Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus
Abstract
:1. Introduction
2. Results
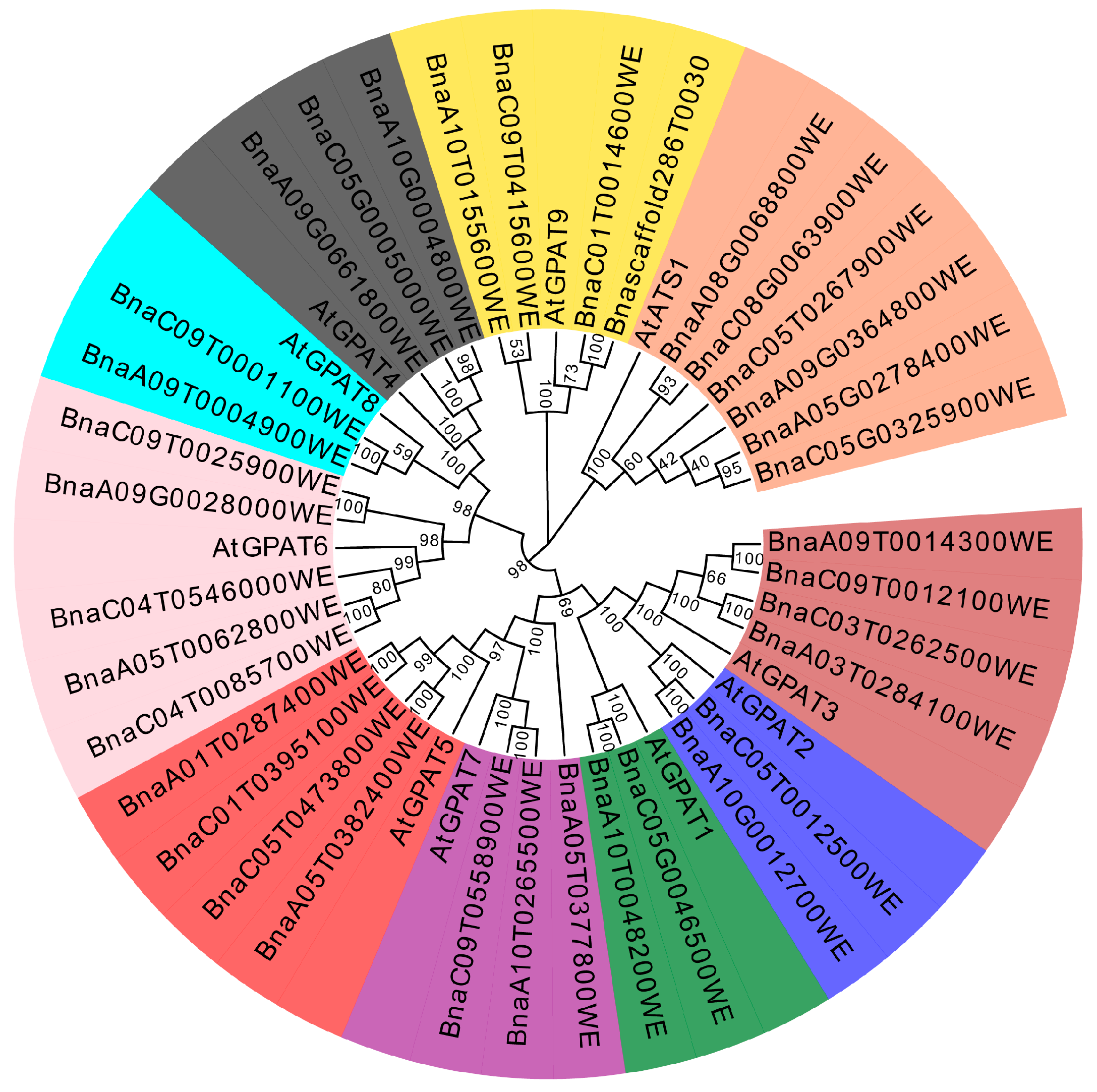
2.1. Identification of an Ortholog of AtGPAT9 in Brassica napus
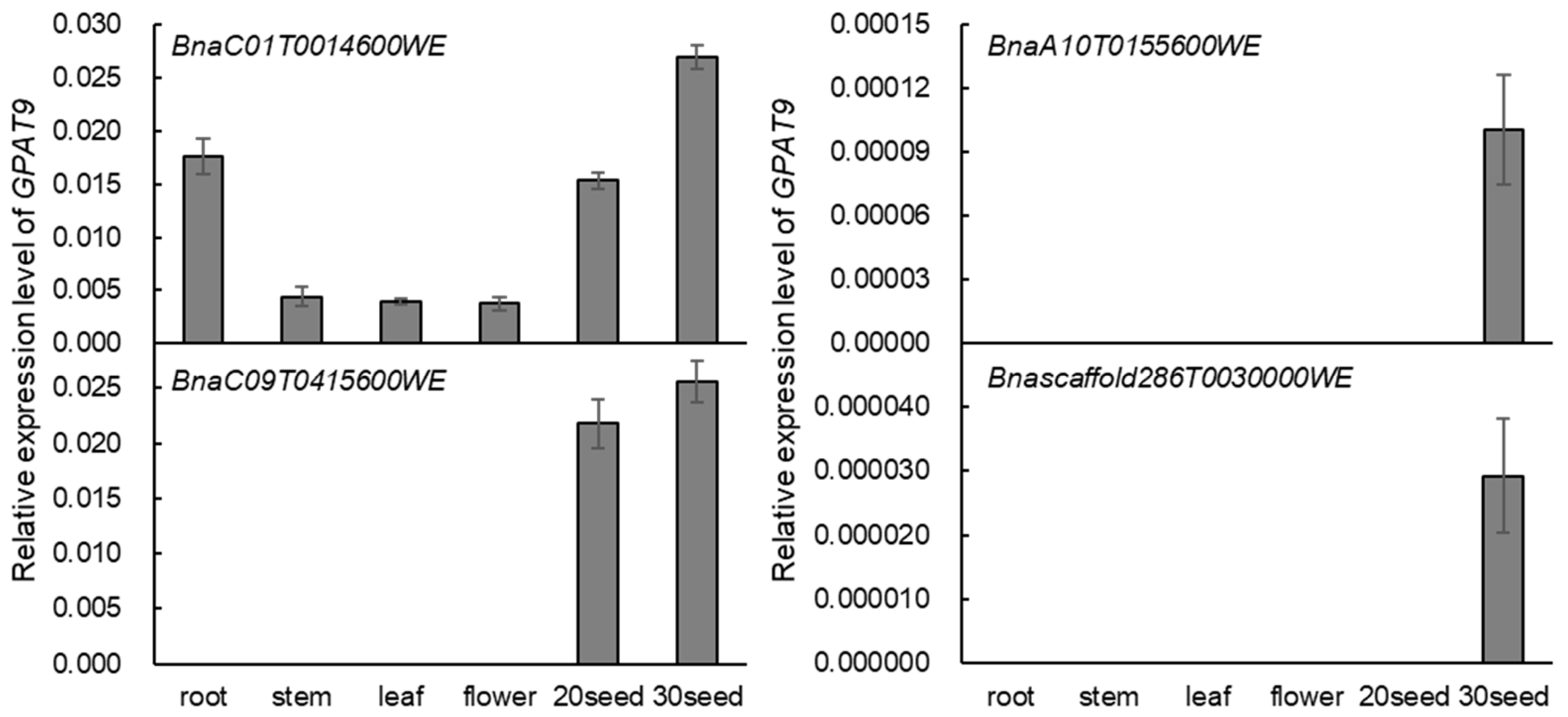
2.2. BnaGPAT9 Is Highly Expressed in Developing Seed and BnaGPAT9 Is Localized to ER
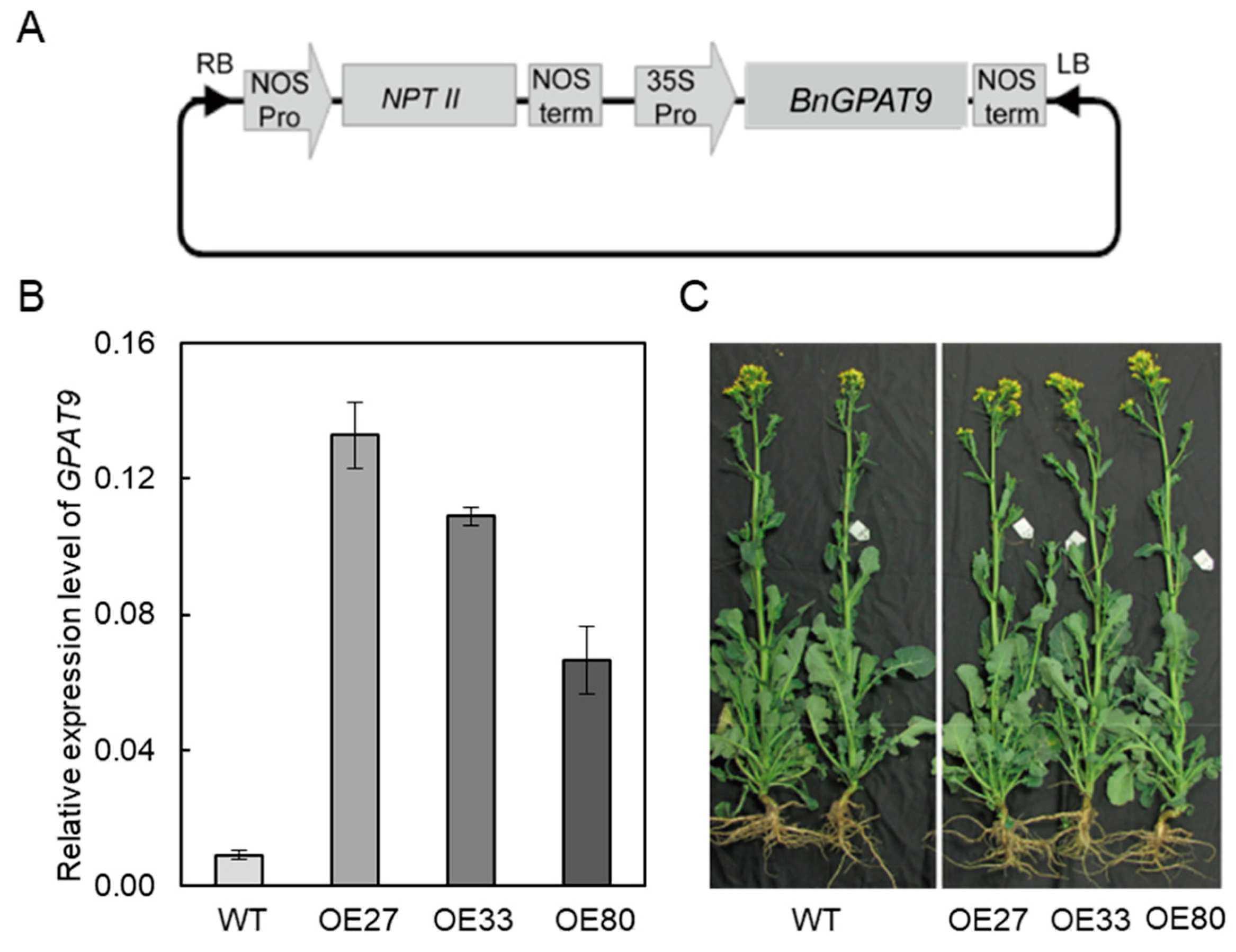
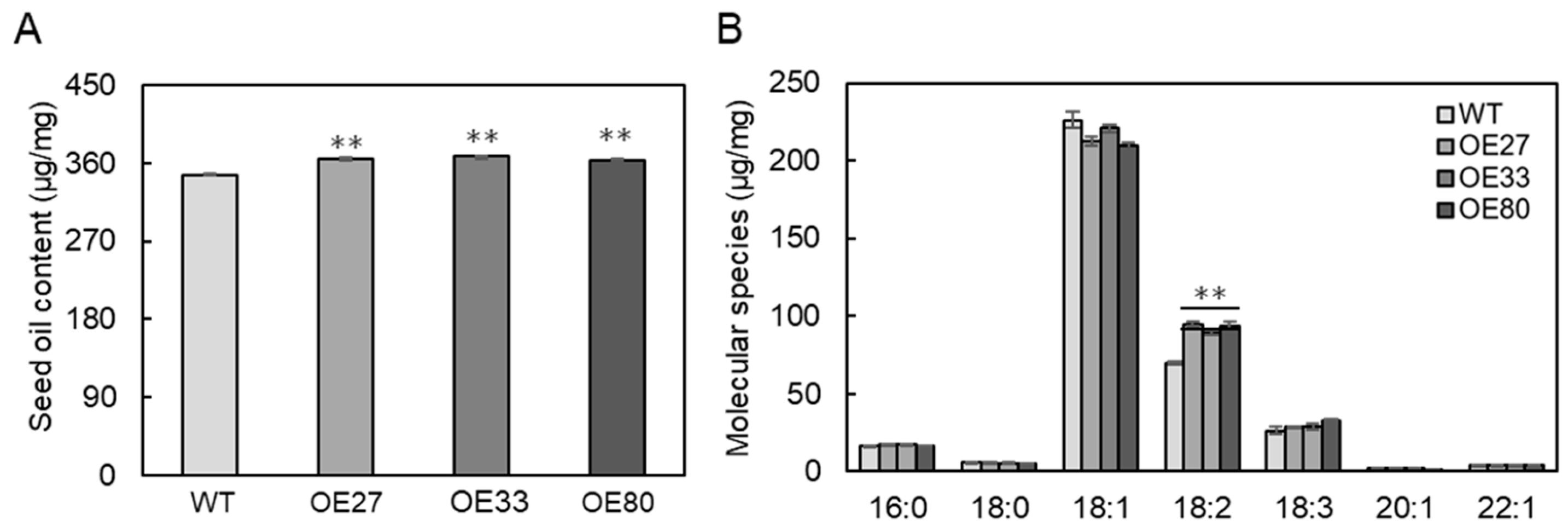
2.3. Overexpression of BnaGPAT9 Enhancing Oil Accumulation in Seeds
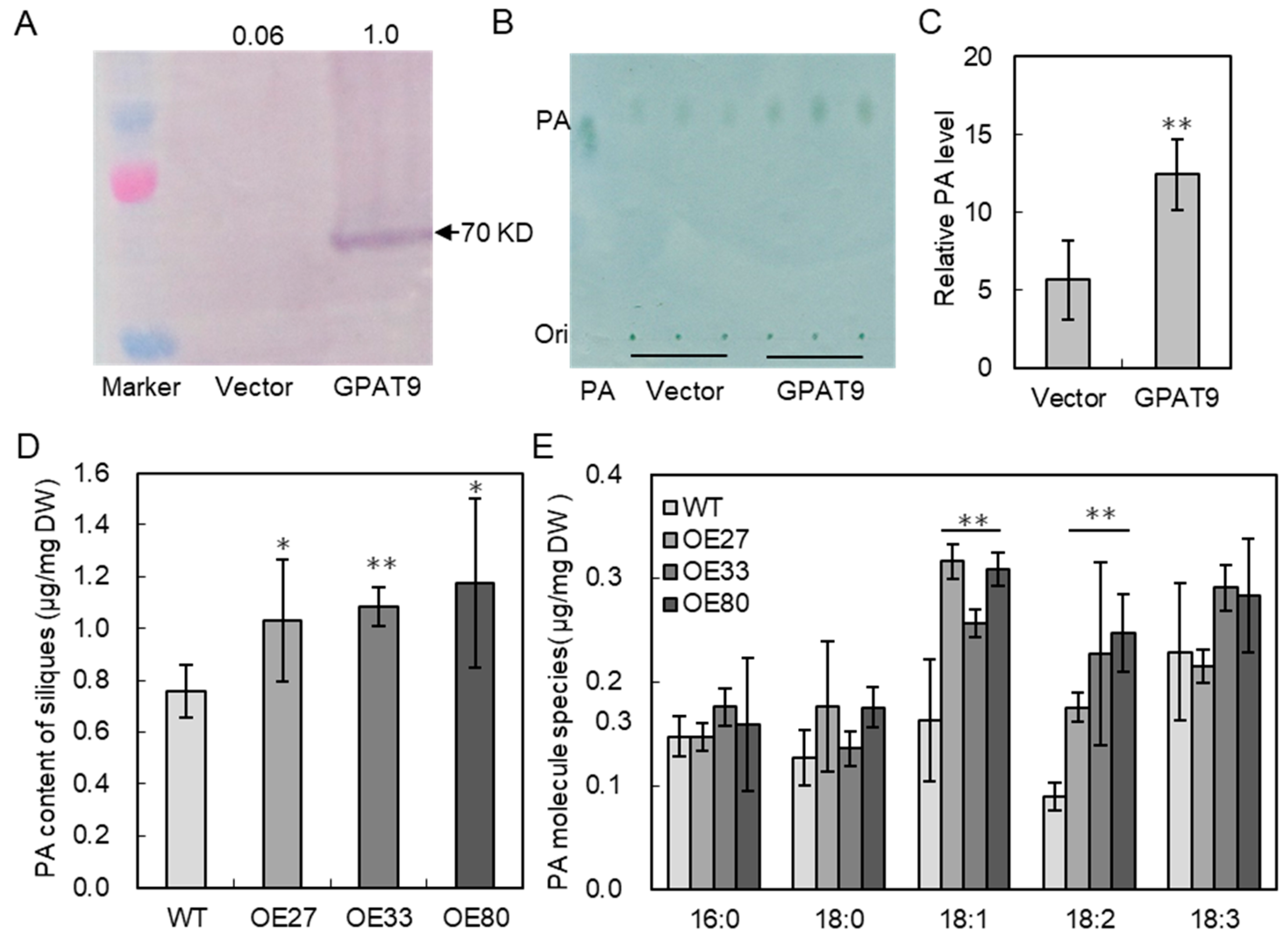
2.4. BnaGPAT9 Promoted PA Production In Vitro and In Vivo
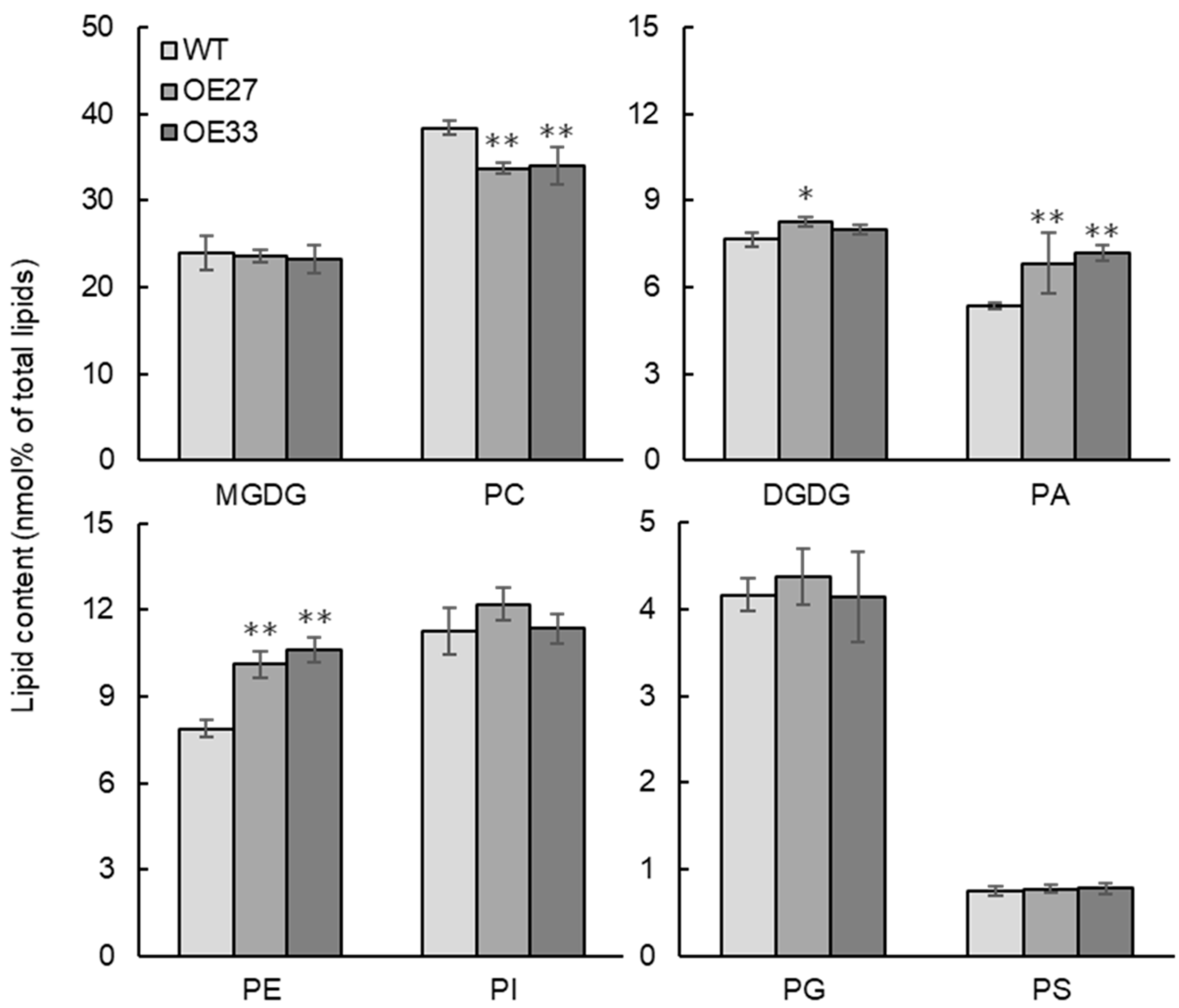
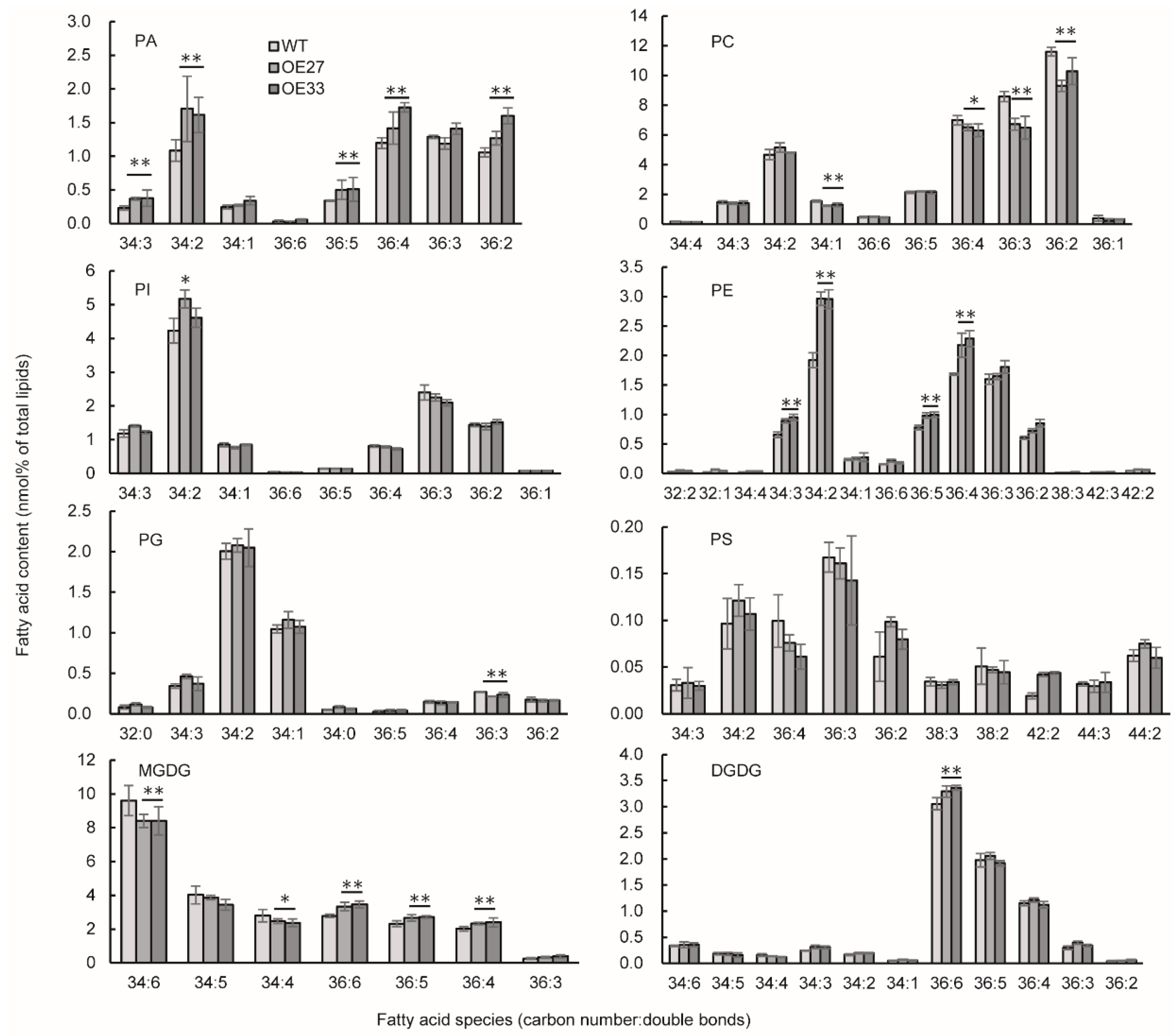
2.5. BnaGPAT9 Affected Membrane Phospholipids and Enhanced Eukaryotic Galactolipids in Developing Seed
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Database Search and Sequence Analysis
4.3. BnaGPAT9 Cloning, Vector Construction, and Plant Transformation
4.4. Quantitative Reverse-Transcription PCR
4.5. Subcellular Localization
4.6. GPAT Activity Assay
4.7. Lipid Extraction and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlsson, A.S.; Yilmaz, J.L.; Green, A.G.; Stymne, S.; Hofvander, P. Replacing fossil oil with fresh oil—With what and for what? Eur. J. Lipid Sci. Technol. 2011, 113, 812–831. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schafer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. Engl. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Palmer, J.D.; Shields, C.R.; Cohen, D.B.; Orton, T.J. Chloroplast DNA evolution and the origin of amphidiploid Brassica species. Theor. Appl. Genet. 1983, 65, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Parkin, I.A.; Sharpe, A.G.; Keith, D.J.; Lydiate, D.J. Identification of the A and C genomes of amphidiploid Brassica napus (oilseed rape). Genome 1995, 38, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, H.K.; Sturtevant, D.; Borisjuk, L.; Munz, E.; Guschina, I.A.; Chapman, K.; Harwood, J.L. Spatial and temporal mapping of key lipid species in Brassica napus seeds. Plant Physiol. 2017, 173, 1998–2009. [Google Scholar] [CrossRef]
- Weselake, R.J.; Taylor, D.C.; Rahman, M.H.; Shah, S.; Laroche, A.; McVetty, P.B.E.; Harwood, J.L. Increasing the flow of carbon into seed oil. Biotechnol. Adv. 2009, 27, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [PubMed]
- Kunst, L.; Browse, J.; Somerville, C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc. Natl. Acad. Sci. USA 1988, 85, 4143–4147. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Huang, A.H. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004, 134, 1206–1216. [Google Scholar] [CrossRef]
- Xu, C.; Yu, B.; Cornish, A.J.; Froehlich, J.E.; Benning, C. Phosphatidylglycerol biosynthesis in chloroplasts of Arabidopsis mutants deficient in acyl-ACP glycerol-3- phosphate acyltransferase. Plant J. 2006, 47, 296–309. [Google Scholar] [CrossRef]
- Hölzl, G.; Dormann, P. Chloroplast lipids and their biosynthesis. Annu. Rev. Plant Biol. 2019, 70, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Li, Y.; Huang, A.H. Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell 2005, 17, 1073–1089. [Google Scholar] [CrossRef]
- Shockey, J.; Regmi, A.; Cotton, K.; Adhikari, N.; Browse, J.; Bates, P.D. Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis. Plant Physiol. 2016, 170, 163–179. [Google Scholar] [CrossRef]
- Singer, S.D.; Chen, G.; Mietkiewska, E.; Tomasi, P.; Jayawardhane, K.; Dyer, J.M.; Weselake, R.J. Arabidopsis GPAT9 contributes to synthesis of intracellular glycerolipids but not surface lipids. J. Exp. Bot. 2016, 67, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, J.; Riekhof, W.; Froehlich, J.E.; Benning, C. A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J. 2003, 22, 2370–2379. [Google Scholar] [CrossRef]
- Xu, C.; Fan, J.; Froehlich, J.E.; Awai, K.; Benning, C. Mutation of the TGD1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell 2005, 17, 3094–3110. [Google Scholar] [CrossRef] [PubMed]
