Abstract
Hericium erinaceus is a valuable mushroom known for its strong bioactive properties. It shows promising potential as an excellent neuroprotective agent, capable of stimulating nerve growth factor release, regulating inflammatory processes, reducing oxidative stress, and safeguarding nerve cells from apoptosis. The active compounds in the mushroom, such as erinacines and hericenones, have been the subject of research, providing evidence of their neuroprotective effects. Further research and standardization processes for dietary supplements focused on H. erinaceus are essential to ensuring effectiveness and safety in protecting the nervous system. Advancements in isolation and characterization techniques, along with improved access to pure analytical standards, will play a critical role in achieving standardized, high-quality dietary supplements based on H. erinaceus. The aim of this study is to analyze the protective and nourishing effects of H. erinaceus on the nervous system and present the most up-to-date research findings related to this topic.
1. Introduction
Neuroprotective action refers to the ability of substances or factors to shield neurons from damage or death. The goal is to prevent the degeneration of nerve cells and maintain proper neural functioning. Neuroprotection can be achieved through a variety of mechanisms, such as reducing oxidative stress, inhibiting inflammation, regulating apoptosis, and improving mitochondrial function and blood flow to the brain. One such aspect is neurotrophic action, which refers to the ability of substances or factors to stimulate the growth, differentiation, and function of neurons. The balance between neurodegenerative and neuroregenerative processes largely depends on the availability and activity of neurotrophic factors that are essential for maintaining the functional organization of neurons. By interacting with receptors on nerve cells and influencing their survival, synaptic functions, and neuronal plasticity, these substances play a crucial role in the development, maintenance, and regeneration of the nervous system. Neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF) can support neuron growth and development as well as protect neurons from damage and degeneration. Therefore, substances that are similar to neurotrophic factors or their inducers can be utilized in the treatment of neurodegenerative diseases [1,2].
Neurodegenerative diseases encompass a broad group of disorders leading to progressive loss of structure and function of nerve cells in various regions of the brain and/or spinal cord, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and Creutzfeldt-Jakob disease. Each of these manifests with characteristic symptoms and pathologies, but they all share a common factor—the gradual loss of nerve cells. These serious and often debilitating conditions affect millions of people worldwide, impacting not only the patients but also their families and caregivers. Associated with the degeneration and death of neurons, neurodegenerative diseases lead to impaired neural communication and brain function and are characterized by a gradual decline in cognitive, motor, and/or autonomic functions. Their courses vary but are often associated with a progressive deterioration of health and loss of independence. The causes of such diseases are complex and multifactorial, involving genetic predispositions, environmental factors, aging processes, and abnormal accumulation of proteins or other pathological changes in the brain. While there is currently no known way to completely cure neurodegenerative diseases, available therapies often aim to alleviate symptoms, delay disease progression, and improve patient quality of life. Ongoing research aims to understand the mechanisms of disease development and develop new therapies. The quest for effective methods of treatment and prevention is a significant area of scientific and clinical research associated with the immense impact of these conditions on public health and the quality of life of many people worldwide.
The use of mushrooms in neuroprotection is a subject of intensive scientific research aiming to identify potential health benefits and understand the mechanisms of their action at the neuronal level. Studies indicate that some mushroom species demonstrate promising neuroprotective properties, opening possibilities for their use in the prevention and treatment of neurological disorders [3,4].
Mushrooms have been utilized in traditional medicine for centuries. Hericium erinaceus (Bull.: Fr.) Pers., also known as lion’s mane mushroom, bearded tooth mushroom, “Yamabushitake” in Japan, and “Houtou” in China, is a medicinal and edible mushroom belonging to the Basidiomycota phylum within the Hericiaceae family. It is a saprotroph, but it can occasionally act as a mild parasite on trees, e.g., on dead or dying trees of the genera Quercus sp., Fagus sp., Acer sp., Juglans sp., and Ulmus sp. [5]. It occurs naturally in Asia, Europe, and North America, and in 2003 it was included in the red list of endangered and extinct species in 13 out of 23 European countries due to the decrease in its natural habitats [6].
H. erinaceus can be cultivated on a large scale using inexpensive substrates, such as artificial and agricultural waste. Commercial cultivation of this species is popular worldwide, especially in Asia and the USA [7]. Due to its beneficial properties, this mushroom is widely used in the diets of East Asian countries [8,9]. In traditional Chinese and Japanese medicine, lion’s mane mushroom has been used for centuries as a remedy for gastrointestinal disorders, liver and kidney diseases, spleen disorders, and heart regulation. Indigenous Americans in North America also used it as a preventive measure against bleeding.
