The Force-Dependent Mechanism of an Integrin α4β7–MAdCAM-1 Interaction
Abstract
1. Introduction
2. Results
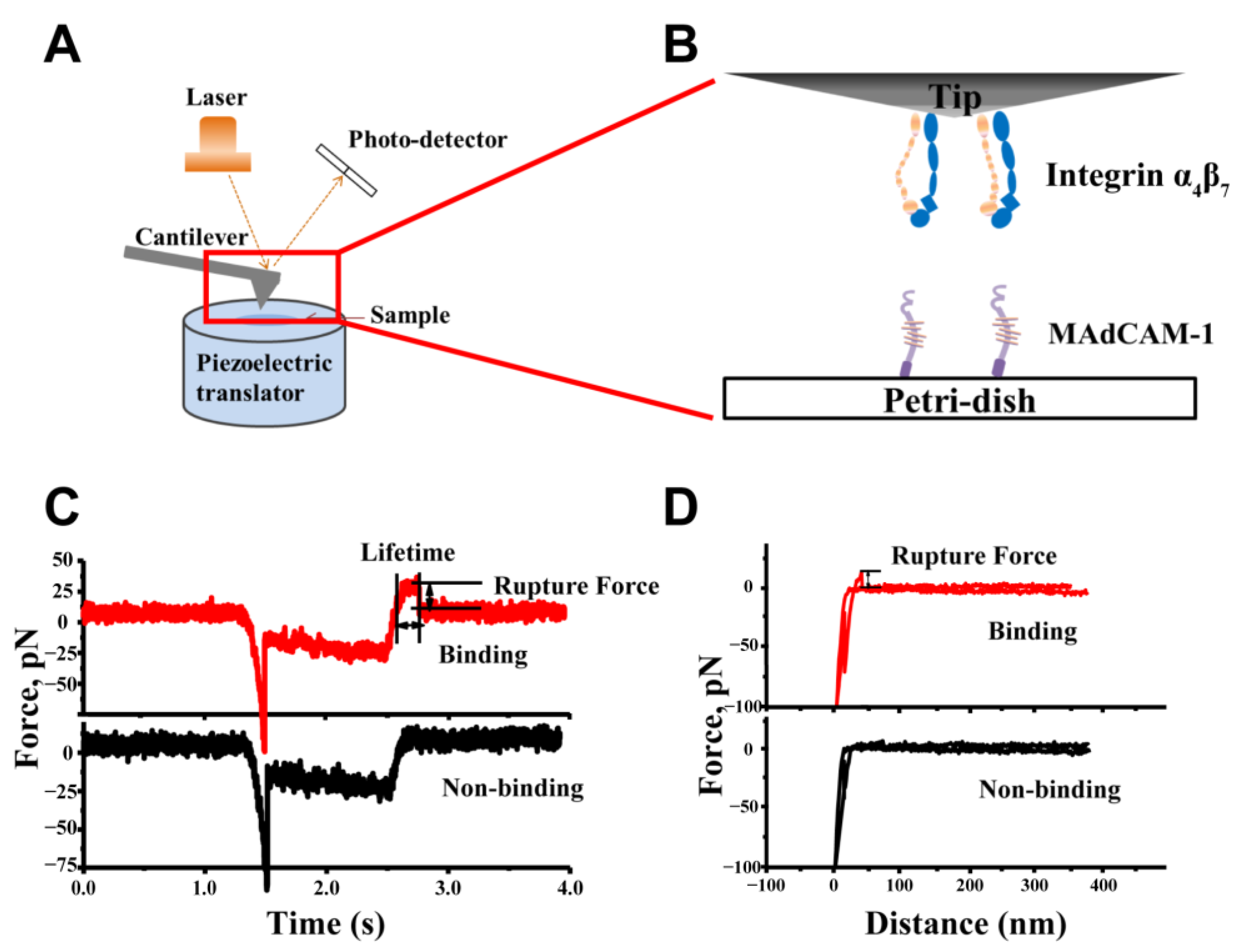
2.1. Specificity of the Integrin α4β7–MAdCAM-1 Interaction
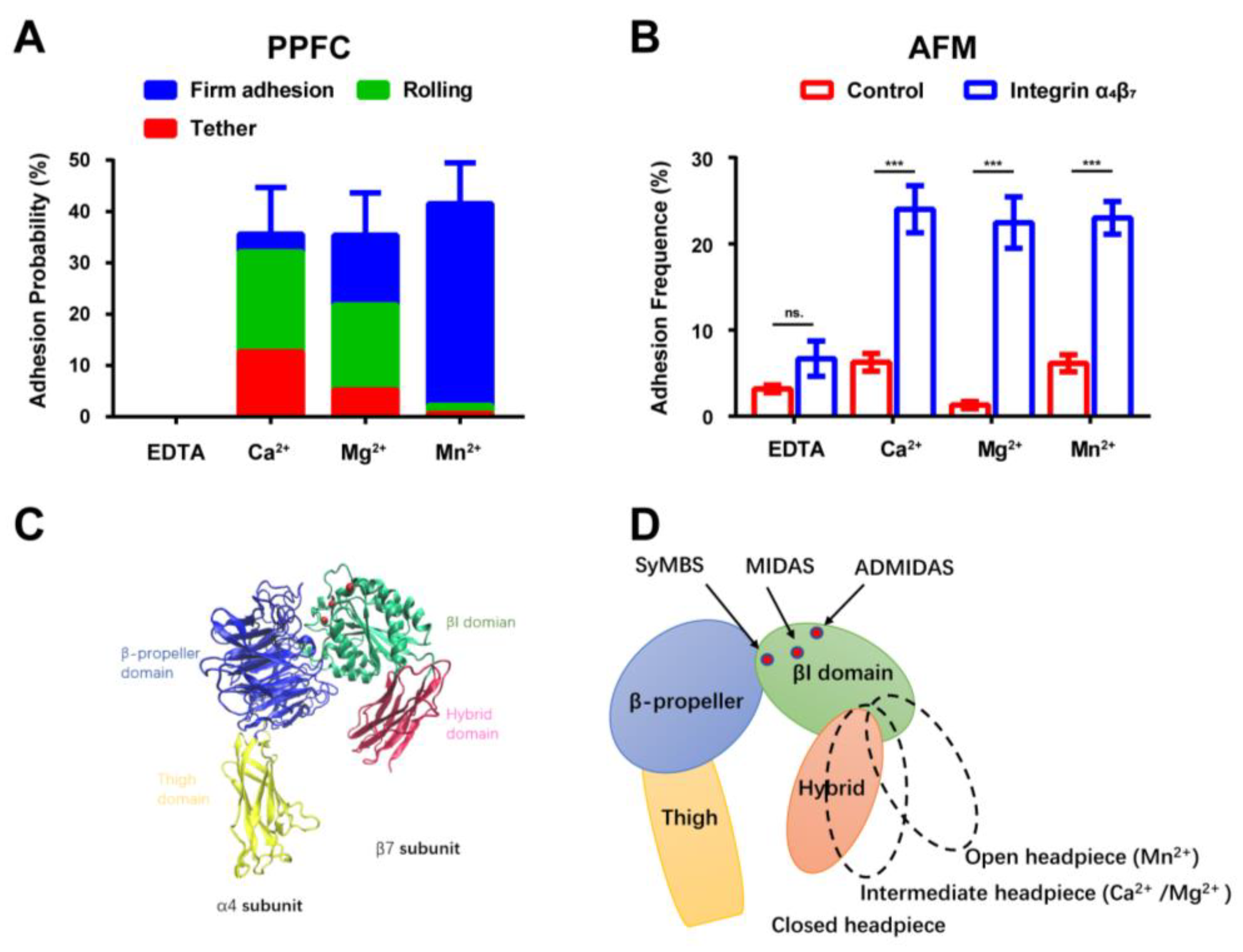
2.2. Binding Affinity of Integrin α4β7 with MAdCAM-1 Remains Consistent in the Presence of Various Metal Ions
2.3. Transition of Catch–Slip Bonds Govern the Lifetime of Interactions below and above the Optimal Force
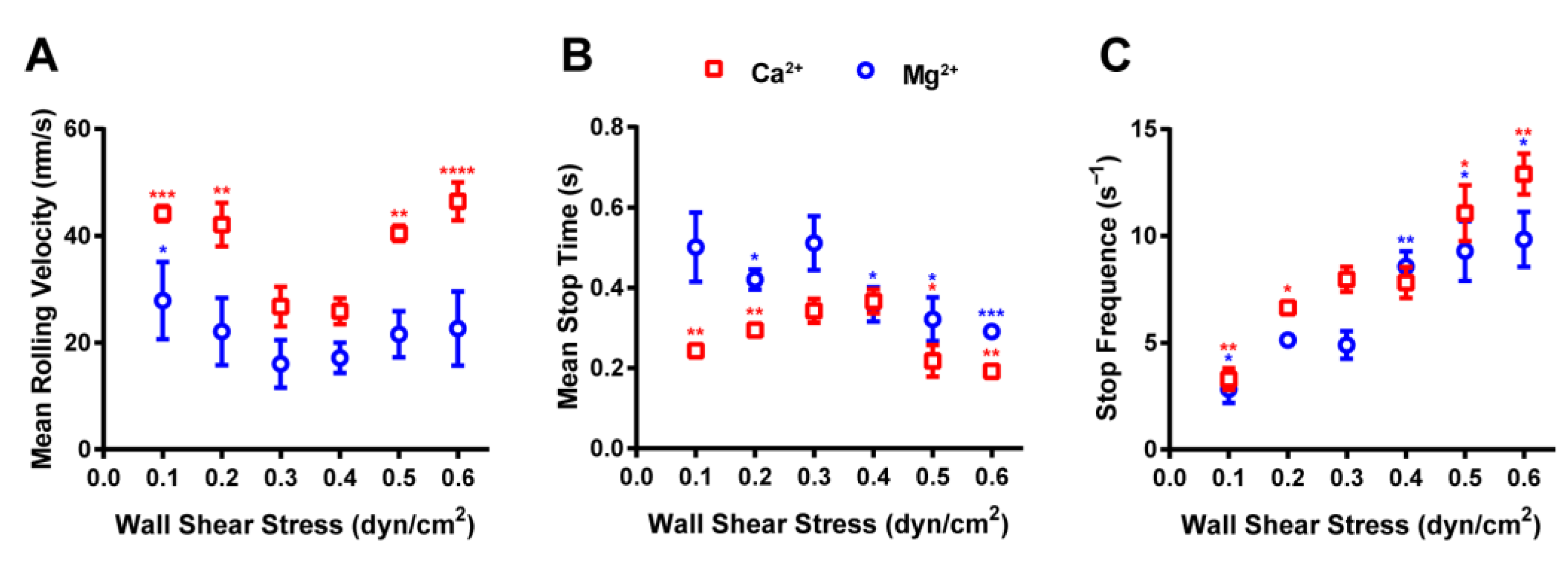
2.4. Rolling Behaviors of RPMI 8226 Cells Mediated by the Integrin α4β7–MAdCAM-1 Interaction under Flow
2.5. Ion and Force Dependence of RPMI 8226 Cell-Rolling Adhesion on MAdCAM-1-Coated Substrate under Flow
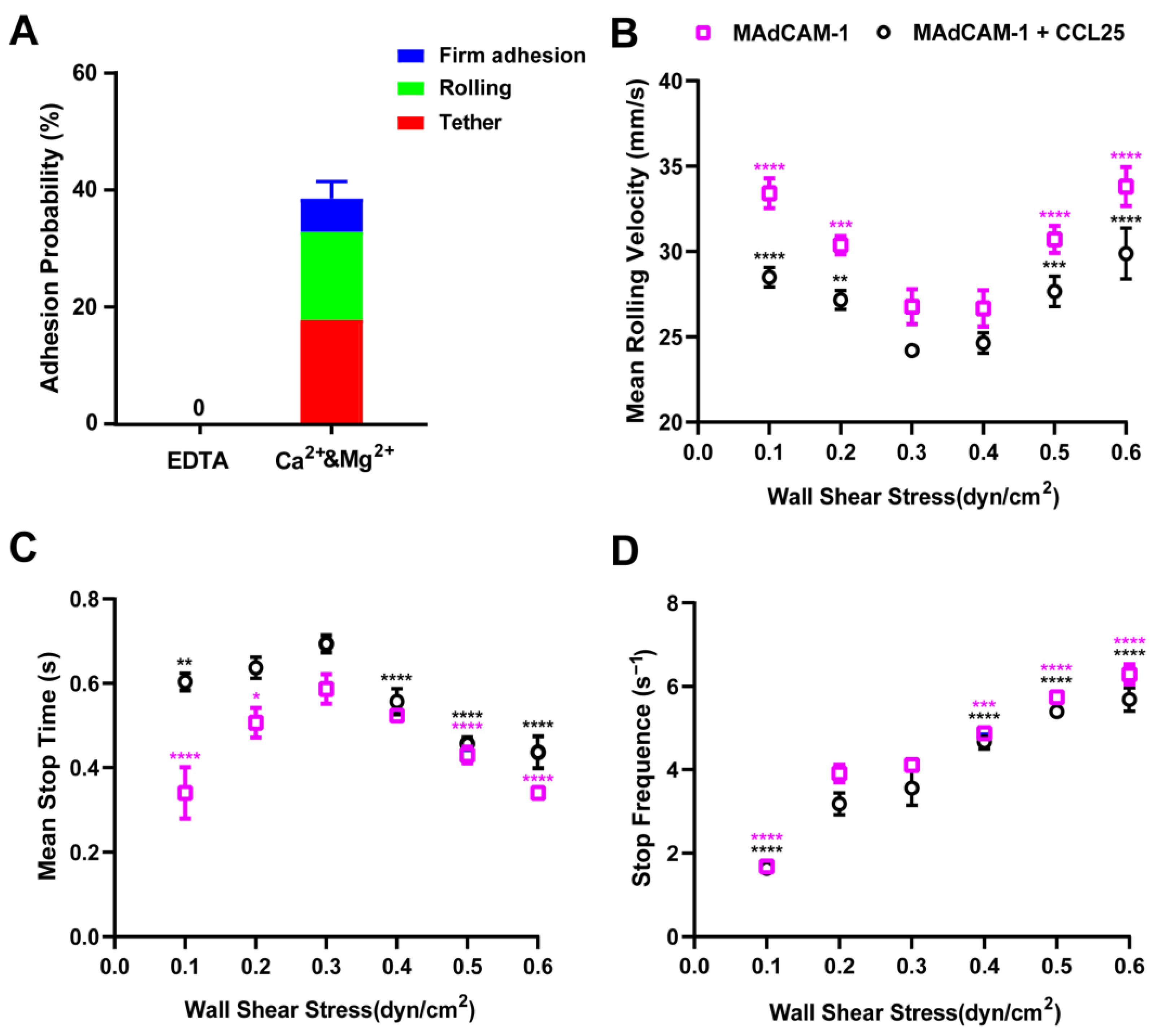
2.6. Rolling Adhesion of RPMI 8226 Cells on the MAdCAM-1- and/or CCL25-Coated Substrate
3. Discussion
4. Materials and Methods
4.1. Proteins and Cells
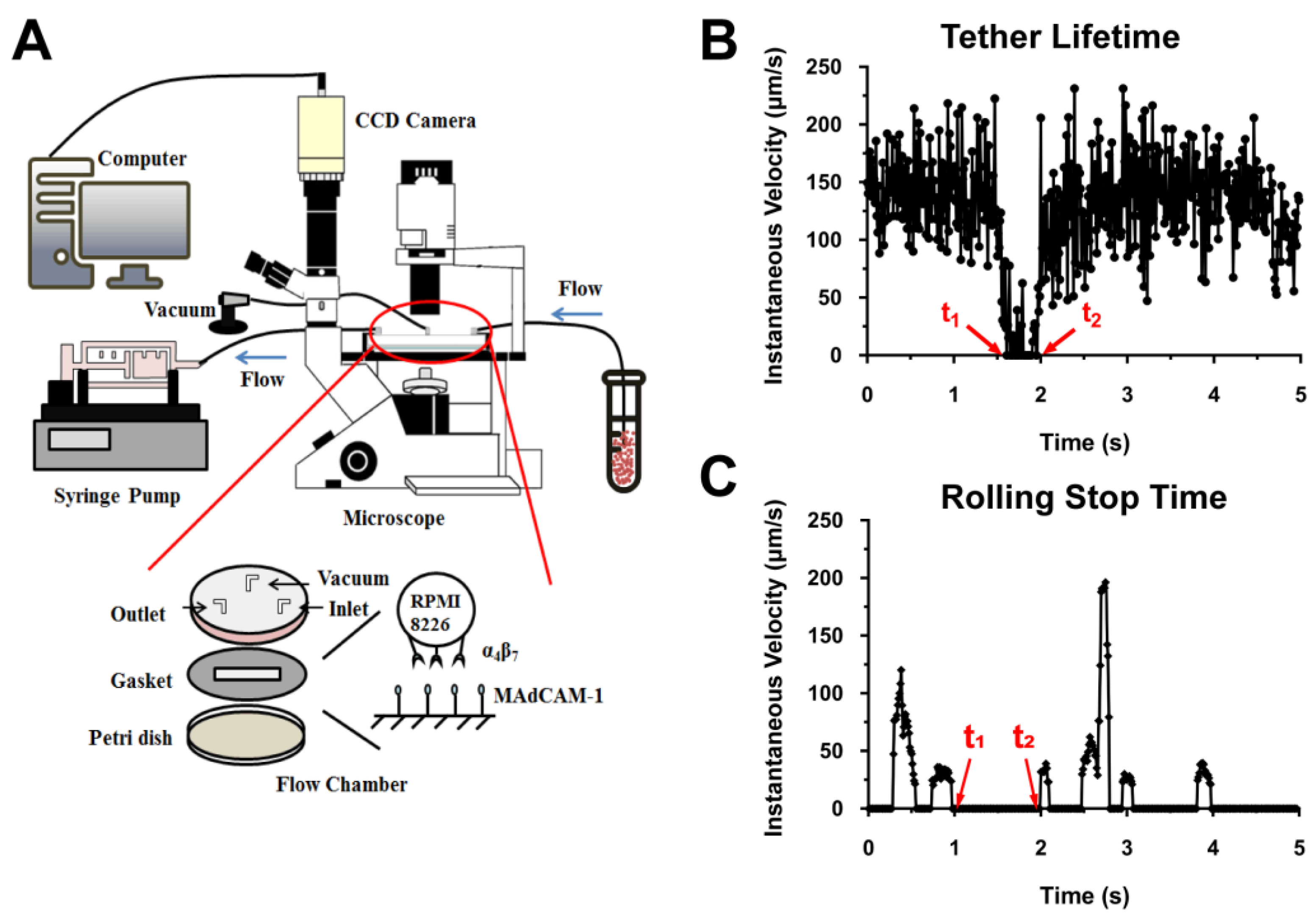
4.2. PPFC Assay
4.3. Transient Tether Lifetime Measurements and Rolling Step Analyses
4.4. AFM System Setup and Functionalization
4.5. AFM Experiments of the Adhesion Frequency and Lifetime Measurement
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, T.T.; Monteleone, G. Immunity, Inflammation, and Allergy in the Gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.; Berg, E.L.; Briskin, M.J.; Andrew, D.P.; Kilshaw, P.J.; Holzmann, B.; Weissman, I.L.; Hamann, A.; Butcher, E.C. α4β7 Integrin Mediates Lymphocyte Binding to the Mucosal Vascular Addressin MAdCAM-1. Cell 1993, 74, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, A.; Zhou, Y.; Kattah, M.G.; Fisher, S.J.; Mahadevan, U. Vedolizumab Antagonizes MAdCAM-1-Dependent Human Placental Cytotrophoblast Adhesion and Invasion In Vitro. Inflamm. Bowel Dis. 2022, 28, 1219–1228. [Google Scholar] [CrossRef]
