Relevance of Phytochemical Taste for Anti-Cancer Activity: A Statistical Inquiry
Abstract
1. Introduction
2. Results
2.1. The Correlation between Anti-Inflammatory/Anti-Cancer Activity and Taste/Chemical Class
2.2. Correlation between Anti-Cancer and Anti-Inflammatory Activity
3. Discussion
3.1. Taste as an Important Determinant of Pharmacodynamic Activity
3.2. Upgrade of the Previously Reported Taste—AIA Associations
3.3. The Pharmacodynamic Activity of Phytochemicals: Taste Has Predictive Primacy over Chemical Classes
3.4. Bitter—Sweet: Are They in Opposition?
3.5. Anti-Inflammatory Activity- Anti-Cancer Activity Association Is Independent of Taste
3.6. Anti-Cancer Activity—Is Bitter Better?
3.7. Anti-Cancer Activity—Is Sweet Worse?
3.8. Limitations of the Study
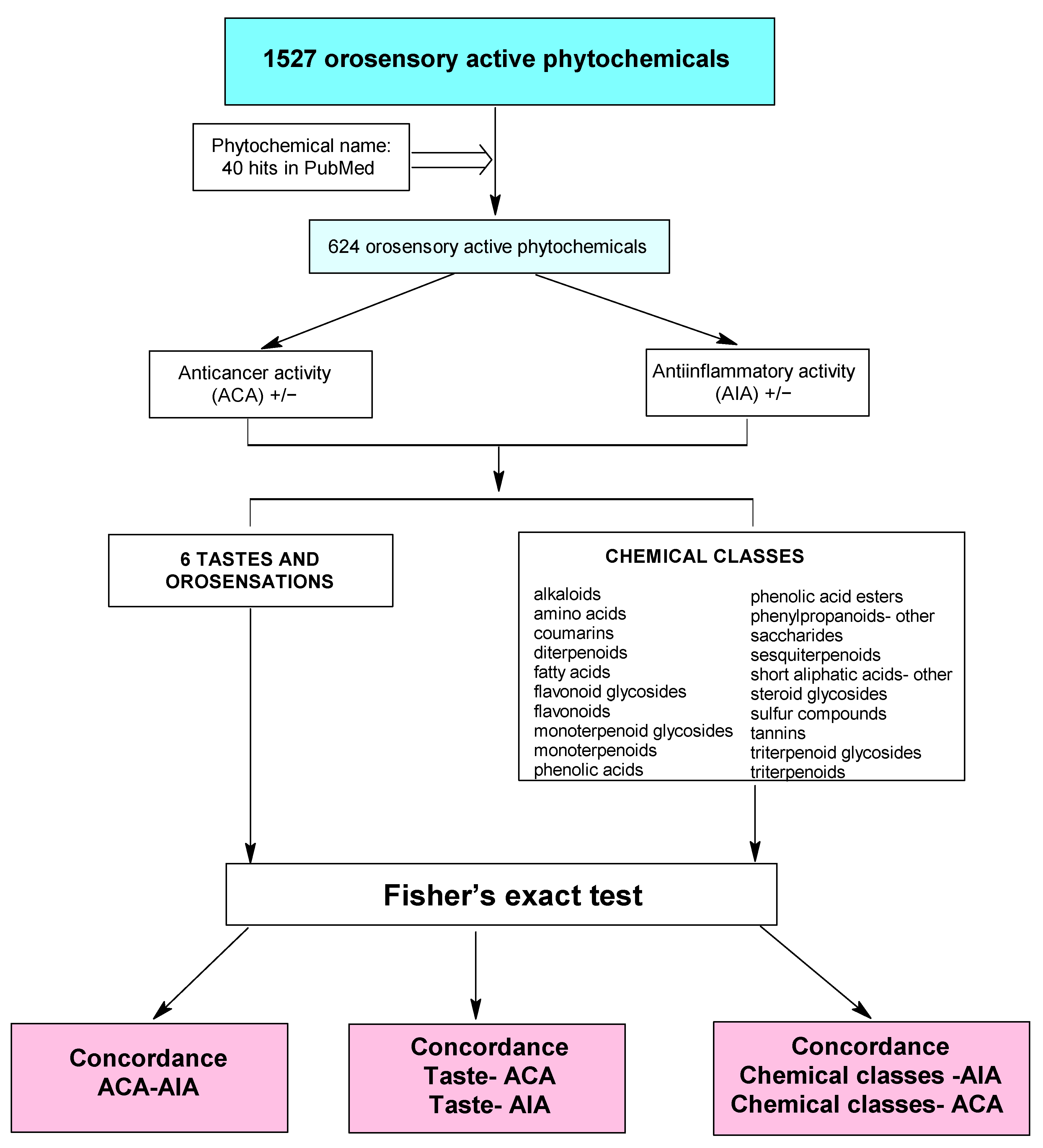
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | anti-cancer activity |
| AIA | anti-inflammatory activity |
| chemClass | chemical class |
| PMTDB | PlantMolecularTasteDB |
| PDA | pharmacodynamic activity |
| TASRs | taste receptors |
References
- Behrens, M.; Lang, T. Extra-Oral Taste Receptors-Function, Disease, and Perspectives. Front. Nutr. 2022, 9, 881177. [Google Scholar] [CrossRef]
- Sanematsu, K.; Yoshida, R.; Shigemura, N.; Ninomiya, Y. Structure, function, and signaling of taste G-protein-coupled receptors. Curr. Pharm. Biotechnol. 2014, 15, 951–961. [Google Scholar] [CrossRef]
- Gilca, M.; Dragos, D. Extraoral Taste Receptor Discovery: New Light on Ayurvedic Pharmacology. Evid. -Based Complement. Altern. Med. 2017, 2017, 5435831. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014, 223, 827–871. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.-H.; Lifshitz, L.M.; ZhuGe, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 2011, 105, 4–13. [Google Scholar] [CrossRef]
- Ziegler, F.; Steuer, A.; Di Pizio, A.; Behrens, M. Physiological activation of human and mouse bitter taste receptors by bile acids. Commun. Biol. 2023, 6, 612. [Google Scholar] [CrossRef]
- Gradinaru, T.-C.; Petran, M.; Dragos, D.; Gilca, M. Plant Molecular Taste DB: A Database of Taste Active Phytochemicals. Front. Pharmacol. 2022, 12, 751712. [Google Scholar] [CrossRef]
- Behrens, M.; Somoza, V. Gastrointestinal taste receptors: Could tastants become drugs? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Di Pizio, A.; Behrens, M.; Krautwurst, D. Beyond the Flavour: The Potential Druggability of Chemosensory G Protein-Coupled Receptors. Int. J. Mol. Sci. 2019, 20, 1402. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, O.; Drago, F. Pharmacological significance of extra-oral taste receptors. Eur. J. Pharmacol. 2021, 910, 174480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xi, R.; Liu, J.; Peng, X.; Zhao, L.; Zhou, X.; Li, J.; Zheng, X.; Xu, X. TAS2R16 Activation Suppresses LPS-Induced Cytokine Expression in Human Gingival Fibroblasts. Front. Immunol. 2021, 12, 726546. [Google Scholar] [CrossRef]
- Tiroch, J.; Sterneder, S.; Di Pizio, A.; Lieder, B.; Hoelz, K.; Holik, A.-K.; Pignitter, M.; Behrens, M.; Somoza, M.; Ley, J.P.; et al. Bitter Sensing TAS2R50 Mediates the trans-Resveratrol-Induced Anti-inflammatory Effect on Interleukin 6 Release in HGF-1 Cells in Culture. J. Agric. Food Chem. 2021, 69, 13339–13349. [Google Scholar] [CrossRef]
- Costa, A.R.; Duarte, A.C.; Costa-Brito, A.R.; Gonçalves, I.; Santos, C.R.A. Bitter taste signaling in cancer. Life Sci. 2023, 315, 121363. [Google Scholar] [CrossRef] [PubMed]
- Zehentner, S.; Reiner, A.T.; Grimm, C.; Somoza, V. The role of bitter taste receptors in cancer: A systematic review. Cancers 2021, 13, 5891. [Google Scholar] [CrossRef]
- Singh, N.; Chakraborty, R.; Bhullar, R.P.; Chelikani, P. Differential expression of bitter taste receptors in non-cancerous breast epithelial and breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 499–503. [Google Scholar] [CrossRef]
