Dipeptidyl Peptidase 4 Stimulation Induces Adipogenesis-Related Gene Expression of Adipose Stromal Cells
Abstract
:1. Introduction
2. Results
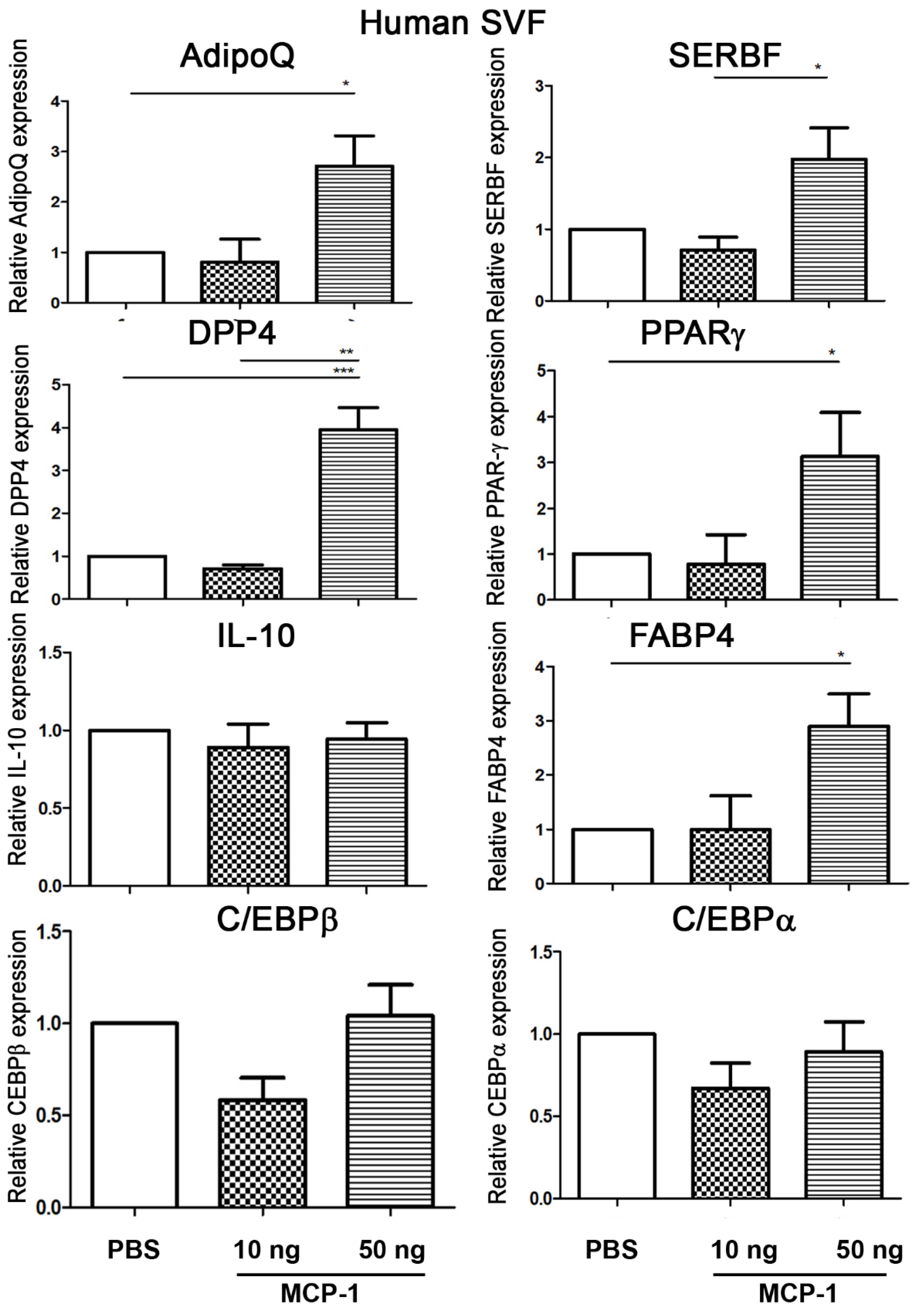
2.1. MCP-1 Treatment Increases Adipogenesis-Related Gene Expression of Adipose SVFs in Humans
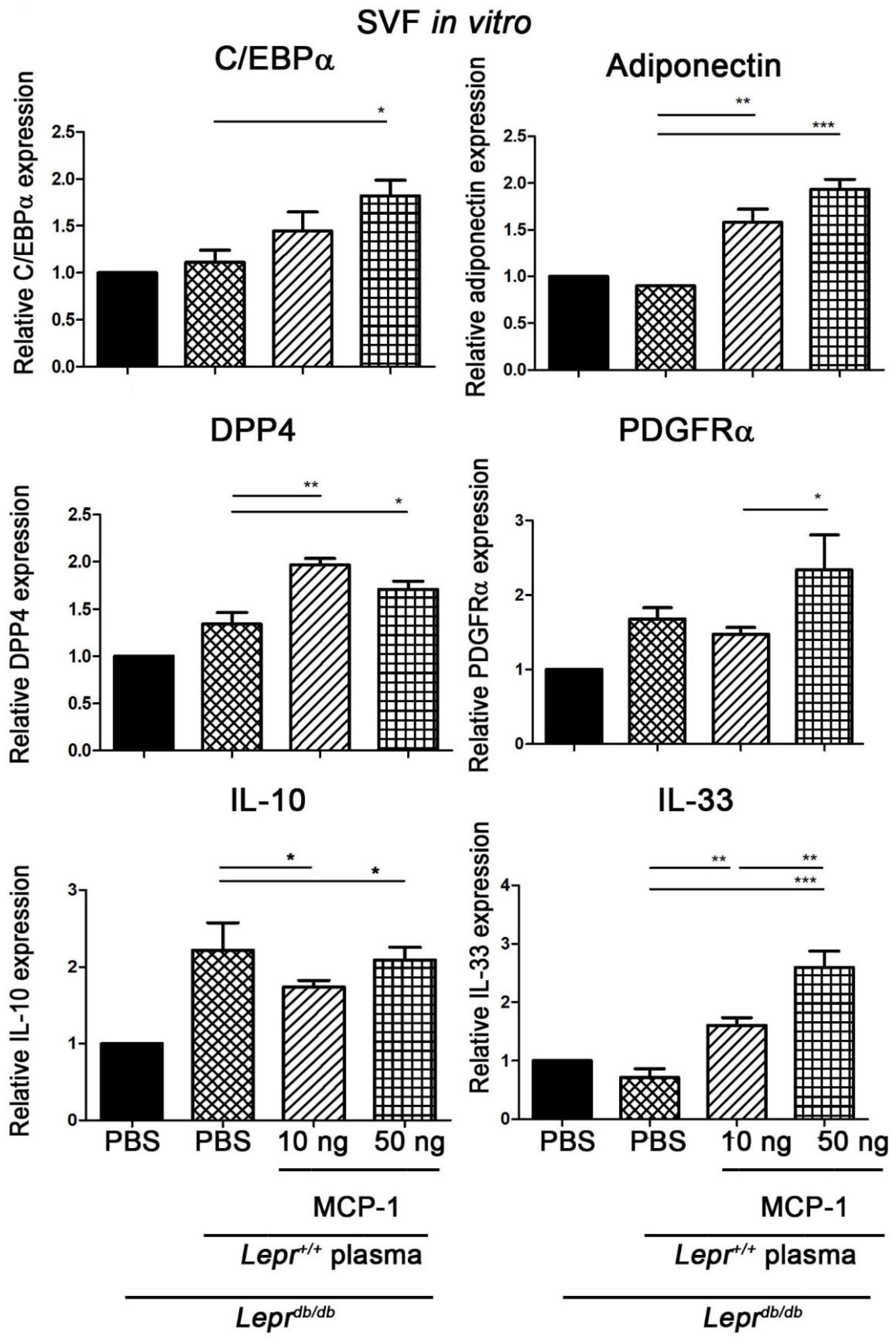
2.2. MCP-1-Supplemented Plasma Enhances the Expression of Adipogenesis-Related Genes in SVFs of Adipose Tissue of Leprdb/db Mice
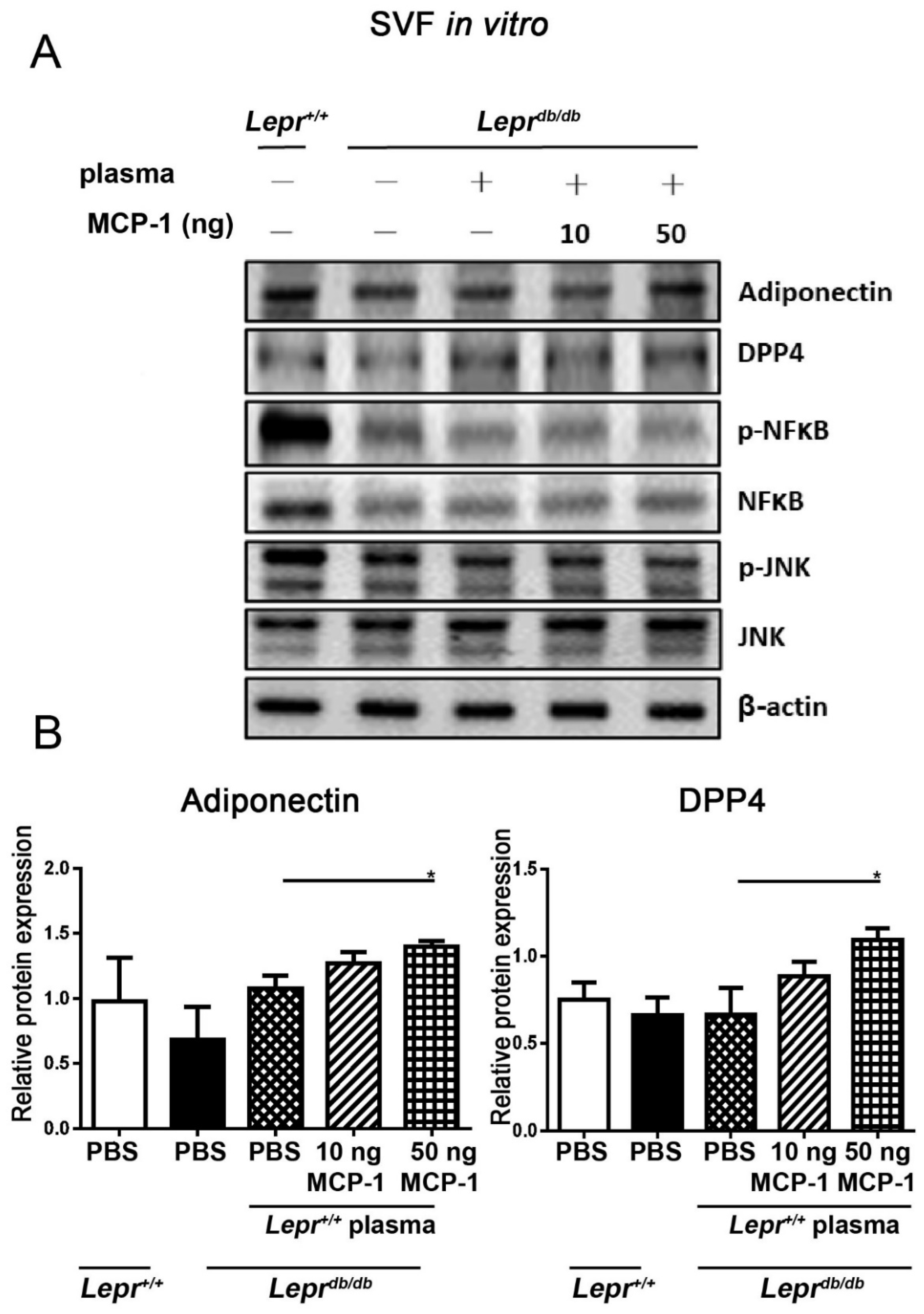
2.3. MCP-1-Supplemented Control Plasma Increases the Protein Expression of Adiponectin and DPP4 in SVFs from Adipose Tissue of Leprdb/db Mice
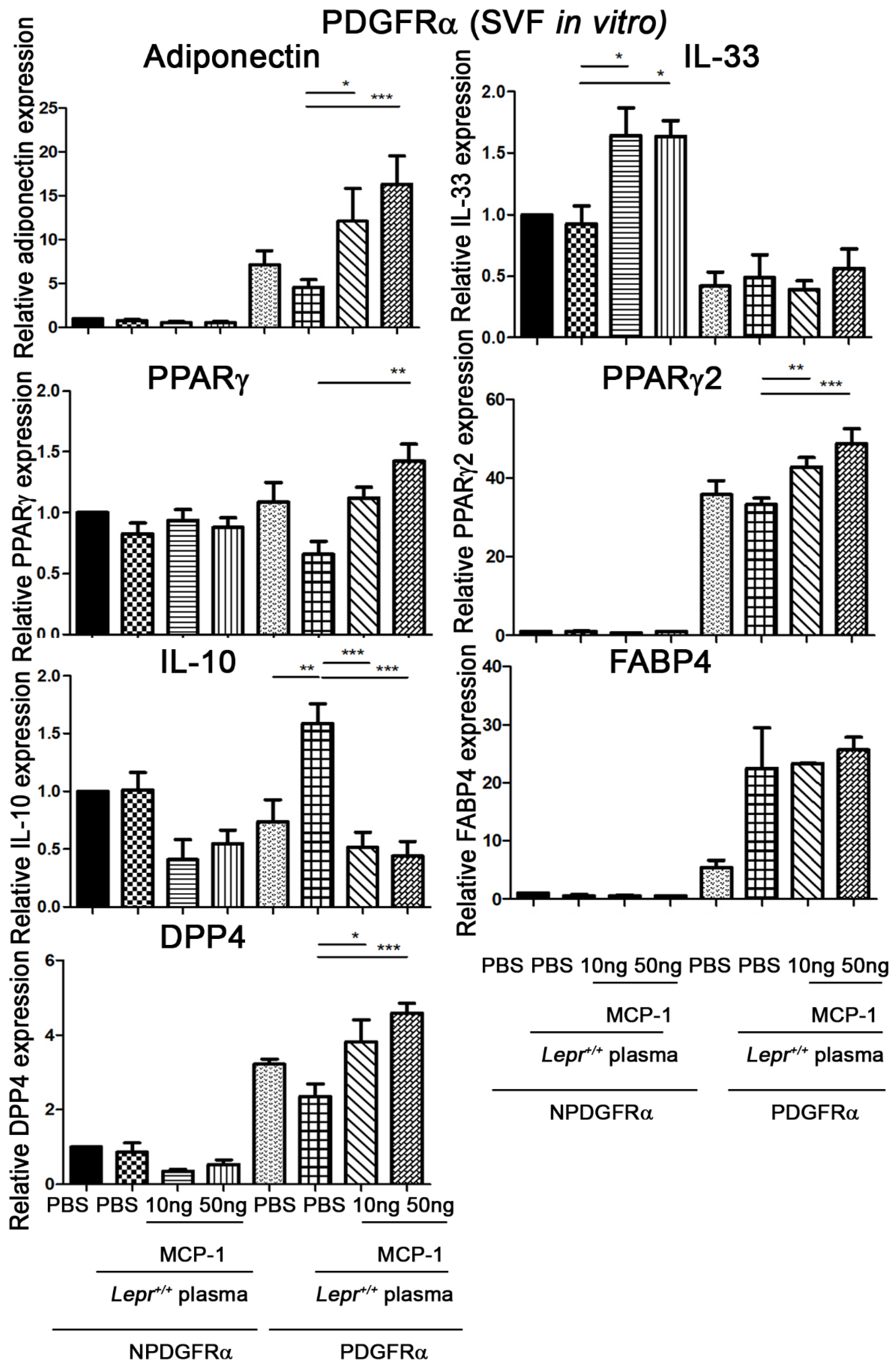
2.4. MCP-1-Supplemented Plasma Increases PPARγ, PPARγ2, Adiponectin, DPP4, and FABP4 mRNA Expression and Decreases IL-10 mRNA Expression in PDGFRα Cells from Adipose Tissue
2.5. MCP-1-Supplemented Plasma Increases MCP-1, PDGFRα, TNFα, Adiponectin, and IL-1β mRNA Expression and Decreases IL-10 and FOXP3 mRNA Expression in DPP4 Cells from Adipose Tissue
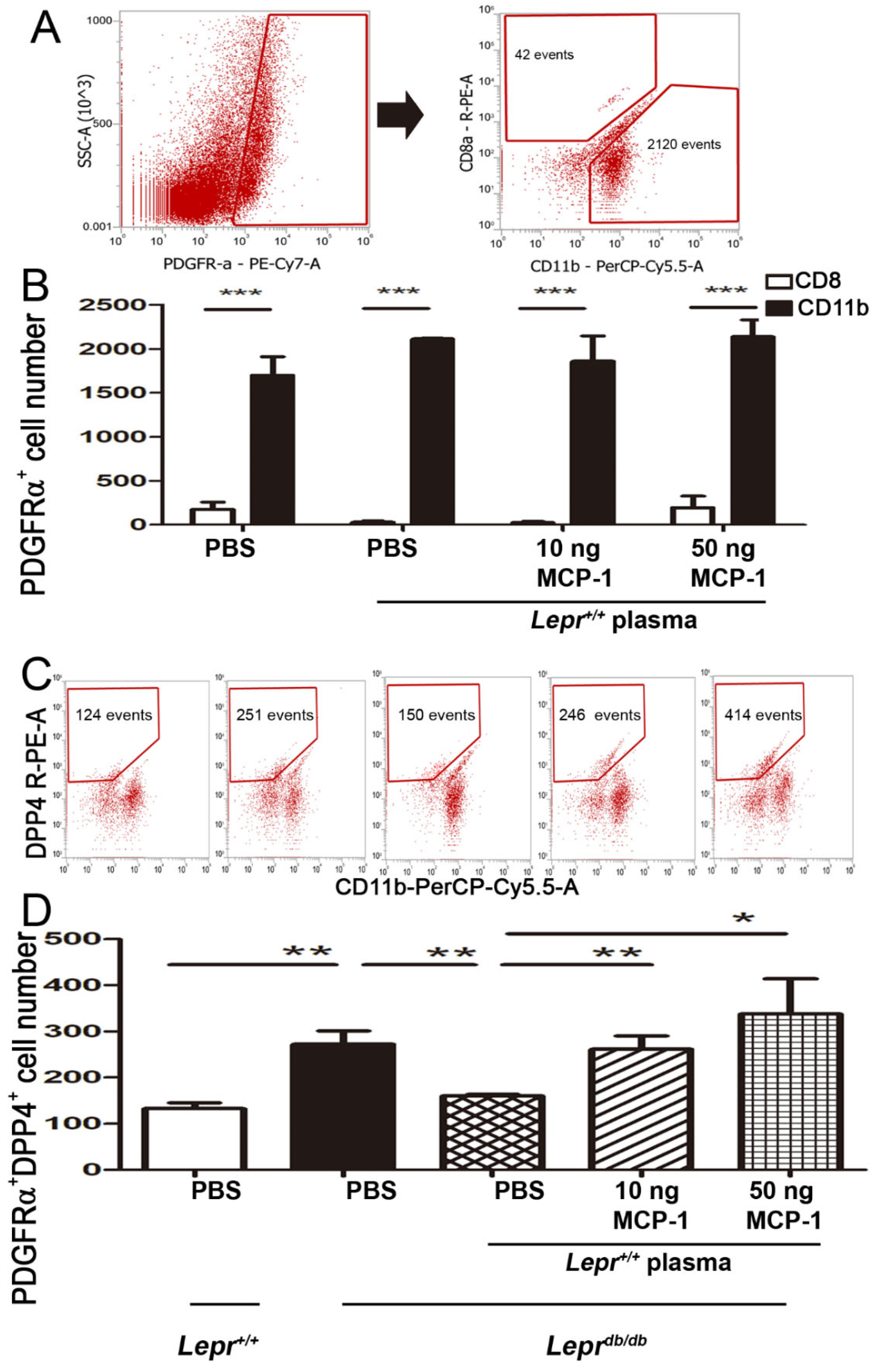
2.6. Injection of MCP-1-Supplemented Plasma into Adipose Tissue of Leprdb/db Mice Increases DPP4+ Cells among PDGFα+ Cells