- Melde, M.; Müller, T.M.; Schneider, I.; Geppert, C.I.; Mühl, L.; Besendorf, L.; Allner, C.; Becker, E.; Atreya, I.; Vitali, F.; et al. α4β7 Integrin-Dependent Adhesion of T Cells to MAdCAM-1 Is Blocked by Vedolizumab in Patients with Chronic Refractory Pouchitis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211054707. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.S.; Wang, J.; Buckman, C.A.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Baumgart, D.C. Targeting Leukocyte Migration and Adhesion in Crohn’s Disease and Ulcerative Colitis. Inflammopharmacology 2012, 20, 1–18. [Google Scholar] [CrossRef]
- Butcher, E.C. Leukocyte-Endothelial Cell Recognition: Three (or More) Steps to Specificity and Diversity. Cell 1991, 67, 1033–1036. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration: The Multistep Paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Adams, D.H.; Eksteen, B.; Curbishley, S.M. Immunology of the Gut and Liver: A Love/Hate Relationship. Gut 2008, 57, 838–848. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zheng, Y.; Pan, Y.; Zhang, K.; Chen, J. Distinct Chemokine Signaling Regulates Integrin Ligand Specificity to Dictate Tissue-Specific Lymphocyte Homing. Dev. Cell 2014, 30, 61–70. [Google Scholar] [CrossRef]
- Besendorf, L.; Müller, T.M.; Geppert, C.I.; Schneider, I.; Mühl, L.; Atreya, I.; Vitali, F.; Atreya, R.; Neurath, M.F.; Zundler, S. Vedolizumab Blocks α4β7 Integrin-Mediated T Cell Adhesion to MAdCAM-1 in Microscopic Colitis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221098899. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Li, M.; Zhang, X.; Gao, Z.; Bai, J.; Wu, Y.; Zhou, S.; Li, M.; Qu, L. α4β7 Integrin (LPAM-1) Is Upregulated at Atherosclerotic Lesions and Is Involved in Atherosclerosis Progression. Cell. Physiol. Biochem. 