Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases
Abstract
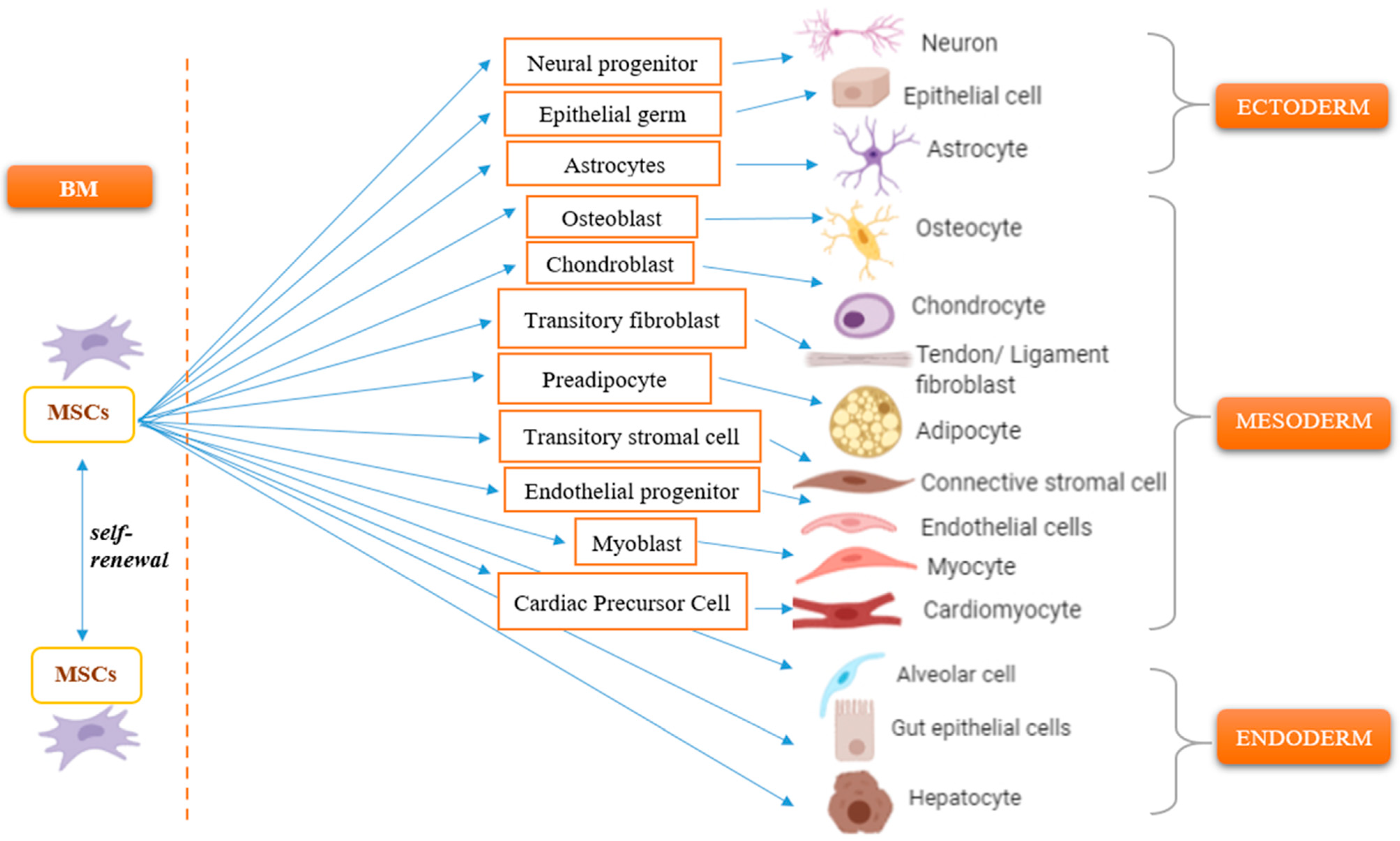
1. Introduction
2. Migratory Response of Mesenchymal Stem Cells
3. Immunomodulatory Properties of MSCs
3.1. Paracrine Activity of MSCs
Extracellular Vesicles Derived from MSCs
3.2. Regulatory Effects of MSCs on Immune Cells
4. Immunogenicity of MSCs
5. Impairment of MSC Biology as a Key Moment in Disease Pathogenesis
6. Pre-Clinical Studies of Mesenchymal Stem Cells
7. Clinical Application of Mesenchymal Stem Cells in the Treatment of Autoimmune and Autoinflammatory Diseases
| Disease | Patients (N) | MSC Type | Outcomes | Reference |
|---|---|---|---|---|
| Steroid-refractory acute graft-versus-host disease | 55 | Allogeneic BM-MSCs |
| [144] |
| Acute graft-versus-host disease resistant to multiple immunosuppressive agents in children | 75 | Allogeneic BM-MSCs |
| [143] |
| Steroid-refractory acute graft-versus-host disease III/IV after hematopoietic stem cell transplantation | 46 | Allogeneic BM-MSCs |
| [145] |
| Multiple sclerosis | 20 | Allogeneic UC-MSC |
| [151] |
| Multiple sclerosis | 9 patients received MSCs (N = 5) or placebo (N = 4) | Autologous BM-MSCs |
| [152] |
| Secondary progressive multiple sclerosis | 10 patients had low-dose (1 × 106 cells/kg) and 9 high-dose (4 × 106 cells/kg) | Autologous AT-MSCs |
| [153] |
| Amyotrophic lateral sclerosis | 23 | Autologous BM-MSCs |
| [154] |
| Amyotrophic lateral sclerosis | 20 | Autologous BM-MSCs |
| [155] |
| Rheumatoid arthritis | 53 | Allogeneic AT-MSCs |
| [156] |
| Rheumatoid arthritis | 64 | Allogeneic UC-MSC |
| [157] |
| Systemic lupus erythematosus with refractory cytopenia | 35 | BM-MSC |
| [158] |
| Systemic lupus erythematosus (severe and drug-refractory) | 81 | Allogeneic 22 BM-MSC, 59 UC-MSCs |
| [159] |
| Lupus nephritis | 18 patients received MSCs (N = 12) or placebo (N = 6) | Allogeneic UC-MSCs |
| [160] |
| Systemic sclerosis | 14 | Allogeneic UC-MSCs |
| [161] |
| Systemic sclerosis | 62 | Autologous AT-MSCs |
| [162] |
| Liver cirrhosis caused by autoimmune diseases (mixed connective tissue disease, primary biliary cirrhosis, primary Sjögren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis) | 26 | Allogeneic (23 patients received UC-MSCs, 2 received cord blood MSCs and 1—BM-MSCs) |
| [163] |
| Idiopathic pulmonary fibrosis | 8 | Allogeneic placenta-derived MSCs |
| [164] |
| Idiopathic pulmonary fibrosis | 9 | Allogeneic BM- MSCs |
| [165] |
| COVID-19 | 7 (1 critically severe type, 4 severe types and 2 common types) | Autologous BM-MSCs |
| [166] |
8. Risks and Challenges of Stem Cell Transplantation
9. Comparison of Allogeneic and Autologous Sources of Mesenchymal Stem Cells
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef]
- Friedenstein, A.; Heersche, J.; Kanis, J. Bone and Mineral Research; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhuge, Y.; Velazquez, O.C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 2009, 106, 984–991. [Google Scholar] [CrossRef]
- Ling, L.; Hou, J.; Liu, D.; Tang, D.; Zhang, Y.; Zeng, Q.; Pan, H.; Fan, L. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Res. Ther. 2022, 13, 79. [Google Scholar] [CrossRef]
- Barhanpurkar-Naik, A.; Mhaske, S.T.; Pote, S.T.; Singh, K.; Wani, M.R. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res. Ther. 2017, 8, 168. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Singh, P.; Kacena, M.A.; Orschell, C.M.; Pelus, L.M. Aging-Related Reduced Expression of CXCR4 on Bone Marrow Mesenchymal Stromal Cells Contributes to Hematopoietic Stem and Progenitor Cell Defects. Stem Cell Rev. Rep. 2020, 16, 684–692. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef]
- Zhang, S.J.; Song, X.Y.; He, M.; Yu, S.B. Effect of TGF-β1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 899–905. [Google Scholar]
- Dubon, M.J.; Yu, J.; Choi, S.; Park, K.S. Transforming growth factor β induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J. Cell. Physiol. 2018, 233, 201–213. [Google Scholar] [CrossRef]
- Camps, J.; Castañé, H.; Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Arenas, M.; Iftimie, S.; Joven, J. On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update. Biomolecules 2021, 11, 971. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Berent-Maoz, B.; Montecino-Rodriguez, E.; Signer, R.A.; Dorshkind, K. Fibroblast growth factor-7 partially reverses murine thymocyte progenitor aging by repression of Ink4a. Blood J. Am. Soc. Hematol. 2012, 119, 5715–5721. [Google Scholar] [CrossRef]