- Awai, K.; Xu, C.; Tamot, B.; Benning, C. A phosphatidic acid-binding protein of the chloroplast inner envelope membrane involved in lipid trafficking. Proc. Natl. Acad. Sci. USA 2006, 103, 10817–10822. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhai, Z.; Yan, C.; Xu, C. Arabidopsis TRIGALACTOSYLDIACYLGLYCEROL5 interacts with TGD1, TGD2, and TGD4 to facilitate lipid transfer from the endoplasmic reticulum to plastids. Plant Cell 2015, 27, 2941–2955. [Google Scholar] [CrossRef]
- Weiss, S.B.; Kennedy, E.P.; Kiyasu, J.Y. The enzymatic synthesis of triglycerides. J. Biol. Chem. 1960, 235, 40–44. [Google Scholar] [CrossRef]
- Kennedy, E.P. Biosynthesis of complex lipids. Fed. Proc. 1961, 20, 934–940. [Google Scholar] [PubMed]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, J.; Taylor, D.C.; Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 2009, 21, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yan, C.; Zhang, X.; Xu, C. Dual role for phospholipid:diacylglycerol acyltransferase: Enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell 2013, 25, 3506–3518. [Google Scholar] [CrossRef]
- Lu, C.; Xin, Z.; Ren, Z.; Miquel, M.; Browse, J. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 18837–18842. [Google Scholar] [CrossRef]