2014, 33, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Cicala, C.; Arthos, J.; Fauci, A.S. HIV-1 Envelope, Integrins and Co-receptor Use in Mucosal Transmission of HIV. J. Transl. Med. 2011, 9 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, J.; Mi, L.Z.; Walz, T.; Sun, H.; Chen, J.; Springer, T.A. Structural Specializations of α4β7, an Integrin That Mediates Rolling Adhesion. J. Cell Biol. 2012, 196, 131–146. [Google Scholar] [CrossRef]
- Briskin, M.J.; McEvoy, L.M.; Butcher, E.C. MAdCAM-1 Has Homology to Immunoglobulin and Mucin-Like Adhesion Receptors and to IgA1. Nature 1993, 363, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Eksteen, B.; Miles, A.E.; Grant, A.J.; Adams, D.H. Lymphocyte Homing in the Pathogenesis of Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Clin. Med. 2004, 4, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Salas, A.; Springer, T.A. Bistable Regulation of Integrin Adhesiveness by a Bipolar Metal Ion Cluster. Nat. Struct. Biol. 2003, 10, 995–1001. [Google Scholar] [CrossRef]
- de Château, M.; Chen, S.; Salas, A.; Springer, T.A. Kinetic and Mechanical Basis of Rolling through an Integrin and Novel Ca2+-Dependent Rolling and Mg2+-Dependent Firm Adhesion Modalities for the α4β7–MAdCAM-1 Interaction. Biochemistry 2001, 40, 13972–13979. [Google Scholar] [CrossRef]
- Dransfield, I.; Cabañas, C.; Craig, A.; Hogg, N. Divalent Cation Regulation of the Function of the Leukocyte Integrin LFA-1. J. Cell Biol. 1992, 116, 219–226. [Google Scholar] [CrossRef]
- Grzesiak, J.J.; Davis, G.E.; Kirchhofer, D.; Pierschbacher, M.D. Regulation of Alpha 2 Beta 1-Mediated Fibroblast Migration on Type I Collagen by Shifts in the Concentrations of Extracellular Mg2+ and Ca2+. J. Cell Biol. 1992, 117, 1109–1117. [Google Scholar] [CrossRef]