2.7. Injection of MCP-1-Supplemented Plasma into Adipose Tissue of Leprdb/db Mice Increases PDGFRα mRNA Expression in Adipose Tissue Macrophages
2.8. Injection of MCP-1-Supplemented Plasma into Adipose Tissue of Leprdb/db Mice Enhances Plasma Adiponectin Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Humans
4.3. Preparation of Stromal Vascular Fractions
4.4. In Vitro Treatment of SVFs
4.5. Isolation of PDGFRα Cells from SVFs with Microbeads
4.6. Isolation of DPP4 Cells from SVFs with Microbeads
4.7. Isolation of Adipose Tissue Macrophages (ATMs) from SVFs with Microbeads
4.8. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.9. Western Immunoblots
4.10. Flow Cytometry Analysis
4.11. In Vivo Injection of MCP-1-Supplemented Plasma into Adipose Tissue
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef]
- Wronkowitz, N.; Gorgens, S.W.; Romacho, T.; Villalobos, L.A.; Sanchez-Ferrer, C.F.; Peiro, C.; Sell, H.; Eckel, J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim. Biophys. Acta 2014, 1842, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, D.S.; Ozcan, L.; Zheng, Z.; Nicoloro, S.M.; Shen, Y.; Chen, E.; Bluher, M.; Czech, M.P.; Tabas, I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 2018, 555, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Stefkovich, M.; Traynor, S.; Cheng, L.; Merrick, D.; Seale, P. Dpp4+ interstitial progenitor cells contribute to basal and high fat diet-induced adipogenesis. Mol. Metab. 2021, 54, 101357. [Google Scholar] [CrossRef] [PubMed]
- Younce, C.; Kolattukudy, P. MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol. Biochem. 2012, 30, 307–320. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Vishvanath, L.; Gupta, R.K. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017, 31, 127–140. [Google Scholar] [CrossRef]
- Berry, R.; Rodeheffer, M.S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 2013, 15, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Spallanzani, R.G.; Zemmour, D.; Xiao, T.; Jayewickreme, T.; Li, C.; Bryce, P.J.; Benoist, C.; Mathis, D. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 2019, 4, eaaw3658. [Google Scholar] [CrossRef]
