Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results
Abstract
:1. Introduction
2. Results
3. Discussion
- (a)
- High Water Solubility: A clear advantage of the ED compound as an antimicrobial agent for chronic wounds is its high water solubility. This facilitates in vitro testing by eliminating the need for potentially harmful solvents. Moreover, water-soluble compounds are generally more suitable for medical applications, reducing risks and enhancing patient safety [17];
- (b)
- Antimicrobial Activity: As indicated in Table 1, the MIC values of ED matched the MBC values, suggesting that ED not only inhibits but also kills the tested microorganisms. Such antimicrobial activity, as opposed to a bacteriostatic mode of action, is essential for topical treatment of wound infections [18];
- (c)
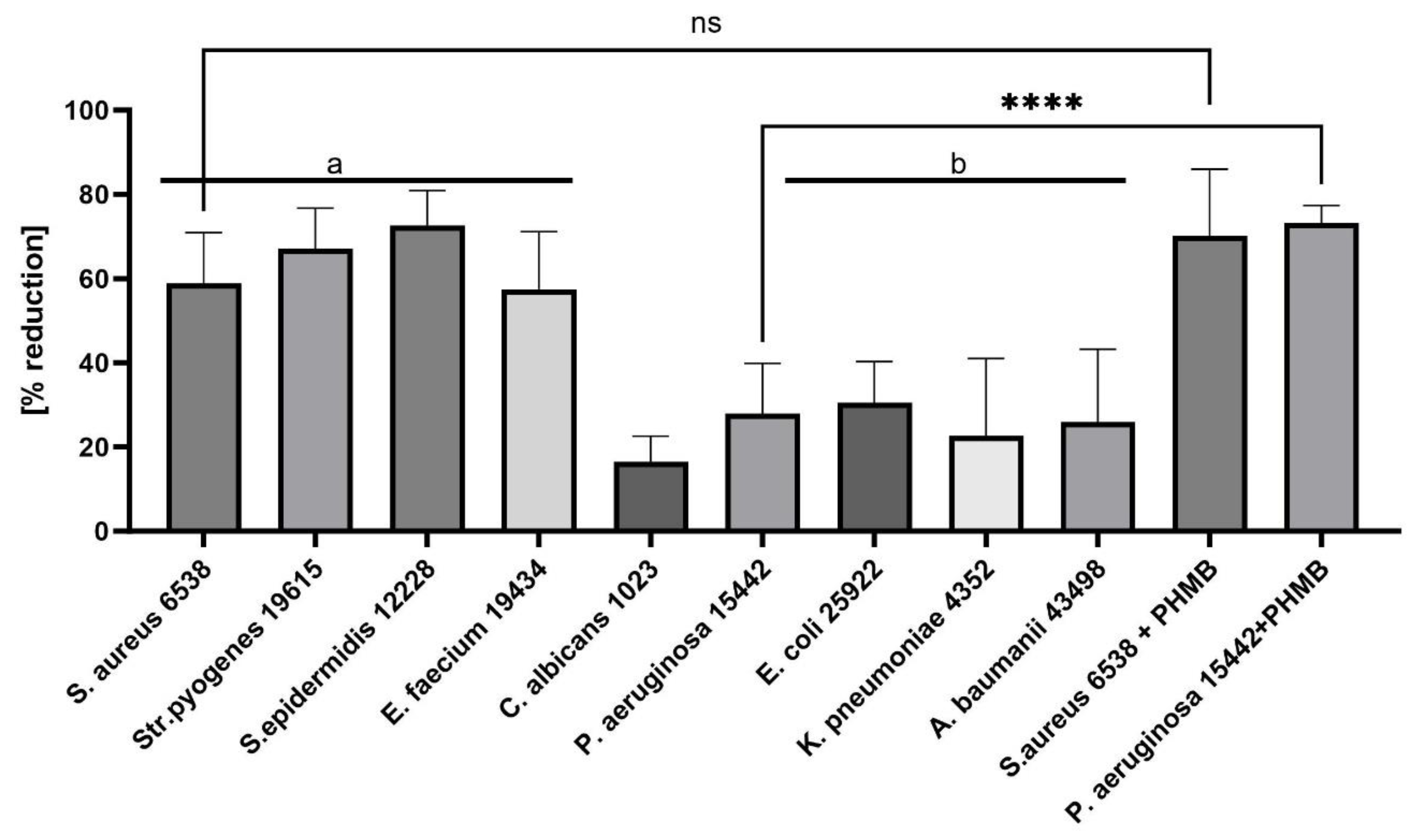
- Gram-Positive Susceptibility: Gram-positive pathogens were more susceptible to the ED agent than Gram-negative bacteria and fungi. The differential susceptibility between these bacterial groups to antimicrobial agents can be attributed to several structural and functional differences, including cell wall composition, outer membrane presence, efflux pumps, target accessibility, and intrinsic resistance mechanisms [19,20]. To pinpoint ED’s exact mechanism of action, further studies are undoubtedly required. These should encompass various lines of investigation, such as assessing ED’s interaction with bacterial membranes using techniques like transmission electron microscopy, determining the compound’s uptake and efflux in both bacterial types, and identifying the precise target or mode of action. Understanding this mechanism can offer insights into optimizing the ED compound or devising adjuvant strategies to boost its activity against Gram-negative pathogens. Previous studies have indicated that interactions with charged compounds differ between these bacterial groups [21,22]. For instance, Abebe et al. synthesized a series of decyl-o-phenanthrolinium organic salts and found that the type of anion significantly influenced their antibacterial activities [23]. Other compounds with broad antimicrobial activity, such as cationic antimicrobial peptides (AMPs), have been identified. These peptides, typically short and positively charged, exhibit activity against a range of Gram-positive bacteria. Although their exact mode of action remains elusive, it is proposed that these peptides interact with and disrupt the cytoplasmic membrane, leading to cell death. Friedrich et al. demonstrated the high activity of positively charged compounds against Gram-positive strains of S. aureus [24]. Fletcher et al. observed that 1,3,4-trisubstituted-1,2,3-triazolium bromide salts had the lowest MIC values against Gram-positive bacteria [25]. Our spectroscopic analysis of the ED structure suggests that the presence of a positive charge is crucial for its selective antibacterial activity, as well as the presence of the hydrophobic isoxazole ring. To explain the non-specific activity of the ED compound, it is worth analyzing reports on positively charged structures, their antibacterial effects, and known mechanisms of action. Da Costa et al. [26] investigated the impact of surface charge modulation of rifampicin-loaded PLA (poly-lactic acid) nanoparticles (NPs) on behavior