- Smith, J.W.; Piotrowicz, R.S.; Mathis, D. A Mechanism for Divalent Cation Regulation of Beta 3-Integrins. J. Biol. Chem. 1994, 269, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Mould, A.P.; Akiyama, S.K.; Humphries, M.J. Regulation of Integrin α5β1-Fibronectin Interactions by Divalent Cations. Evidence for Distinct Classes of Binding Sites for Mn2+, Mg2+, and Ca2+. J. Biol. Chem. 1995, 270, 26270–26277. [Google Scholar] [CrossRef]
- Miles, A.; Liaskou, E.; Eksteen, B.; Lalor, P.F.; Adams, D.H. CCL25 and CCL28 Promote α4β7-Integrin-Dependent Adhesion of Lymphocytes to MAdCAM-1 under Shear Flow. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1257–G1267. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, E.J.; Butcher, E.C. Chemokines and the Tissue-Specific Migration of Lymphocytes. Immunity 2002, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nieves, J.; Ho, J.; Bamias, G.; Ivashkina, N.; Ley, K.; Oppermann, M.; Cominelli, F. Antibody Blockade of CCL25/CCR9 Ameliorates Early but Not Late Chronic Murine Ileitis. Gastroenterology 2006, 131, 1518–1529. [Google Scholar] [CrossRef]
- Berlin, C.; Bargatze, R.F.; Campbell, J.J.; von Andrian, U.H.; Szabo, M.C.; Hasslen, S.R.; Nelson, R.D.; Berg, E.L.; Erlandsen, S.L.; Butcher, E.C. α4 Integrins Mediate Lymphocyte Attachment and Rolling under Physiologic Flow. Cell 1995, 80, 413–422. [Google Scholar] [CrossRef]
- Li, Q.; Wayman, A.; Lin, J.; Fang, Y.; Zhu, C.; Wu, J. Flow-Enhanced Stability of Rolling Adhesion through E-Selectin. Biophys. J. 2016, 111, 686–699. [Google Scholar] [CrossRef]
- Yago, T.; Zarnitsyna, V.I.; Klopocki, A.G.; McEver, R.P.; Zhu, C. Transport Governs Flow-Enhanced Cell Tethering through L-Selectin at Threshold Shear. Biophys. J. 2007, 92, 330–342. [Google Scholar] [CrossRef]
- Li, Q.; Fang, Y.; Ding, X.; Wu, J.; Bond, F.-D. Force-Dependent Bond Dissociation Govern Rolling of HL-60 Cells through E-Selectin. Exp. Cell. Res. 2012, 318, 1649–1658. [Google Scholar] [CrossRef]