- Martin, L.T.P.; Nachtigal, M.W.; Selman, T.; Nguyen, E.; Salsman, J.; Dellaire, G.; Dupré, D.J. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol. Cell. Biochem. 2019, 454, 203–214. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, Y.-S.; Lee, K.E.; Park, T.H.; Kim, Y. Anti-cancer stemness and anti-invasive activity of bitter taste receptors, TAS2R8 and TAS2R10, in human neuroblastoma cells. PLoS ONE 2017, 12, e0176851. [Google Scholar] [CrossRef]
- Singh, N.; Shaik, F.A.; Myal, Y.; Chelikani, P. Chemosensory bitter taste receptors T2R4 and T2R14 activation attenuates proliferation and migration of breast cancer cells. Mol. Cell. Biochem. 2020, 465, 199–214. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Askeland, E.J.; Newton, M.R.; O’Donnell, M.A.; Luo, Y. Bladder Cancer Immunotherapy: BCG and Beyond. Adv. Urol. 2012, 2012, 181987. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Miyahara, E.; Ohshita, A.; Kawabuchi, Y.; Ohta, K.; Shimizu, K.; Minami, K.; Hihara, J.; Sawamura, A.; Toge, T. Locoregional immunotherapy of malignant effusion from colorectal cancer using the streptococcal preparation OK-432 plus interleukin-2: Induction of autologous tumor-reactive CD4+ Th1 killer lymphocytes. Br. J. Cancer 2003, 89, 1876–1884. [Google Scholar] [CrossRef]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Palli, D.; Masala, G.; Del Giudice, G.; Plebani, M.; Basso, D.; Berti, D.; Numans, M.E.; Ceroti, M.; Peeters, P.H.M.; Bueno de Mesquita, H.B.; et al. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int. J. Cancer 2007, 120, 859–867. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Song, C.; Lv, J.; Liu, Y.; Chen, J.G.; Ge, Z.; Zhu, J.; Dai, J.; Du, L.-B.; Yu, C.; Guo, Y.; et al. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw. Open 2019, 2, e195718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, Z.; Wu, W.; Ruan, L.; Yu, C.; Xi, Y.; Wang, L.; Wang, K.; Mo, J.; Zhao, S. Chronic Hepatitis Virus Infection Are Associated With High Risk of Gastric Cancer: A Systematic Review and Cumulative Analysis. Front. Oncol. 2021, 11, 703558. [Google Scholar] [CrossRef] [PubMed]
- Khan, G. Epstein-Barr virus, cytokines, and inflammation: A cocktail for the pathogenesis of Hodgkin’s lymphoma? Exp. Hematol. 2006, 34, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; DE Medeiros Fernandes, T.A.A.; DE Azevedo, J.C.V.; Cobucci, R.N.O.; DE Carvalho, M.G.F.; Andrade, V.S.; DE Araújo, J.M.G. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Tsujimoto, H.; Ueno, H.; Xie, Q.; Shinomiya, N. Helicobacter pylori-Mediated Immunity and Signaling Transduction in Gastric Cancer. J. Clin. Med. 2020, 9, 3699. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Zolondick, A.A.; Gaudino, G.; Xue, J.; Pass, H.I.; Carbone, M.; Yang, H. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches—A narrative review. Precis. Cancer Med. 2021, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Meaza, I.; Wise, J.P.S. Inflammatory effects of hexavalent chromium in the lung: A comprehensive review. Toxicol. Appl. Pharmacol. 2022, 455, 116265. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Huang, X.; Le, Q.-T.; Giaccia, A.J. MiR-210—Micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef]
- Qiao, Y.; He, H.; Jonsson, P.; Sinha, I.; Zhao, C.; Dahlman-Wright, K. AP-1 Is a Key Regulator of Proinflammatory Cytokine TNFα-mediated Triple-negative Breast Cancer Progression. J. Biol. Chem. 2016, 291, 5068–5079. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; Holt, D.; Fulton, A. Prostaglandin E 2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011, 30, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, K.; Han, J.Y.; Lim, J.M.; Song, Y.S. Modulation of inflammatory signaling pathways by phytochemicals in ovarian cancer. Genes Nutr. 2011, 6, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and Mechanisms of Kaempferol in the Management of Cancers through Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef] [PubMed]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: Focus on molecular mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Madka, V.; Rao, C.V. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr. Cancer Drug Targets 2013, 13, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Dragoș, D.; Petran, M.; Gradinaru, T.-C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M. Taste of phytocompounds: A better predictor for ethnopharmacological activities of medicinal plants than the phytochemical class? J. Ethnopharmacol. 2018, 220, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Shi, P.; Gao, C.; Ma, P.; Xing, H.; Ke, Q.; Zhang, D. Data-Driven Elucidation of Flavor Chemistry. J. Agric. Food Chem. 2023, 71, 6789–6802. [Google Scholar] [CrossRef]