- Calandra, T.; Bernhagen, J.; Mitchell, R.A.; Bucala, R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 1994, 179, 1895–1902. [Google Scholar] [CrossRef]
- Calandra, T.; Bernhagen, J.; Metz, C.N.; Spiegel, L.A.; Bacher, M.; Donnelly, T.; Cerami, A.; Bucala, R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377, 68–71. [Google Scholar] [CrossRef]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayer, K.; Chesney, J.; Lohoff, M.; Gemsa, D.; Donnelly, T.; Bucala, R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef]
- Donnelly, S.C.; Haslett, C.; Reid, P.T.; Grant, I.S.; Wallace, W.A.; Metz, C.N.; Bruce, L.J.; Bucala, R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 1997, 3, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Makita, H.; Nishimura, M.; Miyamoto, K.; Nakano, T.; Tanino, Y.; Hirokawa, J.; Nishihira, J.; Kawakami, Y. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharide-induced pulmonary neutrophil accumulation. Am. J. Respir. Crit. Care Med. 1998, 158, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Metz, C.N.; Peng, T.; Bucala, R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF): Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 1999, 274, 18100–18106. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Liao, H.; Chesney, J.; Fingerle-Rowson, G.; Baugh, J.; David, J.; Bucala, R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: Regulatory role in the innate immune response. Proc. Natl. Acad. Sci. USA 2002, 99, 345–350. [Google Scholar] [CrossRef]
- Lehmann, L.; Novender, U.; Schroeder, S.; Pietsch, T.; von Spiegel, T.; Putensen, C.; Hoeft, A.; Stüber, F. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Med. 2001, 27, 1412–1415. [Google Scholar] [CrossRef]
- Gando, S.; Nishihira, J.; Kobayashi, S.; Morimoto, Y.; Nanzaki, S.; Kemmotsu, O. Macrophage migration inhibitory factor is a critical mediator of systemic inflammatory response syndrome. Intensive Care Med. 2001, 27, 1187–1193. [Google Scholar] [CrossRef]
- Calandra, T.; Echtenacher, B.; Roy, D.L.; Pugin, J.; Metz, C.N.; Hültner, L.; Heumann, D.; Männel, D.; Bucala, R.; Glauser, M.P. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000, 6, 164–170. [Google Scholar] [CrossRef]
- Beishuizen, A.; Thijs, L.G.; Haanen, C.; Vermes, I.N. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J. Clin. Endocrinol. Metab. 2001, 86, 2811–2816. [Google Scholar] [CrossRef][Green Version]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. J. Lab. Clin. Med. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Faiotto, V.B.; Franci, D.; Enz Hubert, R.M.; de Souza, G.R.; Fiusa, M.M.L.; Hounkpe, B.W.; Santos, T.M.; Carvalho-Filho, M.A.; De Paula, E.V. Circulating levels of the angiogenesis mediators endoglin, HB-EGF, BMP-9 and FGF-2 in patients with severe sepsis and septic shock. J. Crit. Care 2017, 42, 162–167. [Google Scholar] [CrossRef]
- Heidenreich, R.; Röcken, M.; Ghoreschi, K. Angiogenesis drives psoriasis pathogenesis. Int. J. Exp. Pathol. 2009, 90, 232–248. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and Diabetic Retinal Microvascular Complications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Molnarfi, N.; Benkhoucha, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun. Rev. 2015, 14, 293–303. [Google Scholar] [CrossRef]
- Kmiecik, T.E.; Keller, J.R.; Rosen, E.; Vande Woude, G.F. Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood 1992, 80, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Hisha, H.; Nishino, N.; Adachi, M.; Ikehara, S. Hepatocyte growth factor as a hematopoietic regulator. Blood 1995, 85, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Z.; Hisha, H.; Li, Y.; Lian, Z.; Nishino, T.; Toki, J.; Adachi, Y.; Inaba, M.; Fan, T.X.; Jin, T.; et al. Stimulatory effects of hepatocyte growth factor on hemopoiesis of SCF/c-kit system-deficient mice. Stem Cells 1998, 16, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Futamatsu, H.; Suzuki, J.; Mizuno, S.; Koga, N.; Adachi, S.; Kosuge, H.; Maejima, Y.; Hirao, K.; Nakamura, T.; Isobe, M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: A potential role for induction of T helper 2 cytokines. Circ. Res. 2005, 96, 823–830. [Google Scholar] [CrossRef]
- Skibinski, G.; Skibinska, A.; James, K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 2001, 102, 506–514. [Google Scholar] [CrossRef]