- Yago, T.; Wu, J.; Wey, C.D.; Klopocki, A.G.; Zhu, C.; McEver, R.P. Catch Bonds Govern Adhesion through L-Selectin at Threshold Shear. J. Cell Biol. 2004, 166, 913–923. [Google Scholar] [CrossRef]
- Wayman, A.M.; Chen, W.; McEver, R.P.; Zhu, C. Triphasic Force Dependence of E-Selectin/Ligand Dissociation Governs Cell Rolling under Flow. Biophys. J. 2010, 99, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Clobes, A.M.; Guilford, W.H. Loop 2 of Myosin Is a Force-Dependent Inhibitor of the Rigor Bond. J. Muscle Res. Cell Motil. 2014, 35, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-Bond Mechanism of the Bacterial Adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wu, J.; Fang, Y. Moderate Constraint Facilitates Association and Force-Dependent Dissociation of HA-CD44 Complex. Int. J. Mol. Sci. 2023, 24, 2243. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.C.R.; Gomes, D.E.B.; Bernardi, R.C. Molecular Origins of Force-Dependent Protein Complex Stabilization during Bacterial Infections. J. Am. Chem. Soc. 2023, 145, 70–77. [Google Scholar] [CrossRef]
- Huang, W.; Le, S.; Sun, Y.; Lin, D.J.; Yao, M.; Shi, Y.; Yan, J. Mechanical Stabilization of a Bacterial Adhesion Complex. J. Am. Chem. Soc. 2022, 144, 16808–16818. [Google Scholar] [CrossRef]
- Yago, T.; Lou, J.; Wu, T.; Yang, J.; Miner, J.J.; Coburn, L.; López, J.A.; Cruz, M.A.; Dong, J.F.; McIntire, L.V.; et al. Platelet Glycoprotein Ibα Forms Catch Bonds with Human WT vWF but Not with Type 2B von Willebrand Disease vWF. J. Clin. Investig. 2008, 118, 3195–3207. [Google Scholar] [CrossRef]
- Newham, P.; Craig, S.E.; Seddon, G.N.; Schofield, N.R.; Rees, A.; Edwards, R.M.; Jones, E.Y.; Humphries, M.J. α4 Integrin Binding Interfaces on VCAM-1 and MAdcAM-1. Integrin Binding Footprints Identify Accessory Binding Sites That Play a Role in Integrin Specificity. J. Biol. Chem. 1997, 272, 19429–19440. [Google Scholar] [CrossRef]