In the last decade, there has been a growing interest in the potential use of H. erinaceus in the therapy of various neurological and cognitive disorders. Moreover, it is suggested that they may have a neuroprotective action, which refers to the ability of substances or factors to protect and support the health of nerve cells and prevent their damage. Research shows that H. erinaceus exhibits numerous therapeutic properties, such as antioxidative [10], anti-inflammatory [11], hypolipidemic [12], hemagglutinating [13], antimicrobial [14], hypoglycemic and antidiabetic [15,16,17], and anticancer effects [18,19,20]. Additionally, H. erinaceus induces the synthesis of nerve growth factor (NGF), inhibits β-amyloid (Aβ) cytotoxicity, and protects nerve cells from death caused by oxidative stress or endoplasmic reticulum stress.
Positive effects of H. erinaceus have been observed in the treatment of cognitive disorders, Alzheimer’s disease, ischemic strokes, Parkinson’s disease, and age-related hearing impairment [15,21,22,23,24]. Promising results have also been obtained in the treatment of depressive disorders [25,26,27,28,29]. The studies referred to above were conducted on animal models (Figure 1).
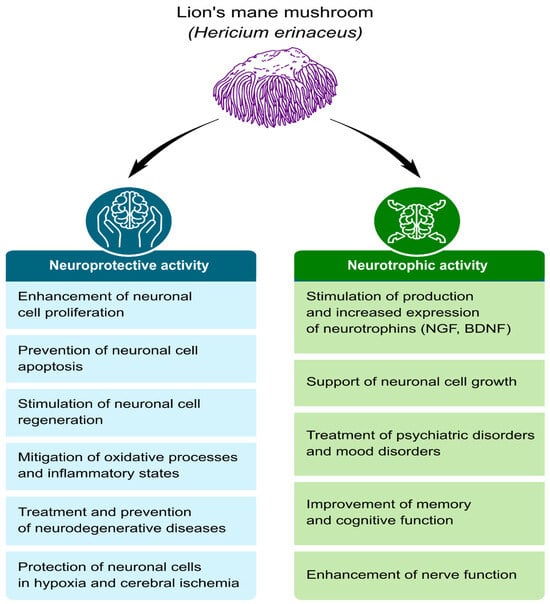
Figure 1.
Examples of the neuroprotective and neurotrophic effects of the Lion’s Mane mushroom in preclinical studies [2,15,26,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
Given the abundance of research dedicated to H. erinaceus, it is necessary to provide a current summary of the latest and most significant research findings regarding this organism and its impact on the vertebrate nervous system.
2. Neurotrophins
Neurotrophins play a significant role in the survival of specific neuron populations and promote the growth and branching of their axons (also known as neurites), which play a crucial role in the development and proper functioning of the nervous system, such as the communication between neurons. Neurotrophins are synthesized in both the central and peripheral nervous systems and are essential for dendritic branching, synaptogenesis, synaptic plasticity, and cellular homeostasis control. In general, this family of proteins plays a key role in neurogenesis processes such as neuronal differentiation, maturation, and survival [62,63]. They act at various stages of neurogenesis, and their concentration can be regulated by other modulating factors [35]. In mammals, within the family of neurotrophins, we can distinguish NGF, BDNF, neurotrophin 3 (NT3), and neurotrophin 4/5 (NT4/5) [29,64].
2.1. NGF and BDNF
Nerve growth factor (NGF) is a neurotrophic factor that regulates the differentiation, growth, survival, and plasticity of cells such as cholinergic neurons in the septum, striatum, and nucleus basalis of Meynert [65]. The highest levels of NGF-mRNA synthesis and NGF protein have been observed in the brain, particularly in the basal forebrain, hippocampus, cerebral cortex, and olfactory bulb [63,66].
NGF is secreted tonically as a monomer in its precursor form (proNGF) and undergoes extracellular maturation by plasmin to become the active form (mNGF). Nerve growth factors are unable to cross the blood-brain barrier and are easily metabolized by peptidases. Consequently, their use as drugs in the treatment of neurodegenerative diseases may be challenging. For this, research is being conducted on compounds that promote NGF synthesis [67].
