inflamed Mouse Bladders. International Journal of Molecular Sciences, 24(22), 16296. https://doi.org/10.3390/ijms242216296