- Viney, J.L.; Jones, S.; Chiu, H.H.; Lagrimas, B.; Renz, M.E.; Presta, L.G.; Jackson, D.; Hillan, K.J.; Lew, S.; Fong, S. Mucosal Addressin Cell Adhesion Molecule-1: A Structural and Functional Analysis Demarcates the Integrin Binding Motif. J. Immunol. 1996, 157, 2488–2497. [Google Scholar] [CrossRef]
- Tan, K.; Casasnovas, J.M.; Liu, J.H.; Briskin, M.J.; Springer, T.A.; Wang, J.H. The Structure of Immunoglobulin Superfamily Domains 1 and 2 of MAdCAM-1 Reveals Novel Features Important for Integrin Recognition. Structure 1998, 6, 793–801. [Google Scholar] [CrossRef]
- Dando, J.; Wilkinson, K.W.; Ortlepp, S.; King, D.J.; Brady, R.L. A Reassessment of the MAdCAM-1 Structure and Its Role in Integrin Recognition. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Takagi, J.; Xie, C.; Xiao, T.; Luo, B.H.; Springer, T.A. The Relative Influence of Metal Ion Binding Sites in the I-Like Domain and the Interface with the Hybrid Domain on Rolling and Firm Adhesion by Integrin α4β7. J. Biol. Chem. 2004, 279, 55556–55561. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Demonstration of Catch Bonds between an Integrin and Its Ligand. J. Cell Biol. 2009, 185, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.B.; Puri, K.D.; Alon, R.; Lawrence, M.B.; von Andrian, U.H.; Springer, T.A. Adhesion through L-Selectin Requires a Threshold Hydrodynamic Shear. Nature 1996, 379, 266–269. [Google Scholar] [CrossRef]
- Rosetti, F.; Chen, Y.; Sen, M.; Thayer, E.; Azcutia, V.; Herter, J.M.; Luscinskas, F.W.; Cullere, X.; Zhu, C.; Mayadas, T.N. A Lupus-Associated Mac-1 Variant Has Defects in Integrin Allostery and Interaction with Ligands under Force. Cell Rep. 2015, 10, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lou, J.; Zhu, C. Forcing Switch from Short- to Intermediate- and Long-Lived States of the alphaA Domain Generates LFA-1/ICAM-1 Catch Bonds. J. Biol. Chem. 2010, 285, 35967–35978. [Google Scholar] [CrossRef]