within a bacterial biofilm. The nanoparticles were functionalized with poly-L-lysine to achieve a positively charged surface. The observed effect was that positively charged nanoparticles had a stronger interaction with S. aureus, under biofilm growth conditions, than negatively charged ones. This led to slower particle migration in the biofilm and better retention of these cationic particles within the biofilms. Although rifampicin showed complete ineffectiveness in biofilms after washing, rifampicin-loaded PLA nanoparticles coated with poly-L-lysine exhibited enhanced antibiotic efficacy compared to uncoated, negatively charged nanoparticles. While negatively charged PLA NPs migrated rapidly and uniformly through the entire S. aureus biofilm, positively charged NPs were primarily bound to the top of biofilms and entrances of dense bacterial clusters, preventing deeper penetration. This suggests electrostatic interactions between the positive charge of the compound and the negatively charged biofilm components, such as bacteria and matrix macromolecules like eDNA. Hasan et al. demonstrated that cationic PLGA (Poly-lactic-co-glycolic acid) nanoparticles loaded with clindamycin significantly reduced the bacterial load in wounds of S. aureus-infected mice compared to anionic NPs [27]. This finding correlated with better wound healing, evidenced by a significantly smaller wound size after 8 days of local treatment. Coating nanoparticles with a poly-L-lysine corona inverted the surface charge of anionic NP-rifampicin, producing positively charged NP-rifampicin that exhibited higher interactions with planktonic S. aureus and biofilms than negatively charged NPs. This study revealed the importance of the ability of antibiotic-loaded NPs to penetrate and remain within biofilms for effective control. The stronger interactions observed with positively charged nanoparticles resulted in better retention of antibiotic-loaded nanoparticles within biofilms, enabling the sustainable delivery of potent rifampicin concentrations at reduced doses. These findings suggest that the carrier retention capacity within biofilms correlates with treatment effectiveness. Thus, positively charged rifampicin-loaded PLA nanoparticles present a promising approach for enhancing antibiotic delivery In S. aureus biofilms. Romero-Urbina et al. [28] reached conclusions aligned with the above due to the presence of a positive charge in the compound. They studied the effects of positively charged silver nanoparticles against both methicillin-sensitive and methicillin-resistant S. aureus. They examined the bactericidal effects of silver nanoparticles and the induced bacterial ultrastructural modifications using aberration-corrected transmission electron microscopy. The study showed that silver nanoparticles cause thinning and increased permeability of the cell wall, destabilization of the peptidoglycan layer, and consequent leakage of intracellular content, leading to bacterial cell lysis. They hypothesized that positively charged silver nanoparticles primarily bind to the negatively charged polyanionic backbones of teichoic acids and related bacterial cell wall glycopolymers, thereby affecting cell wall structure and permeability. This hypothesis could explain the antibacterial effects of silver nanoparticles on S. aureus.