- Rojas, C.; Ballabio, D.; Pacheco Sarmiento, K.; Pacheco Jaramillo, E.; Mendoza, M.; García, F. ChemTastesDB: A curated database of molecular tastants. Food Chem. Mol. Sci. 2022, 4, 100090. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Sun, L.; Wang, X.; Huang, X.; Luo, T. Isoschaftoside Inhibits Lipopolysaccharide-Induced Inflammation in Microglia through Regulation of HIF-1α-Mediated Metabolic Reprogramming. Evid.-Based Complement Altern. Med. 2022, 2022, 5227335. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.P.; de Garcia, E.F.; de Oliveira, M.A.; Candido, L.C.M.; Coelho, F.M.; Costa, V.V.; Batista, N.V.; Queiroz-Junior, C.M.; Brito, L.F.; Sousa, L.P.; et al. cis-Aconitic Acid, a Constituent of Echinodorus grandiflorus Leaves, Inhibits Antigen-Induced Arthritis and Gout in Mice. Planta Med. 2022, 88, 1123–1131. [Google Scholar] [CrossRef]
- Sharma, P.; Yi, R.; Nayak, A.P.; Wang, N.; Tang, F.; Knight, M.J.; Pan, S.; Oliver, B.; Deshpande, D.A. Bitter Taste Receptor Agonists Mitigate Features of Allergic Asthma in Mice. Sci. Rep. 2017, 7, 46166. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Salvator, H.; Mantov, N.; Abrial, C.; Brollo, M.; Faisy, C.; Naline, E.; Couderc, L.J.; Devillier, P. Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Front. Physiol. 2019, 10, 1267. [Google Scholar] [CrossRef]
- Salvestrini, V.; Ciciarello, M.; Pensato, V.; Simonetti, G.; Laginestra, M.A.; Bruno, S.; Pazzaglia, M.; De Marchi, E.; Forte, D.; Orecchioni, S.; et al. Denatonium as a Bitter Taste Receptor Agonist Modifies Transcriptomic Profile and Functions of Acute Myeloid Leukemia Cells. Front. Oncol. 2020, 10, 1225. [Google Scholar] [CrossRef]
- Hung, J.; Perez, S.M.; Dasa, S.S.K.; Hall, S.P.; Heckert, D.B.; Murphy, B.P.; Crawford, H.C.; Kelly, K.A.; Brinton, L.T. A Bitter Taste Receptor as a Novel Molecular Target on Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma. Pharmaceuticals 2023, 16, 389. [Google Scholar] [CrossRef]
- Gilca, M.; Barbulescu, A. Taste of medicinal plants: A potential tool in predicting ethnopharmacological activities? J. Ethnopharmacol. 2015, 174, 464–473. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Durand, M.; Turcotte, I.; Larouche-Anctil, E.; Sylla, M.; Zaidan, S.; Chartrand-Lefebvre, C.; Bunet, R.; Ramani, H.; Sadouni, M. Upregulated IL-32 expression and reduced gut short chain fatty acid caproic acid in people living with HIV with subclinical atherosclerosis. Front. Immunol. 2021, 12, 664371. [Google Scholar] [CrossRef]
- Lee, A.A.; Owyang, C. Sugars, Sweet Taste Receptors, and Brain Responses. Nutrients 2017, 9, 653. [Google Scholar] [CrossRef]
- Smith, N.J.; Grant, J.N.; Moon, J.I.; So, S.S.; Finch, A.M. Critically evaluating sweet taste receptor expression and signaling through a molecular pharmacology lens. FEBS J. 2021, 288, 2660–2672. [Google Scholar] [CrossRef]
- Di Pizio, A.; Ben Shoshan-Galeczki, Y.; Hayes, J.E.; Niv, M.Y. Bitter and sweet tasting molecules: It’s complicated. Neurosci. Lett. 2019, 700, 56–63. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Nissim, I.; Ben Abu, N.; Borgonovo, G.; Bassoli, A.; Niv, M.Y. Bitter or not? BitterPredict, a tool for predicting taste from chemical structure. Sci. Rep. 2017, 7, 12074. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest. 2014, 124, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Maina, I.W.; Workman, A.D.; Cohen, N.A. The role of bitter and sweet taste receptors in upper airway innate immunity: Recent advances and future directions. World J. Otorhinolaryngol.-Head Neck Surg. 2018, 4, 200–208. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. Taste receptors in innate immunity. Cell. Mol. Life Sci. 2015, 72, 217–236. [Google Scholar] [CrossRef]