- Zheng, Z.; Xia, Q.; Dauk, M.; Shen, W.; Selvaraj, G.; Zou, J. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 2003, 15, 1872–1887. [Google Scholar] [CrossRef]
- Beisson, F.; Li, Y.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef]
- Chen, X.; Snyder, C.L.; Truksa, M.; Shah, S.; Weselake, R.J. sn-Glycerol-3-phosphate acyltransferases in plants. Plant Signal. Behav. 2011, 6, 1695–1699. [Google Scholar] [CrossRef]
- Yang, W.; Simpson, J.P.; Li-Beisson, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J.B. A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: Substrate specificity, sn-2 preference, and evolution. Plant Physiol. 2012, 160, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Mauxion, J.P.; Tai, F.W.; Martin, L.B.; Fich, E.A.; Joubes, J.; Rose, J.K.; Domergue, F.; Rothan, C. The glycerol-3-phosphate acyltransferase GPAT6 from tomato plays a central role in fruit cutin biosynthesis. Plant Physiol. 2016, 171, 894–913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, S.; Zhang, D.; Li, Z.; Xie, K.; An, X.; Ma, B.; Hou, Q.; Dong, Z.; Tian, Y.; et al. Genome-wide analysis of maize GPAT gene family and cytological characterization and breeding application of ZmMs33/ZmGPAT6 gene. Theor. Appl. Genet. 2019, 132, 2137–2154. [Google Scholar] [CrossRef] [PubMed]