- (d)
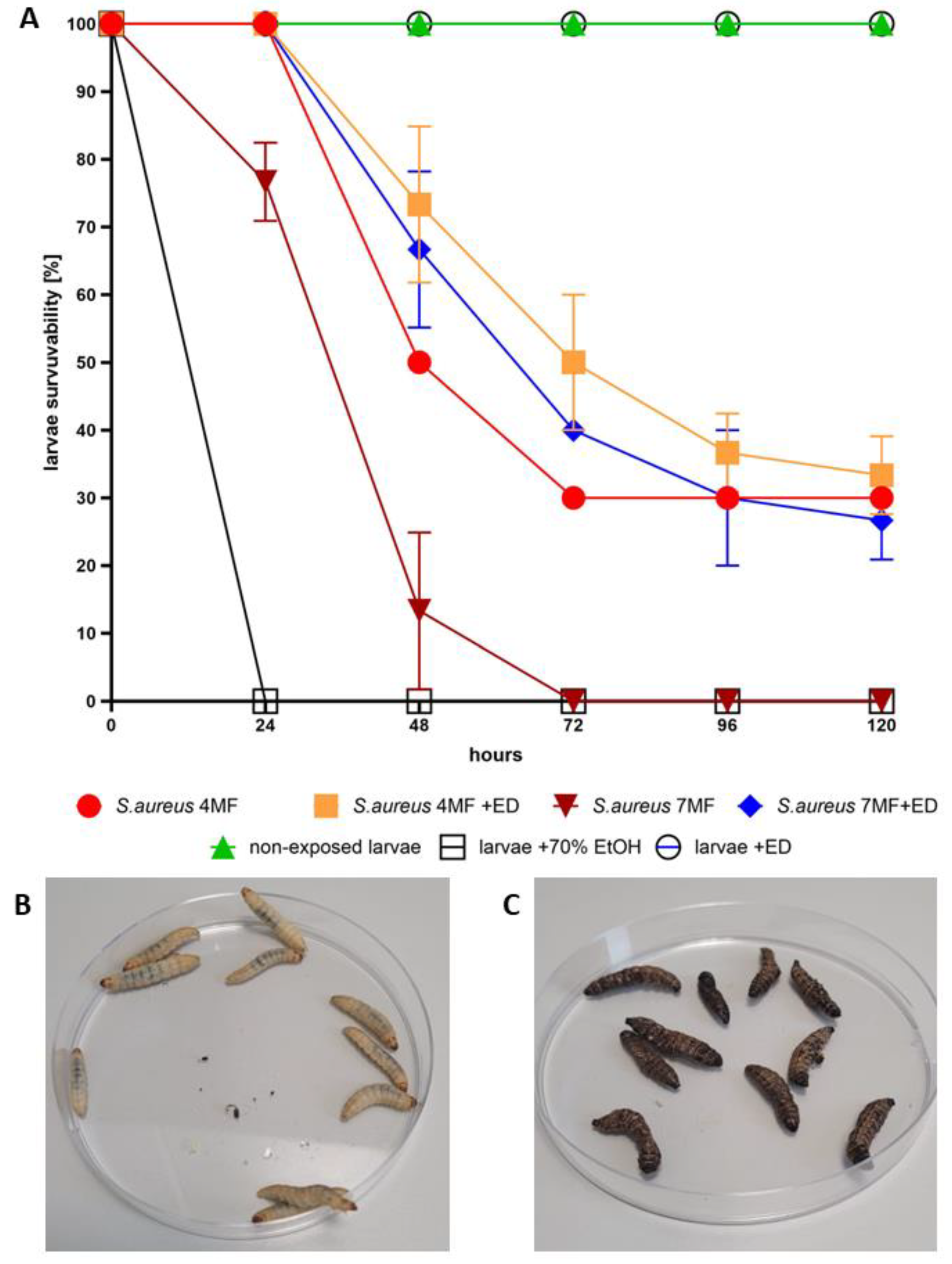
- In Vivo Model: G. mellonella larvae are a widely used in vivo invertebrate infection model for studying bacterial and fungal infections and evaluating the efficacy of antimicrobial drugs [29]. We utilized G. mellonella larvae to assess the antibacterial efficacy and cytotoxicity of ED [30]. The injection of ED into the larvae significantly hindered S. aureus’s ability to cause a lethal infection (Figure 5). Notably, our experiment aimed to mimic the introduction of an antimicrobial agent to a chronic wound. The conditions within G. mellonella tissues, such as specific temperature, hypoxia, and the presence of immune system components, pose challenges not only for the antimicrobial agent due to pharmacokinetic/dynamic issues [31] but also for S. aureus biofilm development compared to standard in vitro models [32]. These conditions resemble those in necrotic, exuding chronic wounds [33].