- Takai, K.; Hara, J.; Matsumoto, K.; Hosoi, G.; Osugi, Y.; Tawa, A.; Okada, S.; Nakamura, T. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood 1997, 89, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Sugawara, T.; Tokoro, Y.; Taniguchi, H.; Fukao, K.; Nakauchi, H.; Takahama, Y. Expression and function of c-Met, a receptor for hepatocyte growth factor, during T-cell development. Scand. J. Immunol. 1998, 47, 296–301. [Google Scholar] [CrossRef] [PubMed]
- van der Voort, R.; Taher, T.E.; Keehnen, R.M.; Smit, L.; Groenink, M.; Pals, S.T. Paracrine regulation of germinal center B cell adhesion through the c-met-hepatocyte growth factor/scatter factor pathway. J. Exp. Med. 1997, 185, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Okazaki, H.; Nakamura, T. Up-regulation of hepatocyte growth factor gene expression by interleukin-1 in human skin fibroblasts. Biochem. Biophys. Res. Commun. 1992, 188, 235–243. [Google Scholar] [CrossRef]
- Soler Palacios, B.; Nieto, C.; Fajardo, P.; González de la Aleja, A.; Andrés, N.; Dominguez-Soto, Á.; Lucas, P.; Cuenda, A.; Rodríguez-Frade, J.M.; Martínez, A.C.; et al. Growth Hormone Reprograms Macrophages toward an Anti-Inflammatory and Reparative Profile in an MAFB-Dependent Manner. J. Immunol. 2020, 205, 776–788. [Google Scholar] [CrossRef]
- Spadaro, O.; Camell, C.D.; Bosurgi, L.; Nguyen, K.Y.; Youm, Y.H.; Rothlin, C.V.; Dixit, V.D. IGF1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell Rep. 2017, 19, 225–234. [Google Scholar] [CrossRef]
- Thibaut, R.; Gage, M.C.; Pineda-Torra, I.; Chabrier, G.; Venteclef, N.; Alzaid, F. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J. 2022, 289, 3024–3057. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Cordoba-Chacon, J.; Kineman, R.D. Growth Hormone and Insulin-Like Growth Factor 1 Regulation of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, 1812–1824. [Google Scholar] [CrossRef]
- Tang, J.; Kozaki, K.; Farr, A.G.; Martin, P.J.; Lindahl, P.; Betsholtz, C.; Raines, E.W. The absence of platelet-derived growth factor-B in circulating cells promotes immune and inflammatory responses in atherosclerosis-prone ApoE-/- mice. Am. J. Pathol. 2005, 167, 901–912. [Google Scholar] [CrossRef]
- Ross, R.; Masuda, J.; Raines, E.W.; Gown, A.M.; Katsuda, S.; Sasahara, M.; Malden, L.T.; Masuko, H.; Sato, H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science 1990, 248, 1009–1012. [Google Scholar] [CrossRef]
- Raines, E. Platelet-derived growth factor in vivo. Biol. Platelet-Deriv. Growth Factor 1993, 5, 74–114. [Google Scholar]
- Raines, E.W. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004, 15, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Gersuk, G.; Westermark, B.; Mohabeer, A.; Challita, P.; Pattamakom, S.; Pattengale, P. Inhibition of Human Natural Killer Cell Activity by Platelet-Derived Growth Factor (PDGF) III. Membrane Binding Studies and Differential Biological Effects of Recombinant PDGF Isoforms. Scand. J. Immunol. 1991, 33, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Hunter, C.A.; Cua, D.J. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242. [Google Scholar] [CrossRef] [PubMed]
- García-Cuesta, E.M.; Santiago, C.A.; Vallejo-Díaz, J.; Juarranz, Y.; Rodríguez-Frade, J.M.; Mellado, M. The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front. Endocrinol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-B.; He, X.-W.; Zhang, L.-J.; Qin, H.-B.; Lin, X.-T.; Liu, X.-H.; Zhou, C.; Liu, H.-S.; Hu, T.; Cheng, H.-C. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol. Rep. 2019, 7, 127–138. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J. Bone Miner. Res. 2003, 18, 1404–1418. [Google Scholar] [CrossRef]
- Wright, L.M.; Maloney, W.; Yu, X.; Kindle, L.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005, 36, 840–853. [Google Scholar] [CrossRef]
- Mirshahi, F.; Pourtau, J.; Li, H.; Muraine, M.; Trochon, V.; Legrand, E.; Vannier, J.-P.; Soria, J.; Vasse, M.; Soria, C. SDF-1 activity on microvascular endothelial cells: Consequences on angiogenesis in in vitro and in vivo models. Thromb. Res. 2000, 99, 587–594. [Google Scholar] [CrossRef]
- Pablos, J.L.; Amara, A.; Bouloc, A.; Santiago, B.; Caruz, A.; Galindo, M.; Delaunay, T.; Virelizier, J.L.; Arenzana-Seisdedos, F. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am. J. Pathol. 1999, 155, 1577–1586. [Google Scholar] [CrossRef]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1–CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006, 129, 200–211. [Google Scholar] [CrossRef]