- Nishida, I.; Tasaka, Y.; Shiraishi, H.; Murata, N. The gene and the RNA for the precursor to the plastid-located glycerol-3-phosphate acyltransferase of Arabidopsis thaliana. Plant Mol. Biol. 1993, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Beisson, F.; Koo, A.J.; Molina, I.; Pollard, M.; Ohlrogge, J. Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA 2007, 104, 18339–18344. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Pollard, M.; Sauveplane, V.; Pinot, F.; Ohlrogge, J.; Beisson, F. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. USA 2009, 106, 22008–22013. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pollard, M.; Li-Beisson, Y.; Beisson, F.; Feig, M.; Ohlrogge, J. A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA 2010, 107, 12040–12045. [Google Scholar] [CrossRef]
- Yunus, I.S.; Cazenave-Gassiot, A.; Liu, Y.C.; Lin, Y.C.; Wenk, M.R.; Nakamura, Y. Phosphatidic acid is a major phospholipid class in reproductive organs of Arabidopsis thaliana. Plant Signal. Behav. 2015, 10, e1049790. [Google Scholar] [CrossRef]
- Kang, H.; Jia, C.; Liu, N.; Aboagla, A.A.A.; Chen, W.; Gong, W.; Tang, S.; Hong, Y. Plastid glycerol-3-phosphate acyltransferase enhanced plant growth and prokaryotic glycerolipid synthesis in Brassica napus. Int. J. Mol. Sci. 2020, 21, 5325. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef]