4. Materials and Methods
4.1. Determination of Minimal Inhibitory Concentration Using Microtiter Plate Method
4.2. Determination of Minimal Biofilm Eradication Concentration Using Microtiter Plate Model
4.3. The Cytotoxicity Assay of Analyzed Compounds towards Fibroblast and Keratinocytes Cell Line In Vitro
4.4. The Synthesis and Purification of Bacterial Cellulose Carrier
4.5. Assessment of ED Compound’s Antibiofilm Activity Using Cellulose-Based Biofilm Model
4.6. Larvae In Vivo Model to Assess Compound’s Cytotoxicity and Capability to Prevent Infection
4.7. The Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, L.; Fan, H.M.; Tao, H.R.; Huang, J.D. Recent Strategies to Combat Biofilms Using Antimicrobial Agents and Therapeutic Approaches. Pathogens 2022, 11, 292. [Google Scholar] [CrossRef]
- Sharma, P.K.; Amin, A.; Kumar, M. Synthetic Methods of Medicinally Important Heterocyclesthiazines: A Review. Open J. Med. Chem. 2020, 14, 71. [Google Scholar] [CrossRef]
- Quadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open J. Med. Chem. 2022, 16, e187410452202280. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-oxadiazole and its derivatives: A review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572. [Google Scholar] [CrossRef]
- Qadir, T.; Kanth, S.A.; Aasif, M.; Fadul, A.N.; Yatoo, G.N.; Jangid, K.; Mir, M.A.; Shah, W.A.; Sharma, P.K. Design, synthesis, and unraveling the antibacterial and antibiofilm potential of 2-azidobenzothiazoles: Insights from a comprehensive in vitro study. Front. Chem. 2023, 11, 1264747. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Therapeutic potential of oxadiazole or furadiazole containing compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Sysak, A.; Obmińska-Mrukowicz, B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur. J. Med. Chem. 2017, 137, 292–309. [Google Scholar] [CrossRef]
- Janardhanan, J.; Chang, M.; Mobashery, S. The oxadiazole antibacterials. Curr. Opin. Microbiol. 2016, 33, 13–17. [Google Scholar] [CrossRef]
- Cheng, K.; Qi, J.; Ren, X.; Zhang, J.; Li, H.; Xiao, H.; Wang, R.; Liu, Z.; Meng, L.; Ma, N.; et al. Developing Isoxazole as a Native Photo-Cross-Linker for Photoaffinity Labeling and Chemoproteomics. Angew. Chem. Int. Ed. 2022, 61, e202209947. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, U.; Junka, A.; Brożyna, M.; Mączyński, M. The In Vitro Impact of Isoxazole Derivatives on Pathogenic Biofilm and Cytotoxicity of Fibroblast Cell Line. Int. J. Mol. Sci. 2023, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, U.; Drozd-Szczygieł, E.; Bąchor, R.; Jerzykiewicz, L.; Wieczorek, R.; Mączyński, M. New water-soluble isoxazole-linked 1,3,4-oxadiazole derivative with delocalized positive charge. RSC Adv. 2021, 11, 29668–29674. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Budiman, A.; Rusdin, A.; Aulifa, D.L. Current Techniques of Water Solubility Improvement for Antioxidant Compounds and Their Correlation with Its Activity: Molecular Pharmaceutics. Antioxidants 2023, 12, 378. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Chetri, S. The culmination of multidrug-resistant efflux pumps vs. meager antibiotic arsenal era: Urgent need for an improved new generation of EPIs. Front Microbiol. 2023, 14, 1149418. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Caudill, E.R.; Hernandez, R.T.; Johnson, K.P.; O’Rourke, J.T.; Zhu, L.; Haynes, C.L.; Feng, Z.V.; Pedersen, J.A. Wall teichoic acids govern cationic gold nanoparticle interaction with Gram-positive bacterial cell walls. Chem. Sci. 2020, 11, 4106–4118. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Abebe, A.; Atlabachew, M.; Ferede, E. The synthesis of decyl-o-phenanthrolinium organic salt and a study of the impact of anion type on in vitro antibacterial activities. Sci. Afr. 2023, 20, e01725. [Google Scholar] [CrossRef]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2020, 44, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.T.; Sobczyk, J.M.; Gwazdacz, S.C.; Blanck, A.J. Antimicrobial 1,3,4-trisubstituted-1,2,3-triazolium salts. Bioorg. Med. Chem. Lett. 2018, 28, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.H.; Lee, B.L.; Jung, Y.; Yoo, J.W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef]
- Romero-Urbina, D.G.; Lara, H.H.; Velázquez-Salazar, J.J.; Arellano-Jiménez, M.J.; Larios, E.; Srinivasan, A.; Lopez-Ribot, J.L.; Yacamán, M.J. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. J. Nanotechnol. 2015, 6, 2396–2405. [Google Scholar]
- Lange, A.; Beier, S.; Huson, D.H.; Parusel, R.; Iglauer, F.; Frick, J.S. Genome sequence of Galleria mellonella (greater wax moth). Genome Announc. 2018, 6, e01220-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Pan, A.J.; Hu, L.F.; Liu, Y.Y.; Cheng, J.; Ye, Y.; Li, J.B. Galleria mellonella as an in vivo model for assessing the efficacy of antimicrobial agents against Enterobacter cloacae infection. J. Microbiol. Immunol. Infect. 2017, 50, 55–61. [Google Scholar] [CrossRef]