- Calderon, T.M.; Eugenin, E.A.; Lopez, L.; Kumar, S.S.; Hesselgesser, J.; Raine, C.S.; Berman, J.W. A role for CXCL12 (SDF-1α) in the pathogenesis of multiple sclerosis: Regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J. Neuroimmunol. 2006, 177, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Q.; Sun, J.; Wang, A.; Shi, Y.; Ju, Y.; Morita, Y.; Song, G. Decreased nuclear stiffness via FAK-ERK1/2 signaling is necessary for osteopontin-promoted migration of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2017, 355, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Chiu, Y.H.; Chen, C.P.; Wang, H.S. Interleukin-1β induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res. Ther. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Wedzinska, A.; Figiel-Dabrowska, A.; Kozlowska, H.; Sarnowska, A. The Effect of Proinflammatory Cytokines on the Proliferation, Migration and Secretory Activity of Mesenchymal Stem/Stromal Cells (WJ-MSCs) under 5% O(2) and 21% O(2) Culture Conditions. J. Clin. Med. 2021, 10, 1813. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, Y.; Akiyama, K.; Chen, C.; Qu, C.; Jin, Y.; Shi, S. IFN-gamma and TNF-alpha synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFkappaB signaling. Stem Cells 2013, 31, 1383–1395. [Google Scholar] [CrossRef]
- Weiss, D.J.; English, K.; Krasnodembskaya, A.; Isaza-Correa, J.M.; Hawthorne, I.J.; Mahon, B.P. The Necrobiology of Mesenchymal Stromal Cells Affects Therapeutic Efficacy. Front. Immunol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Benvenuto, F.; Voci, A.; Carminati, E.; Gualandi, F.; Mancardi, G.; Uccelli, A.; Vergani, L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res. Ther. 2015, 6, 245. [Google Scholar] [CrossRef]
- Vohra, M.; Sharma, A.; Bagga, R.; Arora, S.K. Human umbilical cord-derived mesenchymal stem cells induce tissue repair and regeneration in collagen-induced arthritis in rats. J. Clin. Transl. Res. 2020, 6, 203–216. [Google Scholar]
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R.; et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007, 61, 219–227. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Khong, D.; Chin, L.Y.; Singleton, A.; Parekkadan, B. Therapeutic Delivery Specifications Identified Through Compartmental Analysis of a Mesenchymal Stromal Cell-Immune Reaction. Sci. Rep. 2018, 8, 6816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Min, X.H.; Wang, Q.Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.M.; An, G.; Sha, W.H.; et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- de Witte, S.F.H.; Merino, A.M.; Franquesa, M.; Strini, T.; van Zoggel, J.A.A.; Korevaar, S.S.; Luk, F.; Gargesha, M.; O’Flynn, L.; Roy, D.; et al. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res. Ther. 2017, 8, 140. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Chagastelles, P.C.; Sesterheim, P.; Pranke, P. In Vivo Immunogenic Response to Allogeneic Mesenchymal Stem Cells and the Role of Preactivated Mesenchymal Stem Cells Cotransplanted with Allogeneic Islets. Stem Cells Int. 2017, 2017, 9824698. [Google Scholar] [CrossRef]
- Yang, S.H.; Park, M.J.; Yoon, I.H.; Kim, S.Y.; Hong, S.H.; Shin, J.Y.; Nam, H.Y.; Kim, Y.H.; Kim, B.; Park, C.G. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp. Mol. Med. 2009, 41, 315–324. [Google Scholar] [CrossRef]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. eBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome–enhanced regulatory T-cell production through an antigen-presenting cell–mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zou, X.; Jose, P.A.; Zeng, C. Chapter Two—Extracellular vesicles: Potential impact on cardiovascular diseases. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 105, pp. 49–100. [Google Scholar]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Riazifar, M.; Pone, E.J.; Yeri, A.; Van Keuren-Jensen, K.; Lässer, C.; Lotvall, J.; Zhao, W. Isolation and characterization of microvesicles from mesenchymal stem cells. Methods 2020, 177, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, C.; Chen, L. The Role of Microvesicles Derived from Mesenchymal Stem Cells in Lung Diseases. BioMed Res. Int. 2015, 2015, 985814. [Google Scholar] [CrossRef]