- Mahlakoiv, T.; Flamar, A.L.; Johnston, L.K.; Moriyama, S.; Putzel, G.G.; Bryce, P.J.; Artis, D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019, 4, eaax0416. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Brahs, A.; Dorton, D.; Witfill, K. Platelet-Rich Plasma: A Comprehensive Review of Emerging Applications in Medical and Aesthetic Dermatology. J. Clin. Aesthet. Dermatol. 2021, 14, 44–57. [Google Scholar]
- Frautschi, R.S.; Hashem, A.M.; Halasa, B.; Cakmakoglu, C.; Zins, J.E. Current Evidence for Clinical Efficacy of Platelet Rich Plasma in Aesthetic Surgery: A Systematic Review. Aesthet. Surg. J. 2017, 37, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Por, Y.C.; Yeow, V.K.; Louri, N.; Lim, T.K.; Kee, I.; Song, I.C. Platelet-rich plasma has no effect on increasing free fat graft survival in the nude mouse. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1030–1034. [Google Scholar] [CrossRef]
- Salgarello, M.; Visconti, G.; Rusciani, A. Breast fat grafting with platelet-rich plasma: A comparative clinical study and current state of the art. Plast. Reconstr. Surg. 2011, 127, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Halleux, C.M.; Takahashi, M.; Delporte, M.L.; Detry, R.; Funahashi, T.; Matsuzawa, Y.; Brichard, S.M. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem. Biophys. Res. Commun. 2001, 288, 1102–1107. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Vatner, D.F.; Goedeke, L.; Hirabara, S.M.; Zhang, Y.; Perry, R.J.; Shulman, G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 32584–32593. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.U.; Cohen, J.L.; Vangala, P.; Tencerova, M.; Nicoloro, S.M.; Yawe, J.C.; Shen, Y.; Czech, M.P.; Aouadi, M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014, 19, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.M.; Egro, F.M.; Ejaz, A.; Coleman, S.R.; Greenberger, J.S.; Rubin, J.P. Fat Grafting in Radiation-Induced Soft-Tissue Injury: A Narrative Review of the Clinical Evidence and Implications for Future Studies. Plast. Reconstr. Surg. 2021, 147, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, S.W.; Jahn-Hofmann, K.; Bangari, D.; Cummings, S.; Metz-Weidmann, C.; Schwahn, U.; Wohlfart, P.; Schafer, M.; Bielohuby, M. A siRNA mediated hepatic dpp4 knockdown affects lipid, but not glucose metabolism in diabetic mice. PLoS ONE 2019, 14, e0225835. [Google Scholar] [CrossRef]
- Conarello, S.L.; Li, Z.; Ronan, J.; Roy, R.S.; Zhu, L.; Jiang, G.; Liu, F.; Woods, J.; Zycband, E.; Moller, D.E.; et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 6825–6830. [Google Scholar] [CrossRef]
- Foley, J.E.; Jordan, J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: Mechanistic basis and clinical experience. Vasc. Health Risk Manag. 2010, 6, 541–548. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Dule, S.; Cavallo, M.G. Dipeptidyl Peptidase 4 (DPP4) as A Novel Adipokine: Role in Metabolism and Fat Homeostasis. Biomedicines 2022, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Bird, L. Stromal IL-33 balances fat stores. Nat. Rev. Immunol. 2019, 19, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Geissmann, F. Macrophage ontogeny in the control of adipose tissue biology. Curr. Opin. Immunol. 2020, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Li, S.M.; Fang, L.; Xia, C.J.; Zhang, P.; Xu, R.; Shi, Z.Y.; Zou, Z.; Ge, Q.W.; Wang, P.; et al. Platelet-rich plasma promotes bone formation, restrains adipogenesis and accelerates vascularization to relieve steroids-induced osteonecrosis of the femoral head. Platelets 2021, 32, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.