Currently, it is believed that dysfunctions in extracellular NGF metabolism may lead to accelerated degradation of mature NGF in Alzheimer’s disease. Cholinergic neurons require NGF for proper functioning and plasticity, and NGF metabolism is disrupted in Alzheimer’s disease. Changes in neurotrophin-related signaling pathways are involved in the aging process and contribute to the cognitive decline observed in Alzheimer’s disease. Functional NGF deficiency is believed to be associated with Alzheimer’s disease and plays a role in the pathogenesis of this condition. Studies have evidenced reduced levels of NGF in the basal forebrain in patients with Alzheimer’s disease. Additionally, in patients without dementia symptoms but with amyloid plaques, reduced NGF levels were observed in the frontal cortex [36,65].
NGF has been shown to play a role in maintaining long-term synaptic potentiation (LTP) in the dentate gyrus. NGF blockade in the hippocampus leads to a decrease in LTP and impairment of spatial memory [68]. Additionally, studies have shown that exogenous infusion of NGF into the hippocampus enhances memory, and increased NGF expression correlates with the learning process [69].
BDNF and NGF are the two best-known neurotrophins. In mammals, the highest expression level of BDNF protein is found in the brain, particularly in the hippocampus and cerebral cortex, which are responsible for memory, learning, and higher cognitive processes. Other peripheral sources of BDNF include the lungs, heart, spleen, liver, thymus, gastrointestinal tract, skin, and smooth muscle tissue in blood vessels [70].
BDNF is synthesized as a precursor protein, pro-BDNF, which is converted by proteases into its mature form [37]. BDNF, along with its receptor TrkB, plays a significant role in long-term synaptic potentiation (LTP), which is considered a model process in memory formation [29]. Additionally, BDNF plays an essential role in mood regulation, which closely correlates with the neurogenic actions of antidepressant drugs. Dysfunctions in the BDNF pathway have been linked to Alzheimer’s disease, schizophrenia, Huntington’s disease, and Rett syndrome [29].
2.2. The Mechanisms of NGF and BDNF Activity
The trophic activity of both NGF and BDNF is mediated by interactions with Trk receptors (transmembrane proteins with tyrosine kinase activity) and p75 receptors (a transmembrane glycoprotein that acts as a low-affinity receptor for NGF) [38,71,72]. NGF activates the TrkA receptor, while BDNF activates TrkB [73]. Both neurotrophins also activate the p75 receptor with low affinity, which is located on the plasma membrane of axonal terminals (presynaptic membrane) and is co-expressed with Trk receptors, allowing them to promote enhanced trophic signaling, resulting in increased neuronal survival, neurite growth, and synaptogenesis [29]. The p75NTR receptor lacks a catalytic domain for autoactivation, so its functions depend on interactions with other receptors [74].
Activation of either receptor by NGF leads to different outcomes. The trophic signal that induces cell differentiation and survival is conveyed through the TrkA receptor. It is also believed that the role in learning and memory processes is mediated through the activation of the high-affinity TrkA receptor. Stimulation of only p75NTR receptors can elicit trophic effects, such as promoting neuronal migration and differentiation, but similarly to the stimulation of other TNF family cytokine receptors, it can also lead to sphingolipid hydrolysis, ceramide production, and ultimately, programmed cell death [69,73].
Pro-BDNF exerts its actions through binding to the p75 neurotrophin receptor, while BDNF acts through TrkB receptors, which are widely expressed in various parts of the brain [37]. BDNF, by binding to TrkB, triggers the activation of the Ras/Raf/MAKP, PLCg, and PI3K/Akt signalling cascades, which play roles in neurogenesis, neuronal survival, and plasticity. Reduced BDNF expression has been demonstrated in patients with Alzheimer’s disease in the hippocampus, dentate gyrus, new cortex, and nucleus basalis of Meynert [39]. Defects in the mechanism of pro-BDNF conversion to BDNF, or imbalances between these two forms, have been correlated with impaired cognitive function, psychiatric disorders, and anxiety symptoms [37].
Given the above, neurotrophins, along with their receptors, are considered promising therapeutic targets for the treatment of neurological and neurodegenerative diseases. While exogenous therapies based on neurotrophins have not yielded the expected results in clinical trials, mainly due to the short half-life of neurotrophins and low permeability of the blood-brain barrier (BBB) [29], scientists are exploring alternative interventions based on plants and fungi that can increase endogenous BDNF levels and TrkB receptor activity.
A promising approach may involve the use of natural compounds with potential neuroprotective properties. Nutraceuticals that exhibit multidirectional biological effects may contribute to improved cognitive function. Such substances are safe, readily available, and relatively inexpensive compared to bioengineering methods, making them potentially effective therapeutic agents for maintaining brain health. The discovery of neurotrophin-stimulating compounds, such as hericenones isolated from the fruiting bodies of H. erinaceus and erinacines from mycelia, has made this mushroom species valuable for research purposes.