- Ratajczak, M.Z. The emerging role of microvesicles in cellular therapies for organ/tissue regeneration. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2011, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Lim, H.; Liu, A.; Hu, S.; Lee, J.; Zhuo, H.; Hao, Q.; Matthay, M.A.; Lee, J.W. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 2019, 74, 43–50. [Google Scholar] [CrossRef]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C.; Tian, X.; Wang, J. Micro-vesicles derived from human Wharton’s Jelly mesenchymal stromal cells mitigate renal ischemia-reperfusion injury in rats after cardiac death renal transplantation. J. Cell. Biochem. 2018, 119, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.K.; Ozkocak, D.C.; Poon, I.K.H. Unleashing the therapeutic potential of apoptotic bodies. Biochem. Soc. Trans. 2020, 48, 2079–2088. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic 2021, 22, 174–179. [Google Scholar] [CrossRef]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 31. [Google Scholar] [CrossRef]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; de Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef]
- Luque-Campos, N.; Contreras-López, R.A.; Jose Paredes-Martínez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef]
- Khosravi, M.; Bidmeshkipour, A.; Moravej, A.; Hojjat-Assari, S.; Naserian, S.; Karimi, M.H. Induction of CD4(+)CD25(+)Foxp3(+) regulatory T cells by mesenchymal stem cells is associated with RUNX complex factors. Immunol. Res. 2018, 66, 207–218. [Google Scholar] [CrossRef]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolomé, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.; Sun, L. An Update for Mesenchymal Stem Cell Therapy in Lupus Nephritis. Kidney Dis. 2021, 7, 79–89. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Najar, M.; Fayyad-Kazan, M.; Meuleman, N.; Bron, D.; Fayyad-Kazan, H.; Lagneaux, L. Mesenchymal stromal cells of the bone marrow and natural killer cells: Cell interactions and cross modulation. J. Cell. Commun. Signal. 2018, 12, 673–688. [Google Scholar] [CrossRef]
- Jewett, A.; Man, Y.-G.; Tseng, H.-C. Dual Functions of Natural Killer Cells in Selection and Differentiation of Stem Cells; Role in Regulation of Inflammation and Regeneration of Tissues. J. Cancer 2013, 4, 12–24. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- de Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef] [PubMed]
- Oeller, M.; Laner-Plamberger, S.; Hochmann, S.; Ketterl, N.; Feichtner, M.; Brachtl, G.; Hochreiter, A.; Scharler, C.; Bieler, L.; Romanelli, P.; et al. Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics 2018, 8, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringdén, O.; Volk, H.-D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef]
- Thompson, M.; Mei, S.H.J.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. eClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef]
- Angelova, P.R.; Barilani, M.; Lovejoy, C.; Dossena, M.; Viganò, M.; Seresini, A.; Piga, D.; Gandhi, S.; Pezzoli, G.; Abramov, A.Y.; et al. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol. 2018, 14, 474–484. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, W.; Geng, L.; Zhang, L.; Liu, R.; Chen, W.; Yao, G.; Zhang, H.; Feng, X.; Gao, X.; et al. Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J. Immunol. Res. 2015, 2015, 284215. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, M.; Yang, R.; Liang, X.; Ma, Y.; Tang, Y.; Huang, L.; Ye, J.; Chen, K.; Wang, P.; et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 2011, 13, R29. [Google Scholar] [CrossRef]
- Cipriani, P.; Guiducci, S.; Miniati, I.; Cinelli, M.; Urbani, S.; Marrelli, A.; Dolo, V.; Pavan, A.; Saccardi, R.; Tyndall, A.; et al. Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells: New insight into the pathogenesis of systemic sclerosis. Arthritis Rheum. 2007, 56, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, K.; Proklou, A.; Tsitoura, E.; Lasithiotaki, I.; Kalpadaki, C.; Moraitaki, D.; Sperelakis, I.; Kontakis, G.; Antoniou, K.M.; Tzanakis, N. Impaired mRNA Expression of the Migration Related Chemokine Receptor CXCR4 in Mesenchymal Stem Cells of COPD Patients. Int. J. Inflam. 2017, 2017, 6089425. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Churchman, S.M.; English, A.; Buch, M.H.; Horner, E.A.; Burgoyne, C.H.; Reece, R.; Kinsey, S.; Emery, P.; McGonagle, D.; et al. Mesenchymal stem cells in rheumatoid synovium: Enumeration and functional assessment in relation to synovial inflammation level. Ann. Rheum. Dis. 2010, 69, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Bird, A.K.; Meednu, N.; Dauenhauer, K.; Liesveld, J.; Anolik, J.; Looney, R.J. Bone Marrow-Derived Mesenchymal Stem Cells From Patients With Systemic Lupus Erythematosus Have a Senescence-Associated Secretory Phenotype Mediated by a Mitochondrial Antiviral Signaling Protein-Interferon-beta Feedback Loop. Arthritis Rheumatol. 2017, 69, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein-Achiasaf, L.; Morein, D.; Ben-Yaakov, H.; Liubomirski, Y.; Meshel, T.; Elbaz, E.; Dorot, O.; Pichinuk, E.; Gershovits, M.; Weil, M.; et al. Persistent Inflammatory Stimulation Drives the Conversion of MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells. Cancers 2021, 13, 1472. [Google Scholar] [CrossRef]