- Zhang, X.; Craig, S.E.; Kirby, H.; Humphries, M.J.; Moy, V.T. Molecular Basis for the Dynamic Strength of the Integrin α4β1/VCAM-1 Interaction. Biophys. J. 2004, 87, 3470–3478. [Google Scholar] [CrossRef]
- Shimaoka, M.; Lu, C.; Palframan, R.T.; von Andrian, U.H.; McCormack, A.; Takagi, J.; Springer, T.A. Reversibly Locking a Protein Fold in an Active Conformation with a Disulfide Bond: Integrin αL I Domains with High Affinity and Antagonist Activity In Vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6009–6014. [Google Scholar] [CrossRef]
- Xiao, T.; Takagi, J.; Coller, B.S.; Wang, J.H.; Springer, T.A. Structural Basis for Allostery in Integrins and Binding to Fibrinogen-Mimetic Therapeutics. Nature 2004, 432, 59–67. [Google Scholar] [CrossRef]
- Valdramidou, D.; Humphries, M.J.; Mould, A.P. Distinct Roles of β1 Metal Ion-Dependent Adhesion Site (MIDAS), Adjacent to Midas (ADMIDAS), and Ligand-Associated Metal-Binding Site (LIMBS) Cation-Binding Sites in Ligand Recognition by Integrin α2β1. J. Biol. Chem. 2008, 283, 32704–32714. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J. The Regulation of Integrin Function by Divalent Cations. Cell Adh. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented Immobilization of Antibodies onto Sensing Platforms-A Critical Review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, W.; Fang, J.; Shi, X.; Wu, J.; Fang, Y.; Lin, J. AFM Imaging Reveals Multiple Conformational States of ADAMTS13. J. Biol. Eng. 2019, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.T.; Long, M.; Piper, J.W.; Yago, T.; McEver, R.P.; Zhu, C. Direct Observation of Catch Bonds Involving Cell-Adhesion