3. Activities of Components Isolated from Hericium erinaceus
H. erinaceus is characterized by its high nutritional value. According to a study conducted by Friedman [30], dried fruiting bodies of this mushroom contain approximately 61.1% carbohydrates, 5.1% fatty acids, 20.8% proteins, 6.2% water, and 6.8% ash on a dry weight basis, providing around 374 kcal/100 g. On the other hand, dried mycelium contains about 42.5% proteins, 42.9% carbohydrates, 6.3% fatty acids, 3.9% water, and 4.4% ash, providing around 398 kcal/100 g. In terms of carbohydrates, it is worth noting that lion’s mane mushroom exhibits a high content of arabitol, up to 127 mg/g dry weight, as shown in studies by Mau et al. [75] and Valu et al. [76]. Additionally, detailed research conducted by Valu et al. [76] identified the presence of 19 amino acids and 32 aromatic substances in lion’s mane mushrooms. Both the fruiting bodies and the mycelium of H. erinaceus contain a range of macro- and microelements. According to Eisenhut et al. [77], lion’s mane mushroom contains significant amounts of potassium (254 mg/100 g dry weight) and phosphorus (109 mg/100 g dry weight), while the content of magnesium, zinc, and copper is lower. Furthermore, cobalt, iron, manganese, molybdenum, selenium, sodium, and sulfur have also been detected in lion’s mane mushrooms. It is worth noting that lion’s mane mushrooms may also contain heavy metals such as arsenic, cadmium, copper, and lead. As suggested by Yang et al. [78], the content of heavy metals in mycelium is higher than in the fruiting bodies.
Studies on the content of secondary metabolites in H. erinaceus have been conducted since the 1990s. In 2021, Yang et al. [79] identified as many as 102 compounds present in this mushroom. Among these compounds were organic acids, nucleotides and their analogs, amino acids, carbohydrates and their derivatives, flavonoids, unsaturated fatty acids, terpenoids, phenolic acids, phenylpropanoids, and steroids.
Research has shown the presence of a range of bioactive substances in both the fruiting bodies and mycelium of H. erinaceus (Table 1). These substances can be divided into two main categories. The first category consists of high-molecular-weight compounds, such as polysaccharides such as β-glucans, as well as other polysaccharides and polypeptides, which have a significant impact on strengthening the body’s immune system. The second category consists of low-molecular-weight compounds, such as terpenoids and polyketides, including erinacines and hericenones, which exhibit antioxidative, antidiabetic, anticancer, anti-inflammatory, and hypolipidemic properties, as shown by studies conducted by Thongbai et al. [6] and Ratto et al. [31]. These compounds have the ability to interact at the molecular level by regulating cytokines, protein kinases, and transcription factors. It is particularly interesting that the most abundant compounds, hericenones and erinacines, are capable of effectively crossing the blood-brain barrier (BBB) [80]. They demonstrate neuroprotective and neurotrophic effects, both in vitro and in vivo, in animal models of peripheral nerve injury [32], stroke [33], and Alzheimer’s disease [15,30,34]. H. erinaceus may exhibit pharmacological activity at the tissue, organ, and systemic levels, as suggested by the results of research conducted by Roda et al. [81] (Figure 1).

Table 1.
Biological activity of secondary metabolites isolated from Hericium erinaceus.
3.1. Polysaccharides
Polysaccharides in fungi are mainly present in the cell wall and can constitute up to 20% of the mass in both fruiting bodies and mycelium [6]. Polysaccharides with antitumor properties have been isolated from the basidia of H. erinaceus, such as xylans, glucans, heteroxyloglucans, and galactoxyloglucans [6,88].
In 2004, Jia et al. [89] isolated heteropolysaccharides with a mass of 18 kDa, such as rhamnose, galactose, and glucose, while Zhang [90,91] found some with a mass of 19 kDa containing fructose, galactose, and glucose. In H. erinaceus from Malaysia, arabinoxylans are the main sugar component, with glucose, rhamnose, deoxyribose, and galactose being much less common [92]. Polysaccharides isolated from H. erinaceus served various functions, such as neurogenesis, peripheral nerve regeneration, and muscle regeneration after injuries [50], as well as immunostimulatory, anticancer, and cholesterol-lowering activities [93] Hou et al. [94] isolated water-soluble oligosaccharides from H. erinaceus with antioxidant properties.