- Jorgensen, C. Mesenchymal stem cells immunosuppressive properties: Is it specific to bone marrow-derived cells? Stem Cell Res. Ther. 2010, 1, 15. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Xie, Z.; Yang, R.; Li, Y.; Wu, X.; Su, H.; Deng, W.; Wang, S.; Liu, Z.; et al. Elevated TRAF4 expression impaired LPS-induced autophagy in mesenchymal stem cells from ankylosing spondylitis patients. Exp. Mol. Med. 2017, 49, e343. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Zhou, T.; Liao, C.; Li, H.Y.; Lin, W.; Lin, S.; Zhong, H. Efficacy of mesenchymal stem cells in animal models of lupus nephritis: A meta-analysis. Stem Cell Res. Ther. 2020, 11, 48. [Google Scholar] [CrossRef]
- Garimella, M.G.; Kour, S.; Piprode, V.; Mittal, M.; Kumar, A.; Rani, L.; Pote, S.T.; Mishra, G.C.; Chattopadhyay, N.; Wani, M.R. Adipose-Derived Mesenchymal Stem Cells Prevent Systemic Bone Loss in Collagen-Induced Arthritis. J. Immunol. 2015, 195, 5136–5148. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Corcelli, M.; Dowding, K.; Ranzoni, A.M.; Vlahova, F.; Hau, K.L.; Hunjan, A.; Peebles, D.; Gressens, P.; Hagberg, H.; et al. Embryonic Stem Cell-Derived Mesenchymal Stem Cells (MSCs) Have a Superior Neuroprotective Capacity Over Fetal MSCs in the Hypoxic-Ischemic Mouse Brain. Stem Cells Transl. Med. 2018, 7, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Samarelli, A.V.; Tonelli, R.; Heijink, I.; Martin Medina, A.; Marchioni, A.; Bruzzi, G.; Castaniere, I.; Andrisani, D.; Gozzi, F.; Manicardi, L.; et al. Dissecting the Role of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis: Cause or Solution. Front. Pharmacol. 2021, 12, 692551. [Google Scholar] [CrossRef]
- Takeda, K.; Webb, T.L.; Ning, F.; Shiraishi, Y.; Regan, D.P.; Chow, L.; Smith, M.J.; Ashino, S.; Guth, A.M.; Hopkins, S.; et al. Mesenchymal Stem Cells Recruit CCR2(+) Monocytes To Suppress Allergic Airway Inflammation. J. Immunol. 2018, 200, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Yu, Y.; Yoon, K.A.; Kang, T.W.; Jeon, H.J.; Sim, Y.B.; Choe, S.H.; Baek, S.Y.; Lee, S.; Seo, K.W.; Kang, K.S. Therapeutic effect of long-interval repeated intravenous administration of human umbilical cord blood-derived mesenchymal stem cells in DBA/1 mice with collagen-induced arthritis. J. Tissue Eng. Regen. Med. 2019, 13, 1134–1142. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, W.U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Haynesworth, S.E.; Gerson, S.L.; Rosenthal, N.S.; Caplan, A.I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Kurtzberg, J.; Prockop, S.; Teira, P.; Bittencourt, H.; Lewis, V.; Chan, K.W.; Horn, B.; Yu, L.; Talano, J.A.; Nemecek, E.; et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 229–235. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Dotoli, G.M.; De Santis, G.C.; Orellana, M.D.; de Lima Prata, K.; Caruso, S.R.; Fernandes, T.R.; Rensi Colturato, V.A.; Kondo, A.T.; Hamerschlak, N.; Simões, B.P.; et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017, 52, 859–862. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Badraiq, H.; Cvoro, A.; Galleu, A.; Simon, M.; Miere, C.; Hobbs, C.; Schulz, R.; Siow, R.; Dazzi, F.; Ilic, D. Effects of maternal obesity on Wharton’s Jelly mesenchymal stromal cells. Sci. Rep. 2017, 7, 17595. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res. Ther. 2015, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Lerrer, S.; Liubomirski, Y.; Bott, A.; Abnaof, K.; Oren, N.; Yousaf, A.; Körner, C.; Meshel, T.; Wiemann, S.; Ben-Baruch, A. Co-Inflammatory Roles of TGFβ1 in the Presence of TNFα Drive a Pro-inflammatory Fate in Mesenchymal Stem Cells. Front. Immunol. 2017, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Riordan, N.H.; Morales, I.; Fernández, G.; Allen, N.; Fearnot, N.E.; Leckrone, M.E.; Markovich, D.J.; Mansfield, D.; Avila, D.; Patel, A.N.; et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 2018, 16, 57. [Google Scholar] [CrossRef]