- Bates, P.D.; Durrett, T.P.; Ohlrogge, J.B.; Pollard, M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009, 150, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Karki, N.; Johnson, B.S.; Bates, P.D. Metabolically distinct pools of phosphatidylcholine are involved in trafficking of fatty acids out of and into the chloroplast for membrane production. Plant Cell 2019, 31, 2768–2788. [Google Scholar] [CrossRef]
- Bates, P.D.; Ohlrogge, J.B.; Pollard, M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem. 2007, 282, 31206–31216. [Google Scholar] [CrossRef]
- Bates, P.D.; Browse, J. The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front. Plant Sci. 2012, 3, 147. [Google Scholar] [CrossRef]
- Allen, D.K.; Bates, P.D.; Tjellstrom, H. Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: Past, present and future. Prog. Lipid Res. 2015, 58, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Fatihi, A.; Snapp, A.R.; Carlsson, A.S.; Browse, J.; Lu, C. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012, 160, 1530–1539. [Google Scholar] [CrossRef]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ’16:3’ plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef]
- Kunst, L.; Browse, J.; Somerville, C. Altered chloroplast structure and function in a mutant of Arabidopsis deficient in plastid glycerol-3-phosphate acyltransferase activity. Plant Physiol. 1989, 90, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bahn, S.C.; Qu, G.; Qin, H.; Hong, Y.; Xu, Q.; Zhou, Y.; Hong, Y.; Wang, X. Increased expression of phospholipase Dα1 in guard cells decreases water loss with improved seed production under drought in Brassica napus. Plant Biotechnol. J. 2013, 11, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Chen, W.; Gao, Q.; Qian, L.; Yuan, X.; Tang, S.; Hong, Y. Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus. Int. J. Mol. Sci. 2023, 24, 16111. https://doi.org/10.3390/ijms242216111
Gong W, Chen W, Gao Q, Qian L, Yuan X, Tang S, Hong Y. Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus. International Journal of Molecular Sciences. 2023; 24(22):16111. https://doi.org/10.3390/ijms242216111
Chicago/Turabian StyleGong, Wei, Wenling Chen, Qiang Gao, Lei Qian, Xueyuan Yuan, Shaohua Tang, and Yueyun Hong. 2023. "Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus" International Journal of Molecular Sciences 24, no. 22: 16111. https://doi.org/10.3390/ijms242216111
APA StyleGong, W., Chen, W., Gao, Q., Qian, L., Yuan, X., Tang, S., & Hong, Y. (2023). Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus. International Journal of Molecular Sciences, 24(22), 16111. https://doi.org/10.3390/ijms242216111