3.2. Erinacines
Erinacines are a group of bioactive compounds capable of crossing the blood-brain barrier and inducing the expression of NGF in the brain [95]. These compounds are obtained from the mycelium and fruiting bodies of H. erinaceus and belong to the group of substances known as cyathane-xyloside, which are diterpenoids. These molecules contain five-, six-, and seven-membered rings in their structure, with cyathane-xyloside additionally containing xylose (wood sugar) attached to the aglycone [52].
Three main biological activities of erinacines have been identified. Firstly, they demonstrate the ability to stimulate NGF synthesis. Secondly, they possess antibiotic properties. Thirdly, they can stimulate the κ opioid receptor. So far, nineteen different erinacines have been isolated. Among these compounds, ten exhibit neuroprotective activity, including the stimulation of NGF synthesis (erinacines A, B, C, E, F, and H) and the promotion of neurite outgrowth (T, U, V, and P) (Figure 2, Table 1).
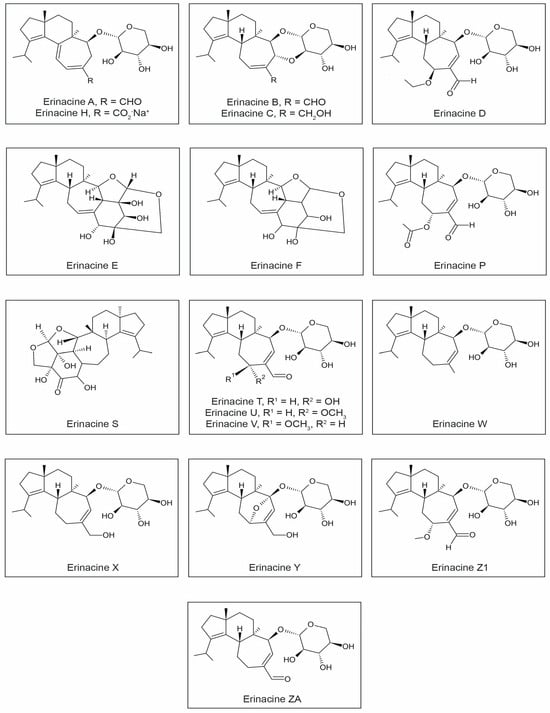
Figure 2.
Chemical structures of erinacines.
Erinacine A, a natural NGF synthesis inducer with low molecular weight, showed effectiveness in several mouse models of age-related neurological diseases using oral administration [20,22,96]. Some erinacines (A and S) also exhibit actions that reduce β-amyloid deposition and increase the expression of the gene encoding the insulin-degrading enzyme. Erinacine A is exclusively present in the fermented mycelium and is absent in H. erinaceus fruiting bodies. Erinacine E has found application in the treatment of neuropathic pain in animal models [35,40,45,46,97,98,99,100,101]. Kenmoku et al. [100,101] demonstrated that in the mycelium of H. erinaceus, erinacine Q serves as a direct substrate for the biosynthesis of erinacine P, which is a parent precursor for other important erinacines and striatins (striatal derivatives). In a study by Ma et al. [47], erinacines W, X, Y, and ZA showed significant neurotrophic effects on PC12 cells (Figure 2).
3.3. Ergothioneine
The fruiting bodies and mycelium of H. erinaceus in the presence of ergothioneine, an organic compound belonging to the group of amino acids and betaine, have been observed. It exhibits antioxidant and cytoprotective properties. A growing number of scientific reports suggest the potential application of therapy based on L-ergothioneine in the treatment of cardiovascular diseases [102], musculoskeletal disorders, pre-eclampsia [103], and neurodegenerative diseases [81,104,105].
3.4. Hericenones
From the fruiting bodies of H. erinaceus, several hericenones (A, B, C, D, E, F, G, H, I, J) (Figure 3) have been isolated, which are phenolic derivatives with diverse biological activities. H. erinaceus is the sole source of these valuable acids [40,83,84,106]. Hericenones A and B exhibit cytotoxic properties against HeLa cervical cancer cells. Hericenones C-E and H stimulate nerve growth factor (NGF) synthesis and NGF gene expression through the activation of the protein kinase A signaling pathway. The activity of individual hericenones differs depending on the length and presence of double bonds in the fatty acid chains. The highest NGF-stimulating ability was observed in hericenones E, which possess two double bonds in the chain [35].