- Di Pizio, A.; Waterloo, L.A.W.; Brox, R.; Löber, S.; Weikert, D.; Behrens, M.; Gmeiner, P.; Niv, M.Y. Rational design of agonists for bitter taste receptor TAS2R14: From modeling to bench and back. Cell. Mol. Life Sci. 2020, 77, 531–542. [Google Scholar] [CrossRef]
- Nakagita, T.; Taketani, C.; Narukawa, M.; Hirokawa, T.; Kobayashi, T.; Misaka, T. Ibuprofen, a Nonsteroidal Anti-Inflammatory Drug, is a Potent Inhibitor of the Human Sweet Taste Receptor. Chem. Senses 2020, 45, 667–673. [Google Scholar] [CrossRef]
- Cardenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry 2015, 113, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Liem, D.G.; Russell, C.G. The Influence of Taste Liking on the Consumption of Nutrient Rich and Nutrient Poor Foods. Front. Nutr. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.; Simon, S.A. Physiology of Taste Processing in the Tongue, Gut, and Brain. Compr. Physiol. 2021, 11, 2489–2523. [Google Scholar] [CrossRef]
- Cavallo, C.; Cicia, G.; Del Giudice, T.; Sacchi, R.; Vecchio, R. Consumers’ Perceptions and Preferences for Bitterness in Vegetable Foods: The Case of Extra-Virgin Olive Oil and Brassicaceae-A Narrative Review. Nutrients 2019, 11, 1164. [Google Scholar] [CrossRef]
- Beckett, E.L.; Martin, C.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. Bitter taste genetics—The relationship to tasting, liking, consumption and health. Food Funct. 2014, 5, 3040–3054. [Google Scholar] [CrossRef]
- Freeman, C.R.; Zehra, A.; Ramirez, V.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Impact of sugar on the body brain and behavior. Front. Biosci. 2018, 23, 4704. [Google Scholar] [CrossRef]
- Aravindaram, K.; Yang, N.-S. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef]
- Chinnasamy, P.; Arumugam, R. In silico prediction of anticarcinogenic bioactivities of traditional anti-inflammatory plants used by tribal healers in Sathyamangalam wildlife Sanctuary, India. Egypt. J. Basic Appl. Sci. 2018, 5, 265–279. [Google Scholar] [CrossRef][Green Version]
- Pallavi, B.; Sharma, P.; Baig, N.; Kumar Madduluri, V.; Sah, A.K.; Saumya, U.; Dubey, U.S.; Shukla, P. Quinoline Glycoconjugates as Potentially Anticancer and Anti-Inflammatory Agents: An Investigation Involving Synthesis, Biological Screening, and Docking. ChemistrySelect 2020, 5, 9878–9882. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A.-A. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1797–1806. [Google Scholar] [CrossRef]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: A preliminary randomized controlled clinical trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; R, P.; et al. A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. 2016, 9, 683–691. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, D.H.; Kim, S.-W.; Kim, M.-J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S., II; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc(TM)) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer 2016, 16, 173–186. [Google Scholar] [CrossRef]
- Elwood, P.; Protty, M.; Morgan, G.; Pickering, J.; Delon, C.; Watkins, J. Aspirin and cancer: Biological mechanisms and clinical outcomes. Open Biol. 2022, 12, 220124. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, T.; Gan, Y.; Li, W.; Wang, C.; Gong, Y.; Lu, Z. Associations between aspirin use and the risk of cancers: A meta-analysis of observational studies. BMC Cancer 2018, 18, 288. [Google Scholar] [CrossRef]
- Sebastian, N.T.; Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.A.; Huang, Z.; Burns, A.; Sukhatme, V.; Lowe, M.C.; Ramalingam, S.S.; et al. The Association of Improved Overall Survival with NSAIDs in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin. Lung Cancer 2023, 24, 287–294. [Google Scholar] [CrossRef]
- Rahme, E.; Ghosn, J.; Dasgupta, K.; Rajan, R.; Hudson, M. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer 2005, 5, 159. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Ren, T.; Xu, X.; Jin, J. Association of aspirin and nonaspirin NSAIDs therapy with the incidence risk of hepatocellular carcinoma: A systematic review and meta-analysis on cohort studies. Eur. J. Cancer Prev. 2022, 31, 35–43. [Google Scholar] [CrossRef]