- Meesters, K.; Alemayehu, T.; Benou, S.; Buonsenso, D.; Decloedt, E.H.; Pillay-Fuentes Lorente, V.; Downes, K.J.; Allegaert, K. Pharmacokinetics of Antimicrobials in Children with Emphasis on Challenges Faced by Low and Middle Income Countries, a Clinical Review. Antibiotics 2023, 12, 17. [Google Scholar]
- Paleczny, J.; Brożyna, M.; Dudek-Wicher, R.; Dydak, K.; Oleksy-Wawrzyniak, M.; Madziała, M.; Bartoszewicz, M.; Junka, A. The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections. Int. J. Mol. Sci. 2022, 23, 11564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Dydak, K.; Junka, A.; Dydak, A.; Brożyna, M.; Paleczny, J.; Fijalkowski, K.; Kubielas, G.; Aniołek, O.; Bartoszewicz, M. In Vitro Efficacy of Bacterial Cellulose Dressings Chemisorbed with Antiseptics against Biofilm Formed by Pathogens Isolated from Chronic Wounds. Int. J. Mol. Sci. 2021, 22, 3996. [Google Scholar] [CrossRef] [PubMed]

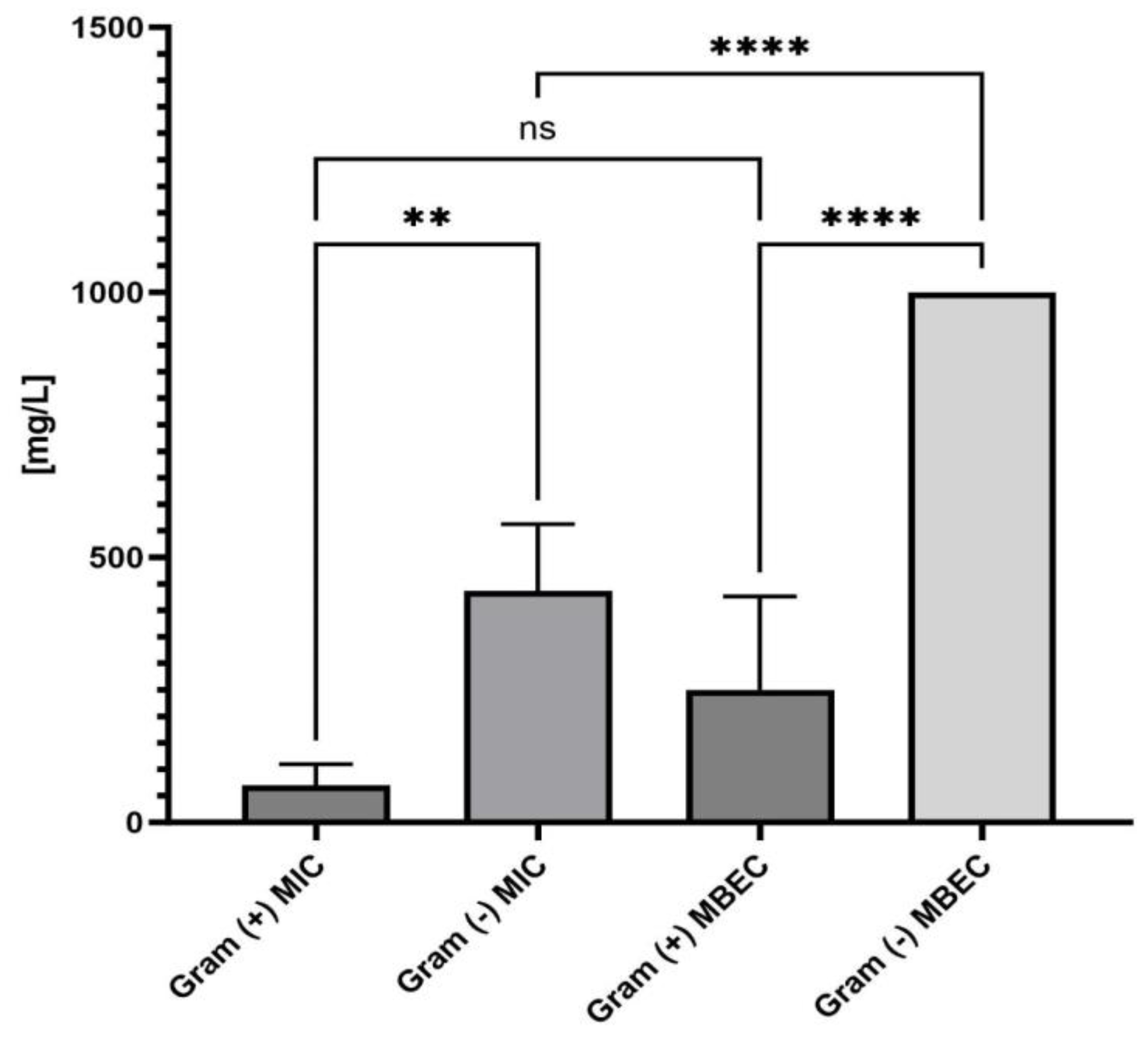

| MIC = MBC [mg/L] | MBEC | ||
|---|---|---|---|
| Gram (+) bacteria | S. aureus 6538 | 62.5 | 125 |
| Str. pyogenes 19615 | 62.5 | 250 | |
| S.epidermidis 12228 | 31.25 | 125 | |
| E. faecium 19434 | 125 | 500 | |
| fungus | C.albicans 1023 | 250 [Minimal Fungicidal Concentration] | 1000 |
| Gram (−) bacteria | P.aeruginosa 15442 | 500 | >range of concentrations |
| E.coli 25922 | 500 | >range of concentrations | |
| K.pneumoniae 4352 | 500 | >range of concentrations | |
| A.baumanii 43498 | 250 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, B.; Bąchor, U.; Drozd-Szczygieł, E.; Brożyna, M.; Dąbrowski, P.; Junka, A.; Mączyński, M. Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results. Int. J. Mol. Sci. 2023, 24, 16033. https://doi.org/10.3390/ijms242216033
Dudek B, Bąchor U, Drozd-Szczygieł E, Brożyna M, Dąbrowski P, Junka A, Mączyński M. Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results. International Journal of Molecular Sciences. 2023; 24(22):16033. https://doi.org/10.3390/ijms242216033
Chicago/Turabian StyleDudek, Bartłomiej, Urszula Bąchor, Ewa Drozd-Szczygieł, Malwina Brożyna, Piotr Dąbrowski, Adam Junka, and Marcin Mączyński. 2023. "Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results" International Journal of Molecular Sciences 24, no. 22: 16033. https://doi.org/10.3390/ijms242216033
APA StyleDudek, B., Bąchor, U., Drozd-Szczygieł, E., Brożyna, M., Dąbrowski, P., Junka, A., & Mączyński, M. (2023). Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results. International Journal of Molecular Sciences, 24(22), 16033. https://doi.org/10.3390/ijms242216033