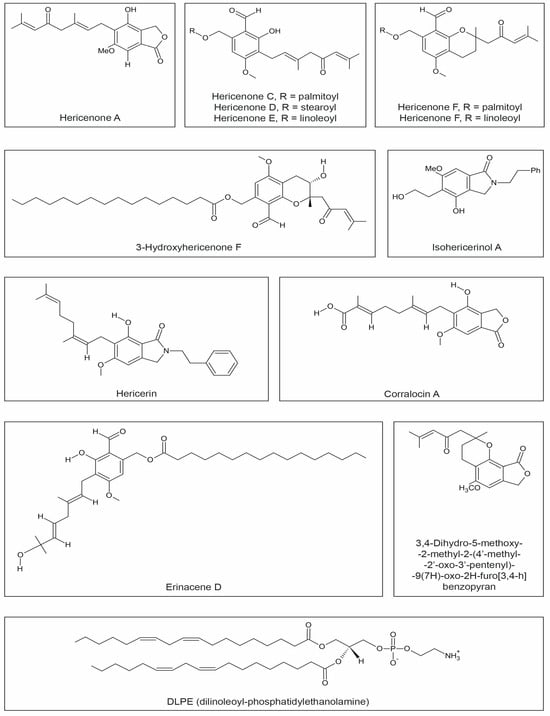
Figure 3.
Chemical structures of hericenones, 3-hydroxyhericenone F, isohericerinol A, hericerin, corralocin A, erinacene D, 3,4-Dihydro-5-methoxy-2-methyl-2-(40-methyl-20-oxo-30-pentyl)-9(7H)-oxo-2h-furo[3,4-h]benzopyran, and DLPE.
Approximately 3-hydroxyhericenone F (Figure 3) demonstrates protective activity on cells against endoplasmic reticulum (ER) stress-induced apoptosis, which is implicated in neuronal apoptosis in many neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and prion diseases [84]. Moreover, other phenolic compounds, such as hericenones A-D, hericenals A and B, hericenol A, erinacerines A and B, and hericerin, have been isolated from H. erinaceus. Hericenes B and C exhibited neuroprotective properties against ER stress induced by tunicamycin [106,107] (Figure 3, Table 1).
3.5. Dilinoleyl-Phosphatidylethanolamine (DLPE)
DLPE (Figure 2), isolated from the fruiting bodies of H. erinaceus, is a phospholipid containing two unsaturated fatty acids, namely linoleic acids. It exhibits properties that protect neurons from cell death caused by ER stress [15], wherein a protective mechanism involving the PKC (protein kinase C) pathway is engaged. There is a connection between this protective mechanism and the activation of protein kinase C (PKC) by phospholipids, mainly phosphatidylserine and unsaturated fatty acids [38,85]. Some researchers suggest that PKC reduces cell mortality by reducing the toxicity of amyloid beta [108,109], while others believe that PKC contributes to apoptosis induction [110].
3.6. Other Compounds
Li et al. [111] isolated new aromatic compounds from H. erinaceus, such as erinacene D (Figure 2), and demonstrated that this compound has the ability to inhibit the transcriptional activity of NF-kB induced by tumor necrosis factor-alpha (TNFα), confirming their potential role in NF-kB activity inhibition (Table 1).
Steroids such as six erinarols (A-F), five ergostane-type sterol fatty acid esters, ten ergostane-type sterols, and four erinarols (G-J) have also been isolated from the fruiting bodies of H. erinaceus. All these compounds exhibit anti-inflammatory and antiproliferative properties [112].
Furthermore, nucleoside components have been isolated, showing diverse forms of biological activity, such as anticancer and antiviral effects, as well as potential use in gene therapy. Adenosine, inosine, and guanosine exhibit a variety of pharmacological effects, including immune regulation, neuroprotection, and potential treatment for cardiovascular diseases. Among the compounds isolated from H. erinaceus, flavonoids have also been identified, which can be utilized in pharmacology, including for neuroprotection, treatment of myocardial ischemia, improvement of cognitive function, prevention of chronic gastric ulcers, protection of reproductive tissue, as well as acting as anti-inflammatory and anticancer drugs [79].
4. The Neuroprotective and Neurotrophic Potential of H. erinaceus Components
The impact of extracts derived from H. erinaceus has been extensively investigated for many years, both in in vitro and in vivo studies as well as clinical research. This subsection summarizes the physiological mechanisms and behavioral effects induced by the action of various types of extracts obtained from H. erinaceus (Table 2).

Table 2.
The impact of various extracts from Hericium erinaceus on studied cell lines or organisms.