- Majidi, A.; Na, R.; Jordan, S.J.; DeFazio, A.; Obermair, A.; Friedlander, M.; Grant, P.; Webb, P.M. Common analgesics and ovarian cancer survival: The Ovarian cancer Prognosis And Lifestyle (OPAL) Study. J. Natl. Cancer Inst. 2023, 115, 570–577. [Google Scholar] [CrossRef]
- Sasamoto, N.; Babic, A.; Vitonis, A.F.; Titus, L.; Cramer, D.W.; Trabert, B.; Tworoger, S.S.; Terry, K.L. Common Analgesic Use for Menstrual Pain and Ovarian Cancer Risk. Cancer Prev. Res. 2021, 14, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Curhan, G.; Hankinson, S.E.; Kantoff, P.; Atkins, M.B.; Stampfer, M.; Choueiri, T.K. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch. Intern. Med. 2011, 171, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Poole, E.M.; White, E.; Visvanathan, K.; Adami, H.-O.; Anderson, G.L.; Brasky, T.M.; Brinton, L.A.; Fortner, R.T.; Gaudet, M.; et al. Analgesic Use and Ovarian Cancer Risk: An Analysis in the Ovarian Cancer Cohort Consortium. J. Natl. Cancer Inst. 2019, 111, 137–145. [Google Scholar] [CrossRef]
- Cryer, B.; Feldman, M. Cyclooxygenase-1 and Cyclooxygenase-2 Selectivity of Widely Used Nonsteroidal Anti-Inflammatory Drugs. Am. J. Med. 1998, 104, 413–421. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, C.; Zeng, L.; Liu, G.; Jiang, W.; Zhang, X.; Chen, S.; Guo, J.; Jian, X.; Ouyang, J.; et al. High COX-2 expression in cancer-associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol. Carcinog. 2020, 59, 265–280. [Google Scholar] [CrossRef]
- Du, J.; Feng, J.; Luo, D.; Peng, L. Prognostic and Clinical Significance of COX-2 Overexpression in Laryngeal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 854946. [Google Scholar] [CrossRef]
- Rigas, B.; Goldman, I.S.; Levine, L. Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 1993, 122, 518–523. [Google Scholar]
- McLemore, T.L.; Hubbard, W.C.; Litterst, C.L.; Liu, M.C.; Miller, S.; McMahon, N.A.; Eggleston, J.C.; Boyd, M.R. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988, 48, 3140–3147. [Google Scholar] [PubMed]
- Schrey, M.P.; Patel, K. V Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. Br. J. Cancer 1995, 72, 1412–1419. [Google Scholar] [CrossRef][Green Version]
- Hong, D.S.; Parikh, A.; Shapiro, G.I.; Varga, A.; Naing, A.; Meric-Bernstam, F.; Ataman, Ö.; Reyderman, L.; Binder, T.A.; Ren, M.; et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE 2 -receptor E-type 4 (EP4), in patients with advanced cancers. J. Immunother. Cancer 2020, 8, e000222. [Google Scholar] [CrossRef] [PubMed]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E(2) Receptor 4 in Cancer Therapeutics and Immunotherapy. Front. Pharmacol. 2020, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Take, Y.; Koizumi, S.; Nagahisa, A. Prostaglandin E Receptor 4 Antagonist in Cancer Immunotherapy: Mechanisms of Action. Front. Immunol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Carey, R.M.; McMahon, D.B.; Miller, Z.A.; Kim, T.; Rajasekaran, K.; Gopallawa, I.; Newman, J.G.; Basu, D.; Nead, K.T.; White, E.A.; et al. T2R bitter taste receptors regulate apoptosis and may be associated with survival in head and neck squamous cell carcinoma. Mol. Oncol. 2022, 16, 1474–1492. [Google Scholar] [CrossRef]
- Carey, R.M.; Kim, T.; Cohen, N.A.; Lee, R.J.; Nead, K.T. Impact of sweet, umami, and bitter taste receptor (TAS1R and TAS2R) genomic and expression alterations in solid tumors on survival. Sci. Rep. 2022, 12, 8937. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Singh, N.; Upadhyaya, J.; Sikarwar, A.S.; Arakawa, M.; Dakshinamurti, S.; Bhullar, R.P.; Duan, K.; Chelikani, P. Analysis of the expression of human bitter taste receptors in extraoral tissues. Mol. Cell. Biochem. 2017, 426, 137–147. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018, 560, 499–503. [Google Scholar] [CrossRef]
- Dong, H.; Kong, X.; Wang, X.; Liu, Q.; Fang, Y.; Wang, J. The Causal Effect of Dietary Composition on the Risk of Breast Cancer: A Mendelian Randomization Study. Nutrients 2023, 15, 2586. [Google Scholar] [CrossRef]