Molecules. Nature 2003, 423, 190–193. [Google Scholar] [CrossRef]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Waldron, T.T.; Astrof, N.; Shimaoka, M.; Springer, T.A. The Connection between Metal Ion Affinity and Ligand Affinity in Integrin I Domains. Biochim. Biophys. Acta 2007, 1774, 1148–1155. [Google Scholar] [CrossRef][Green Version]
- Xiong, J.P.; Stehle, T.; Goodman, S.L.; Arnaout, M.A. Integrins, Cations and Ligands: Making the Connection. J. Thromb. Haemost. 2003, 1, 1642–1654. [Google Scholar] [CrossRef]
- Xiong, J.P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal Structure of the Extracellular Segment of Integrin αVβ3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef]
- Xiong, J.P.; Stehle, T.; Zhang, R.; Joachimiak, A.; Frech, M.; Goodman, S.L.; Arnaout, M.A. Crystal Structure of the Extracellular Segment of Integrin αVβ3 in Complex with an Arg–Gly–Asp Ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Luo, Z.; Sun, D.; Yang, B.; Li, Q. The Force-Dependent Mechanism of an Integrin α4β7–MAdCAM-1 Interaction. Int. J. Mol. Sci. 2023, 24, 16062. https://doi.org/10.3390/ijms242216062
Su Y, Luo Z, Sun D, Yang B, Li Q. The Force-Dependent Mechanism of an Integrin α4β7–MAdCAM-1 Interaction. International Journal of Molecular Sciences. 2023; 24(22):16062. https://doi.org/10.3390/ijms242216062
Chicago/Turabian StyleSu, Youmin, Zhiqing Luo, Dongshan Sun, Bishan Yang, and Quhuan Li. 2023. "The Force-Dependent Mechanism of an Integrin α4β7–MAdCAM-1 Interaction" International Journal of Molecular Sciences 24, no. 22: 16062. https://doi.org/10.3390/ijms242216062
APA StyleSu, Y., Luo, Z., Sun, D., Yang, B., & Li, Q. (2023). The Force-Dependent Mechanism of an Integrin α4β7–MAdCAM-1 Interaction. International Journal of Molecular Sciences, 24(22), 16062. https://doi.org/10.3390/ijms242216062