4.1. Growth, Regeneration, and Protection of Nerve Cells
Since the 1990s, several in vitro studies have been conducted, demonstrating the stimulatory effects on the synthesis of nerve growth factor (NGF) by hericenones (C, D, E, H) and erinacines (A-F, H) extracted from H. erinaceus (Table 1) [40,43,44,45,48]. However, these studies did not provide a clear understanding of the underlying mechanisms of action or how they influence neurite growth. Rupcic et al. [41] analyzed the effects of secondary metabolites from H. erinaceus on 1321N1 cells and confirmed previous findings suggesting that erinacines A, B, C, and E stimulate NGF synthesis. Moreover, they were the first to demonstrate that erinacine Z1 also increases the expression of this neurotrophin. Additionally, they found that erinacine C also increases the expression of BDNF. The ability of erinacine to stimulate the transcription of both neurotrophins suggests the existence of a common regulatory factor that plays a role in inducing both NGF and BDNF. Similar neurotrophin expression stimulation was observed by Ryu et al. [86]. They isolated hericerin, isohericerinol A, and corallocin A from H. erinaceus. Isohericerinol A and corallocin A increased the expression of BDNF, while hericerin and isohericerinol A increased the level of NGF.
Rat pheochromocytoma (PC12) cells are often used as an in vitro model in research on neurodegenerative diseases because they have the ability to form synapses and produce proteins related to the nervous system [9,60]. Phan et al. (2014) [35] conducted a study to examine neurite growth activity and analyze signaling pathways involved in neurogenesis after H. erinaceus induction in PC12 cells. They found that hericenone E exhibits neurotrophic effects in these cells. This action is associated with the stimulation of nerve growth factor (NGF) synthesis and subsequently increased phosphorylation of the TrkA receptor by NGF, leading to the activation of ERK and Akt signaling pathways. Additionally, they analyzed additional signaling events and found that hericenone E activates the ERK1/2 and PI3K/Akt cascades independently of the presence of NGF.
The stimulation of ERK activity by hericenone E independently of NGF suggests that this compound is involved in additional signaling pathways directly regulated by hericenone E. These signaling pathways complement the ERK and Akt pathways, leading to neuronal differentiation and neurite growth. Zhang et al. [46] demonstrated the significant effects of erinacines T, U, V, and P on neurite growth in PC12 cells. Furthermore, Ma et al. [47], using genetic engineering techniques, synthesized new types of erinacines (W, X, Y, and ZA) in Saccharomyces cerevisiae, which also exhibited neurotrophic effects on PC12 cells. Studies on the effect of H. erinaceus on neurite elongation have been conducted by Rahman et al. [10], Zhang et al. [14], and Lai et al. [2], and all of them confirmed this effect (Table 2).
Cheng et al. [59] investigated the effects of different concentrations of H. erinaceus polysaccharide extracts (HEPS) and Aβ1-40 on PC12 cells to induce cytotoxicity. The accumulation of Aβ plays a significant role in initiating and developing Alzheimer’s disease (AD). Neurotoxic mechanisms associated with this disease include oxidative stress and mitochondrial dysfunction, leading to apoptosis and neuronal dysfunction. The researchers found that HEPS promoted the survival of PC12 cells under toxic conditions induced by Aβ. Moreover, they observed increased effectiveness in removing free radicals and reactive oxygen species (ROS). As a result, HEPS protected PC12 cells from Aβ-induced apoptosis. In vivo experiments were also conducted on APPswe/PS1dE9 mice to assess the impact of erinacine A on Alzheimer’s disease. They showed that after 30 days of oral administration of H. erinaceus mycelium, the insulin-degrading enzyme (IDE) expression was enhanced, and the amount of Aβ amyloid plaques in the brain was reduced. Additionally, an increased ratio of nerve growth factor (NGF) to its precursor pro-NGF and an increase in the number of new neurons in the dentate gyrus (DG) were observed. Furthermore, after 81 days of H. erinaceus supplementation, improvements were seen in the impaired brain regions of transgenic mice, resulting in the reversal of behavioral deficits [22].
The neuroprotective and therapeutic efficacy of erinacine A in improving pathological conditions and behavioral deficits in Parkinson’s disease (PD) and Alzheimer’s disease (AD) was also confirmed in an animal model (C57BL/6 mice) by Lee et al. [23]. The researchers conducted studies on the potential use of erinacine A in the treatment of Parkinson’s disease. In vitro experiments were performed on N2a cells, and in vivo experiments were performed on a C57BL/6 mouse model. These studies were the first to demonstrate that erinacine A can reduce MPTP-induced neurotoxicity by activating cell survival pathways such as PAK1, AKT, LIMK2, and MEK and by reducing cell death pathways such as IRE1α, TRAF2, ASK1, GADD45, and p21. Li et al. [96] developed an in vitro model to confirm the effectiveness of EAHE and demonstrated that, in the absence of NGF, EAHE extract from the mycelium was capable of inducing neurite outgrowth in primary cultures of rat cortical neurons in a concentration-dependent manner (Table 2).