- Llaha, F.; Gil-Lespinard, M.; Unal, P.; de Villasante, I.; Castañeda, J.; Zamora-Ros, R. Consumption of Sweet Beverages and Cancer Risk. A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 516. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.; Tong, J.; Lacmanovic, V.; Agbonghae, C.; Minaya, D.M.; Czaja, K. The Dose Makes the Poison: Sugar and Obesity in the United States—A Review. Polish J. food Nutr. Sci. 2019, 69, 219–233. [Google Scholar] [CrossRef]
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Fucà, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frigè, G.; Belfiore, A.; et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLOS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Lee, H.J. Effects of phenylethyl isothiocyanate and its metabolite on cell-cycle arrest and apoptosis in LNCaP human prostate cancer cells. Int. J. Food Sci. Nutr. 2010, 61, 324–336. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.-H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef]
- Gach, K.; Wyrębska, A.; Fichna, J.; Janecka, A. The role of morphine in regulation of cancer cell growth. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 384, 221–230. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yang, C.; Huang, X.; Han, M.; Kang, F.; Li, J. Morphine promotes the malignant biological behavior of non-small cell lung cancer cells through the MOR/Src/mTOR pathway. Cancer Cell Int. 2021, 21, 622. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Qin, Y.; Jiang, Y.; Wei, Y.; Chen, J.; Xie, Y. Exogenous morphine inhibits the growth of human gastric tumor in vivo. Ann. Transl. Med. 2020, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Maryam, A.; Tian, X.; Khan, M.; Ma, T. Santamarine Inhibits NF-κB and STAT3 Activation and Induces Apoptosis in HepG2 Liver Cancer Cells via Oxidative Stress. J. Cancer 2017, 8, 3707–3717. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Yan, J.; Khan, M.; Yu, X. Santamarine Inhibits NF-κB Activation and Induces Mitochondrial Apoptosis in A549 Lung Adenocarcinoma Cells via Oxidative Stress. Biomed Res. Int. 2017, 2017, 4734127. [Google Scholar] [CrossRef] [PubMed]
- Al-Attas, A.A.M.; El-Shaer, N.S.; Mohamed, G.A.; Ibrahim, S.R.M.; Esmat, A. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. J. Ethnopharmacol. 2015, 176, 365–374. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, D.-S.; Li, B.; Choi, Y.H.; Lee, S.-H.; Kim, Y.-C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279. [Google Scholar] [CrossRef] [PubMed]
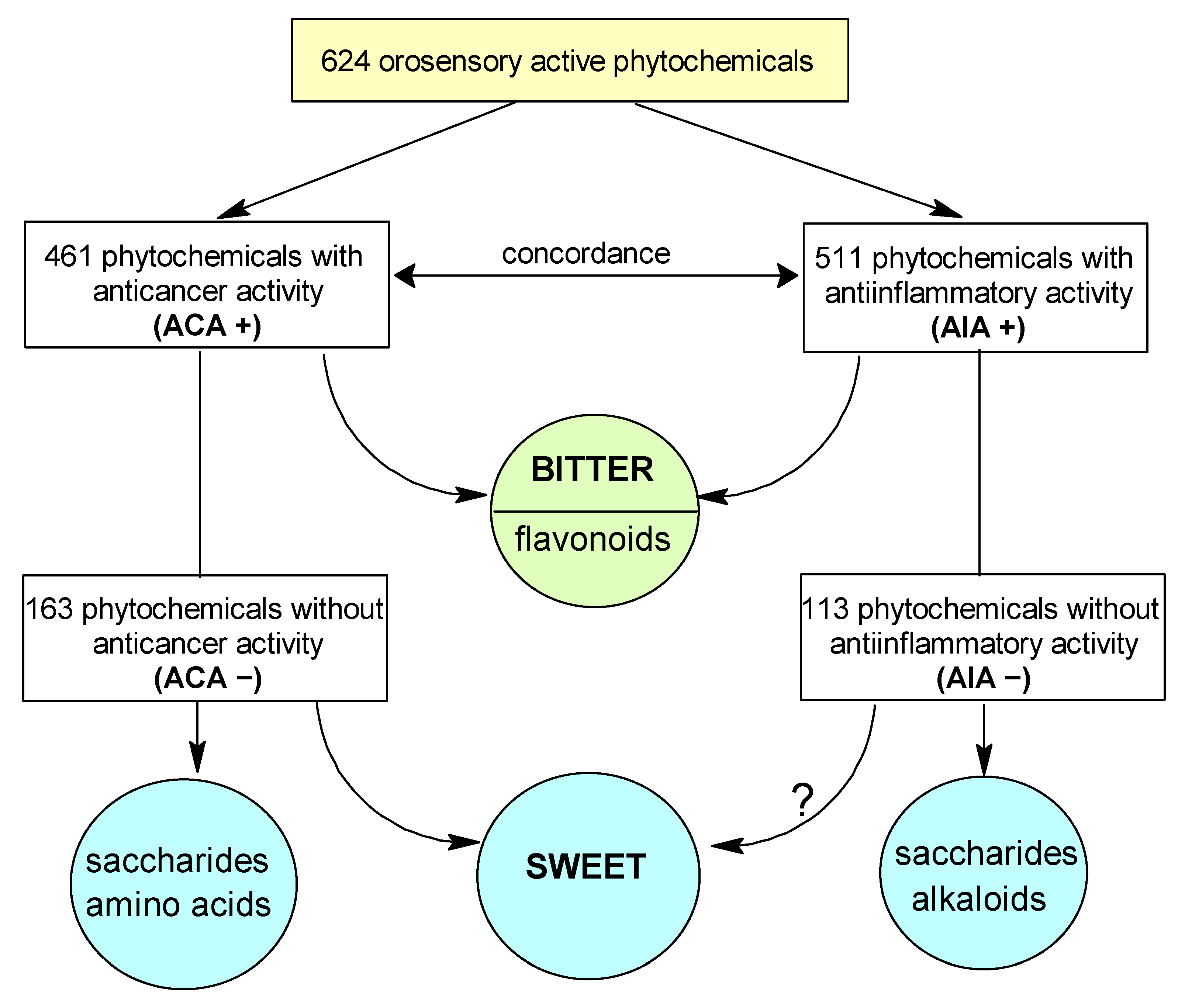
| Taste/Orosensation | PDA | (a, b, c, d) | Odds Ratio (OR) | 95% Confidence Interval for OR | p-Value |
|---|---|---|---|---|---|
| Astringent | ACA | (75, 17, 386, 146) | 1.67 | 0.97-2.99 | 0.073 |
| Bitter (flavonoids only) | ACA | (57, 0, 5, 2) | Infinity | __ | 0.01 |
| Bitter | ACA | (324, 90, 137, 73) | 1.92 | 1.32–2.77 | 7 × 10−4 |
| Pungent | ACA | (64, 18, 397, 145) | 1.3 | 0.75–2.32 | 0.42 |
| Salty | ACA | (2, 2, 459, 161) | 0.35 | 0.04–3.4 | 0.28 |
| Sour | ACA | (29, 19, 432, 144) | 0.51 | 0.28–0.95 | 0.039 |
| Sweet | ACA | (55, 45, 406, 118) | 0.36 | 0.23–0.56 | 1 × 10−5 |
| Umami | ACA | (4, 5, 457, 158) | 0.28 | 0.07–1.11 | 0.057 |
| Astringent | AIA | (82, 10, 429, 103) | 1.97 | 1.01–4.13 | 0.056 |
| Bitter | AIA | (353, 61, 158, 52) | 1.9 | 1.25–2.88 | 0.003 |
| Pungent | AIA | (70, 12, 441, 101) | 1.34 | 0.71–2.66 | 0.44 |
| Salty | AIA | (3, 1, 508, 112) | 0.66 | 0.07–17.56 | 0.55 |
| Sour | AIA | (37, 11, 474, 102) | 0.72 | 0.36–1.53 | 0.43 |
| Sweet | AIA | (73, 27, 438, 86) | 0.53 | 0.32–0.88 | 0.016 |
| Umami | AIA | (6, 3, 505, 110) | 0.44 | 0.11–2.16 | 0.21 |
| Chemical Class | PDA | (a, b, c, d) | Odds Ratio (OR) | 95% Confidence Interval for OR | p-Value |
|---|---|---|---|---|---|
| alkaloids | ACA | (71, 37, 390, 126) | 0.62 | 0.4–0.98 | 0.04 |
| flavonoids | ACA | (62, 2, 399, 161) | 12.48 | 3.58–76.76 | 7 × 10−7 |
| flavonoid glycosides | ACA | (33, 4, 428, 159) | 3.06 | 1.15–10.28 | 0.032 |
| saccharides | ACA | (14, 18, 447, 145) | 0.25 | 0.12–0.52 | 0.0002 |