-C.; Chen, P.-H.; Tang, C.-H.; Chen, L.-W. Dipeptidyl Peptidase 4 Stimulation Induces Adipogenesis-Related Gene Expression of Adipose Stromal Cells. Int. J. Mol. Sci. 2023, 24, 16101. https://doi.org/10.3390/ijms242216101
Lai H-C, Chen P-H, Tang C-H, Chen L-W. Dipeptidyl Peptidase 4 Stimulation Induces Adipogenesis-Related Gene Expression of Adipose Stromal Cells. International Journal of Molecular Sciences. 2023; 24(22):16101. https://doi.org/10.3390/ijms242216101
Chicago/Turabian StyleLai, Hsiao-Chi, Pei-Hsuan Chen, Chia-Hua Tang, and Lee-Wei Chen. 2023. "Dipeptidyl Peptidase 4 Stimulation Induces Adipogenesis-Related Gene Expression of Adipose Stromal Cells" International Journal of Molecular Sciences 24, no. 22: 16101. https://doi.org/10.3390/ijms242216101
APA StyleLai, H.-C., Chen, P.-H., Tang, C.-H., & Chen, L.-W. (2023). Dipeptidyl Peptidase 4 Stimulation Induces Adipogenesis-Related Gene Expression of Adipose Stromal Cells. International Journal of Molecular Sciences, 24(22), 16101. https://doi.org/10.3390/ijms242216101