- Llufriu, S.; Sepúlveda, M.; Blanco, Y.; Marín, P.; Moreno, B.; Berenguer, J.; Gabilondo, I.; Martínez-Heras, E.; Sola-Valls, N.; Arnaiz, J.A.; et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE 2014, 9, e113936. [Google Scholar] [CrossRef]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Syková, E.; Rychmach, P.; Drahorádová, I.; Konrádová, Š.; Růžičková, K.; Voříšek, I.; Forostyak, S.; Homola, A.; Bojar, M. Transplantation of Mesenchymal Stromal Cells in Patients With Amyotrophic Lateral Sclerosis: Results of Phase I/IIa Clinical Trial. Cell Transpl. 2017, 26, 647–658. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Yaghmour, N.E.; Ginzberg, A.; Karussis, D. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front. Biosci. 2021, 26, 693–706. [Google Scholar] [CrossRef]
- Alvaro-Gracia, J.M.; Jover, J.A.; Garcia-Vicuna, R.; Carreno, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martinez-Taboada, V.M.; Taylor, P.; Martin-Martin, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, S.; Li, S.; Li, M.; Shi, J.; Bai, W.; Wang, Q.; Zheng, L.; Liu, Y. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug Des. Devel. Ther. 2019, 13, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Liang, J.; Zhang, H.; Sun, L. Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone Marrow Transplant. 2013, 48, 544–550. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Liang, J.; Wang, H.; Hua, B.; Feng, X.; Gilkeson, G.S.; Farge, D.; Shi, S.; Sun, L. A Long-Term Follow-Up Study of Allogeneic Mesenchymal Stem/Stromal Cell Transplantation in Patients with Drug-Resistant Systemic Lupus Erythematosus. Stem Cell Rep. 2018, 10, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Zhang, P.; Guo, Y.; Lim, T.O. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann. Rheum. Dis. 2017, 76, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, J.; Tang, X.; Wang, D.; Feng, X.; Wang, F.; Hua, B.; Wang, H.; Sun, L. Sustained benefit from combined plasmapheresis and allogeneic mesenchymal stem cells transplantation therapy in systemic sclerosis. Arthritis Res. Ther. 2017, 19, 165. [Google Scholar] [CrossRef]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhao, C.; Wang, D.; Ma, X.; Zhao, S.; Wang, S.; Niu, L.; Sun, L. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int. J. Rheum. Dis. 2017, 20, 1219–1226. [Google Scholar] [CrossRef]
- Chambers, D.C.; Enever, D.; Ilic, N.; Sparks, L.; Whitelaw, K.; Ayres, J.; Yerkovich, S.T.; Khalil, D.; Atkinson, K.M.; Hopkins, P.M. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J. Am. Soc. Nephrol. 2018, 29, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jin, L.; Ding, M.; He, J.; Yang, L.; Cui, S.; Wang, X.; Ma, J.; Liu, A. Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: A systematic review and meta-analysis. Stem Cell Res. Ther. 2022, 13, 118. [Google Scholar] [CrossRef]
- Wong, S.C.; Medrano, L.C.; Hoftman, A.D.; Jones, O.Y.; McCurdy, D.K. Uncharted waters: Mesenchymal stem cell treatment for pediatric refractory rheumatic diseases; a single center case series. Pediatr. Rheumatol. Online J. 2021, 19, 87. [Google Scholar] [CrossRef]
- Swart, J.F.; de Roock, S.; Nievelstein, R.A.J.; Slaper-Cortenbach, I.C.M.; Boelens, J.J.; Wulffraat, N.M. Bone-marrow derived mesenchymal stromal cells infusion in therapy refractory juvenile idiopathic arthritis patients. Rheumatology 2019, 58, 1812–1817. [Google Scholar] [CrossRef]
- Taufiq, H.; Shaik Fakiruddin, K.; Muzaffar, U.; Lim, M.N.; Rusli, S.; Kamaluddin, N.R.; Esa, E.; Abdullah, S. Systematic review and meta-analysis of mesenchymal stromal/stem cells as strategical means for the treatment of COVID-19. Ther. Adv. Respir. Dis. 2023, 17, 17534666231158276. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef]
- Rivera-Cruz, C.M.; Shearer, J.J.; Figueiredo Neto, M.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 4015039. [Google Scholar] [CrossRef]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Körner, C.; Ben-Baruch, A. Tumor-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Mojsilović, S.; Jauković, A.; Kukolj, T.; Obradović, H.; Okić Đorđević, I.; Petrović, A.; Bugarski, D. Tumorigenic Aspects of MSC Senescence-Implication in Cancer Development and Therapy. J. Pers. Med. 2021, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hornsby, P.J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007, 67, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Vjetrovic, J.; Shankaranarayanan, P.; Mendoza-Parra, M.A.; Gronemeyer, H. Senescence-secreted factors activate Myc and sensitize pretransformed cells to TRAIL-induced apoptosis. Aging Cell 2014, 13, 487–496. [Google Scholar] [CrossRef]
- Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Finicelli, M.; Melone, M.A.; Peluso, G.; Galderisi, U. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging 2019, 11, 5817–5828. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Borzì, R.M. Molecular Mechanisms Contributing to Mesenchymal Stromal Cell Aging. Biomolecules 2020, 10, 340. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell–based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Swart, J.F.; Wulffraat, N.M. Mesenchymal stromal cells for treatment of arthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 589–603. [Google Scholar] [CrossRef]
- Fu, X.; Xu, B.; Jiang, J.; Du, X.; Yu, X.; Yan, Y.; Li, S.; Inglis, B.M.; Ma, H.; Wang, H.; et al. Effects of cryopreservation and long-term culture on biological characteristics and proteomic profiles of human umbilical cord-derived mesenchymal stem cells. Clin. Proteom. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Sierra Parraga, J.M.; Rozenberg, K.; Eijken, M.; Leuvenink, H.G.; Hunter, J.; Merino, A.; Moers, C.; Møller, B.K.; Ploeg, R.J.; Baan, C.C.; et al. Effects of Normothermic Machine Perfusion Conditions on Mesenchymal Stromal Cells. Front. Immunol. 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ravikumar, M.; Ling, L.; Nurcombe, V.; Cool, S.M. Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy. Stem Cell Rep. 2021, 16, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Baildam, E.M.; Oldershaw, R.A. Juvenile idiopathic arthritis: From aetiopathogenesis to therapeutic approaches. Pediatr. Rheumatol. Online J. 2021, 19, 135. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef]
| Growth Factor | Role in Immune Regulation Inflammation and Disease | Reference |
|---|---|---|
| Monocyte chemotactic protein-1 (MCP1) |
| [14,15,16,17,18] |
| Macrophage migration inhibitory factor (MIF) |
| [19,20,21,22,23,24,25,26,27,28,29] |
| Basic fibroblast growth factor-2 (FGF2) |
| [30,31] |
| Vascular endothelial growth factor (VEGF) |
| [32,33,34,35,36] |
| Hepatocyte growth factor (HGF) |
| [37,38,39,40,41,42,43,44,45,46] |
| Insulin-like growth factor-1 (IGF1) |
| [47,48,49,50] |
| Platelet-derived growth factor (PDGF) |
| [51,52,53,54,55] |
| Transforming growth factor-β1 (TGFβ1) |
| [56,57] |
| Stromal cell-derived factor-1/C-X-C motif chemokine-12 (SDF-1α/CXCL12/) |
| [58,59,60,61,62,63,64,65,66] |
| Property of MSC | Mechanism |
|---|---|
| Suppression of T-cell activity |
|
| Inhibition of B-cells |
|
| Activation of regulatory T-cells |
|
| Inhibition of NK cells |
|
| Induction of macrophages with anti-inflammatory immunophenotype |
|
| Regulating lymphopoiesis |
|
| Interaction with DC |
|
| Paracrine effects of MSCs |
|
| Disease | Study Methodology | MSC Characteristics | References |
|---|---|---|---|
| Rheumatoid arthritis (subtype was not defined despite clinical importance and is a limitation of the study) |
|
| [126] |
| Rheumatoid arthritis (subtype was not defined despite clinical importance and is a limitation of the study) |
|
| [122] |
| Ankylosing spondylitis |
|
| [123] |
| Systemic lupus erythematosus |
|
| [127] |
| Systemic sclerosis |
|
| [124] |
| Parkinson’s disease |
|
| [121] |
| Idiopathic pulmonary fibrosis |
|
| [120] |
| Chronic obstructive pulmonary disease |
|
| [125] |
| Allogeneic | Autologous | |
|---|---|---|
| Availability |
|
|
| Quality |
|
|
| Cell quality in accordance with good manufacturing practice |
|
|
| Quantity |
|
|
| Immune response on MSCs transplantation |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Pain, C.; Baildam, E.M.; Oldershaw, R.A. Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 16040. https://doi.org/10.3390/ijms242216040
Zaripova LN, Midgley A, Christmas SE, Beresford MW, Pain C, Baildam EM, Oldershaw RA. Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases. International Journal of Molecular Sciences. 2023; 24(22):16040. https://doi.org/10.3390/ijms242216040
Chicago/Turabian StyleZaripova, Lina N., Angela Midgley, Stephen E. Christmas, Michael W. Beresford, Clare Pain, Eileen M. Baildam, and Rachel A. Oldershaw. 2023. "Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases" International Journal of Molecular Sciences 24, no. 22: 16040. https://doi.org/10.3390/ijms242216040
APA StyleZaripova, L. N., Midgley, A., Christmas, S. E., Beresford, M. W., Pain, C., Baildam, E. M., & Oldershaw, R. A. (2023). Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases. International Journal of Molecular Sciences, 24(22), 16040. https://doi.org/10.3390/ijms242216040