| amino acids | ACA | (9, 21, 452, 142) | 0.14 | 0.06–0.3 | 3 × 10−7 |
| monoterpenoids | ACA | (21, 6, 440, 157) | 1.25 | 0.51–3.44 | 0.82 |
| triterpenoids | ACA | (20, 2, 441, 161) | 3.65 | 0.97–23.34 | 0.082 |
| coumarins | ACA | (20, 1, 441, 162) | 7.33 | 1.33–155.28 | 0.022 |
| sesquiterpenoids | ACA | (18, 3, 443, 160) | 2.16 | 0.68–9.33 | 0.31 |
| phenolic others | ACA | (16, 3, 445, 160) | 1.92 | 0.6–8.33 | 0.43 |
| phenolic acids | ACA | (14, 4, 447, 159) | 1.24 | 0.42–4.45 | 1 |
| short aliphatic acids | ACA | (9, 9, 452, 154) | 0.34 | 0.13–0.9 | 0.028 |
| tannins | ACA | (14, 1, 447, 162) | 5.07 | 0.89–109.2 | 0.13 |
| diterpenoids | ACA | (12, 2, 449, 161) | 2.15 | 0.54–14.29 | 0.54 |
| sulfur compounds | ACA | (8, 6, 453, 157) | 0.46 | 0.15–1.45 | 0.21 |
| monoterpenoid glycosides | ACA | (12, 1, 449, 162) | 4.32 | 0.74–94.12 | 0.2 |
| steroid glycosides | ACA | (10, 1, 451, 162) | 3.59 | 0.59–79.2 | 0.3 |
| fatty acids | ACA | (8, 2, 453, 161) | 1.42 | 0.32–9.89 | 1 |
| phenolic acid esters | ACA | (7, 3, 454, 160) | 0.82 | 0.21–3.96 | 0.73 |
| phenylpropanoids (others) * | ACA | (7, 3, 454, 160) | 0.82 | 0.21–3.96 | 0.73 |
| triterpenoid glycosides | ACA | (9, 1, 452, 162) | 3.22 | 0.52–71.78 | 0.47 |
| alkaloids | AIA | (74, 34, 437, 79) | 0.39 | 0.25–0.64 | 0.0002 |
| flavonoids | AIA | (63, 1, 448, 112) | 15.72 | 3.03–324.24 | 5 × 10−5 |
| flavonoid glycosides | AIA | (34, 3, 477, 110) | 2.61 | 0.87–10.89 | 0.12 |
| saccharides | AIA | (16, 16, 495, 97) | 0.2 | 0.09–0.41 | 2 × 10−5 |
| amino acids | AIA | (25, 5, 486, 108) | 1.11 | 0.44–3.33 | 1 |
| monoterpenoids | AIA | (26, 1, 485, 112) | 5.99 | 1.11–125.95 | 0.043 |
| triterpenoids | AIA | (19, 3, 492, 110) | 1.42 | 0.45–6.1 | 0.78 |
| coumarins | AIA | (20, 1, 491, 112) | 4.56 | 0.83–96.63 | 0.15 |
| sesquiterpenoids | AIA | (18, 3, 493, 110) | 1.34 | 0.42–5.79 | 0.78 |
| phenolic others | AIA | (16, 3, 495, 110) | 1.18 | 0.37–5.17 | 1 |
| phenolic acids | AIA | (16, 2, 495, 111) | 1.79 | 0.46–11.67 | 0.75 |
| short aliphatic acids | AIA | (11, 7, 500, 106) | 0.33 | 0.13–0.93 | 0.029 |
| tannins | AIA | (14, 1, 497, 112) | 3.15 | 0.55–68.05 | 0.33 |
| diterpenoids | AIA | (13, 1, 498, 112) | 2.92 | 0.5–63.36 | 0.48 |
| sulfur compounds | AIA | (10, 4, 501, 109) | 0.54 | 0.17–2.04 | 0.3 |
| monoterpenoid glycosides | AIA | (12, 1, 499, 112) | 2.69 | 0.46–58.69 | 0.48 |
| steroid glycosides | AIA | (8, 3, 503, 110) | 0.58 | 0.16–2.76 | 0.43 |
| fatty acids | AIA | (7, 3, 504, 110) | 0.51 | 0.13–2.46 | 0.4 |
| phenolic acid esters | AIA | (9, 1, 502, 112) | 2.01 | 0.32–44.8 | 0.7 |
| phenylpropanoids (others) * | AIA | (8, 2, 503, 111) | 0.88 | 0.2–6.16 | 1 |
| triterpenoid glycosides | AIA | (10, 0, 501, 113) | Infinity | __ | 0.22 |
| Pooled Chemical Class | PDA | (a, b, c, d) | Odds Ratio (OR) | 95% Confidence Interval for OR | p-Value |
|---|---|---|---|---|---|
| alkaloids | AIA | (76, 34, 435, 79) | 0.41 | 0.25–0.66 | 0.0003 |
| amino acids | AIA | (25, 5, 486, 108) | 1.11 | 0.44–3.33 | 1 |
| coumarins | AIA | (22, 1, 489, 112) | 5.03 | 0.92–106.32 | 0.01 |
| diterpenoids | AIA | (16, 1, 495, 112) | 3.62 | 0.64–77.5 | 0.33 |
| fatty compounds | AIA | (16, 6, 495, 107) | 0.58 | 0.23–1.64 | 0.26 |
| flavonoids | AIA | (97, 4, 414, 109) | 6.37 | 2.5–20.81 | 9 × 10−6 |
| monoterpenoids | AIA | (48, 3, 463, 110) | 3.8 | 1.29–15.65 | 0.014 |
| phenolic acids | AIA | (27, 3, 484, 110) | 2.04 | 0.67–8.62 | 0.33 |
| phenolic others | AIA | (19, 3, 492, 110) | 1.42 | 0.45–6.1 | 0.78 |
| phenylpropanoids | AIA | (9, 2, 502, 111) | 1 | 0.23–6.84 | 1 |
| saccharides | AIA | (16, 16, 495, 97) | 0.2 | 0.09–0.41 | 2 × 10−5 |
| sesquiterpenoids | AIA | (18, 5, 493, 108) | 0.79 | 0.3–2.43 | 0.59 |
| short aliphatic acids | AIA | (11, 7, 500, 106) | 0.33 | 0.13–0.93 | 0.029 |
| steroids | AIA | (13, 3, 498, 110) | 0.96 | 0.29–4.26 | 1 |
| sulfur compounds | AIA | (10, 5, 501, 108) | 0.43 | 0.15–1.42 | 0.16 |
| tannins | AIA | (14, 1, 497, 112) | 3.15 | 0.55–68.05 | 0.33 |
| triterpenoids | AIA | (29, 3, 482, 110) | 2.2 | 0.73–9.26 | 0.24 |
| alkaloids | ACA | (73, 37, 388, 126) | 0.64 | 0.41–1.01 | 0.056 |
| amino acids | ACA | (9, 21, 452, 142) | 0.14 | 0.06–0.3 | 3 × 10−7 |
| coumarins | ACA | (21, 2, 440, 161) | 3.84 | 1.03–24.49 | 0.055 |
| diterpenoids | ACA | (13, 4, 448, 159) | 1.15 | 0.39–4.16 | 1 |
| fatty compounds | ACA | (15, 7, 446, 156) | 0.75 | 0.3–2 | 0.62 |
| flavonoids | ACA | (95, 6, 366, 157) | 6.78 | 3.08–17.41 | 5 × 10−8 |
| monoterpenoids | ACA | (40, 11, 421, 152) | 1.31 | 0.67–2.73 | 0.51 |
| phenolic acids | ACA | (23, 7, 438, 156) | 1.17 | 0.51–2.99 | 0.83 |
| phenolic others | ACA | (19, 3, 442, 160) | 2.29 | 0.73–9.83 | 0.22 |
| phenylpropanoids | ACA | (8, 3, 453, 160) | 0.94 | 0.25–4.44 | 1 |
| saccharides | ACA | (14, 18, 447, 145) | 0.25 | 0.12–0.52 | 0.0002 |
| sesquiterpenoids | ACA | (18, 5, 443, 158) | 1.28 | 0.49–3.93 | 0.81 |
| short aliphatic acids | ACA | (9, 9, 452, 154) | 0.34 | 0.13–0.9 | 0.028 |
| steroids | ACA | (15, 1, 446, 162) | 5.44 | 0.96–116.79 | 0.083 |
| sulfur compounds | ACA | (8, 7, 453, 156) | 0.39 | 0.14–1.16 | 0.078 |
| tannins | ACA | (14, 1, 447, 162) | 5.07 | 0.89–109.2 | 0.13 |
| triterpenoids | ACA | (29, 3, 432, 160) | 3.58 | 1.19–14.97 | 0.024 |
| Taste | (a, b, c, d) | Odds Ratio (OR) | 95% Confidence Interval for OR | p-Value |
|---|---|---|---|---|
| All tastes | (81, 32, 82, 429) | 13.16 | 8.25–21.32 | 3 × 10−30 |
| Astringent | (9, 1, 8, 74) | 75.56 | 10.69–1847.1 | 3 × 10−7 |
| Bitter | (40, 21, 50, 303) | 11.44 | 6.27–21.31 | 4 × 10−16 |
| Pungent | (8, 4, 10, 60) | 11.45 | 2.93–51.13 | 0.0004 |
| Sour | (10, 1, 9, 28) | 28.62 | 4.05–700.41 | 0.0001 |
| Sweet | (22, 5, 23, 50) | 9.33 | 3.26–30.75 | 1 × 10−5 |
| Chemical Class | (a, b, c, d) | Odds Ratio (OR) | 95% Confidence Interval for OR | p-Value |
|---|---|---|---|---|
| alkaloids | (24, 13, 10, 61) | 10.93 | 3.97–32.64 | 2 × 10−7 |
| flavonoids | (1, 1, 0, 62) | Inf | 0.79–Inf | 0.031 |
| flavonoid glycosides | (2, 2, 1, 32) | 25.21 | 0.98–1904.49 | 0.026 |
| saccharides | (12, 6, 4, 10) | 4.73 | 0.89–30.52 | 0.073 |
| amino acids | (5, 16, 0, 9) | Inf | 0.41-Inf | 0.29 |
| monoterpenoids | (1, 5, 0, 21) | Inf | 0.09-Inf | 0.22 |
| triterpenoids | (1, 1, 2, 18) | 7.55 | 0.08–738.06 | 0.26 |
| coumarins | (0, 1, 1, 19) | 0 | 0–770.62 | 1 |
| sesquiterpenoids | (2, 1, 1, 17) | 23.02 | 0.74–2065.5 | 0.041 |
| phenolic others | (2, 1, 1, 15) | 20.38 | 0.65–1840.77 | 0.051 |
| phenolic acids | (2, 2, 0, 14) | Inf | 0.75-Inf | 0.039 |
| short aliphatic acids | (6, 3, 1, 8) | 13.25 | 1–819.63 | 0.05 |
| tannins | (1, 0, 0, 14) | Inf | 0.36-Inf | 0.067 |
| diterpenoids | (0, 2, 1, 11) | 0 | 0–233.15 | 1 |
| sulfur compounds | (3, 3, 1, 7) | 5.99 | 0.33–417.16 | 0.24 |
| monoterpenoid glycosides | (0, 1, 1, 11) | 0 | 0–464.61 | 1 |
| steroid glycosides | (1, 0, 2, 8) | Inf | 0.07-Inf | 0.27 |
| fatty acids | (1, 1, 2, 6) | 2.65 | 0.03–273.2 | 1 |
| phenolic acid esters | (1, 2, 0, 7) | Inf | 0.06-Inf | 0.3 |
| phenylpropanoids | (1, 2, 1, 6) | 2.65 | 0.03–273.2 | 1 |
| triterpenoid glycosides | (0, 1, 0, 9) | 0 | 0-Inf | 1 |
| No AIA/ACA | AIA/ACA | |
|---|---|---|
| No Taste/Chemical class | a | b |
| Taste/Chemical class | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grădinaru, T.-C.; Gilca, M.; Vlad, A.; Dragoș, D. Relevance of Phytochemical Taste for Anti-Cancer Activity: A Statistical Inquiry. Int. J. Mol. Sci. 2023, 24, 16227. https://doi.org/10.3390/ijms242216227
Grădinaru T-C, Gilca M, Vlad A, Dragoș D. Relevance of Phytochemical Taste for Anti-Cancer Activity: A Statistical Inquiry. International Journal of Molecular Sciences. 2023; 24(22):16227. https://doi.org/10.3390/ijms242216227
Chicago/Turabian StyleGrădinaru, Teodora-Cristiana, Marilena Gilca, Adelina Vlad, and Dorin Dragoș. 2023. "Relevance of Phytochemical Taste for Anti-Cancer Activity: A Statistical Inquiry" International Journal of Molecular Sciences 24, no. 22: 16227. https://doi.org/10.3390/ijms242216227
APA StyleGrădinaru, T.-C., Gilca, M., Vlad, A., & Dragoș, D. (2023). Relevance of Phytochemical Taste for Anti-Cancer Activity: A Statistical Inquiry. International Journal of Molecular Sciences, 24(22), 16227. https://doi.org/10.3390/ijms242216227