Chen et al. [52] and Tzeng et al. [133] conducted animal studies that showed the neuroprotective effects of erinacine S in neurodegenerative diseases. Further, Lin et al. [82] performed in vitro experiments on primary neurons from mice and rats. This study demonstrated that after incubation with erinacine S, there was a significant increase in neurite outgrowth in both central nervous system (CNS) and peripheral nervous system (PNS) neurons. They also analyzed the mechanism of this phenomenon using the RNA-seq technique and confirmed through ELISA that erinacine S stimulates the accumulation of neurosteroids. It was also shown that neurosteroids stimulate neurite outgrowth, induce neurogenesis, and prevent neuronal apoptosis, which explains the neuroprotective effect of erinacine S in Alzheimer’s disease and neuronal regeneration. Additionally, it was suggested that erinacine E exhibits strong and selective agonistic activity on κ-opioid receptors present in the peripheral endings of major ascending neurons. This activity indicates the potential use of erinacine E as an analgesic substance. Moreover, it was suggested that κ-receptor agonists may have the potential to act as neuroprotective agents in conditions such as brain hypoxia and ischemia (Table 2) [43,44].
Park et al. [58] analyzed the neurotrophic activity of compounds derived from H. erinaceus fruiting bodies in rat hippocampal cells. It was shown that the addition of the polymer could partially delay the apoptosis of nerve cells and lead to an increase in the number of cells containing neurites, ultimately contributing to the growth of nerve cells. Studies were conducted on the neurotrophic effects of dried H. erinaceus fruiting bodies on neurons in rat hippocampal slices by analyzing cellular impulse activity. The results demonstrated that the fruiting bodies exhibited neurotrophic or stimulating effects on neurons at concentrations that had no effect on the growth of nerve cells in vitro and did not induce toxic effects or damage to nerve cells. Neurons in the hippocampus, which are part of the limbic system, play a significant role in the regulation of motivational-emotional responses, memory, and other cognitive functions [32,134]. A characteristic feature of this structure is its remarkable sensitivity to even minor changes in the composition of the intercellular fluid substance, which is much greater than in the case of neurons in the cerebral cortex and cerebellum. The extract also promoted the normal development of cultured cerebellar cells and showed a regulatory effect on myelination processes in vitro after myelin damage. The myelin sheath plays an important role in transmitting nerve signals. Damage to the compact myelin structure leads to impairment and serious nervous system diseases [116].
Shimbo et al. [123] tested the effect of erinacine A on different brain regions in rats and observed an increase in NGF synthesis in certain regions. This could be attributed to two factors: the stimulating effect of erinacine A on noradrenaline synthesis, leading to increased NGF secretion in the hippocampus, and the locus coeruleus (LC) region. Erinacine A may act at the neurotransmitter-neurotrophin level, as evidenced by studies showing that adrenergic receptors have been identified in astrocytes, and noradrenaline (NA) regulates neurotrophin synthesis in the whole brain and in astrocytes in the hippocampus. Given that adrenergic receptors are present in hippocampal neurons (associated with the hippocampus), it is possible that NA regulates neurotrophin synthesis in hippocampal glial and nerve cells. Another mechanism suggests that erinacine A increases the levels of neurotrophin 3 (NT-3) in the LC as well as enhances the survival of noradrenergic neurons and NA synthesis in the LC. Noradrenaline in the LC stimulates NGF synthesis in the hippocampus.
Neurotrophin-3, in turn, induces the formation of new receptors for the tyrosine kinase C enzyme, recognizing other neurotrophic factors, such as NGF, and simultaneously activating them. A study conducted by Mori et al. [36] showed that oral administration of H. erinaceus to mice for 7 days increased the expression of genes encoding NGF fivefold in the hippocampus. Interestingly, this effect was observed only in the hippocampus, not in the cerebral cortex, which suggests a significant influence of NGF on memory and learning, especially considering that in mammals, neurogenesis occurs in the hippocampus, specifically in the dentate gyrus area [135]. Furthermore, H. erinaceus extracts have shown the ability to induce phosphorylation of c-Jun N-terminal kinase (JNK) and its substrate, c-Jun protein, as well as increase c-Fos expression. These results suggest that H. erinaceus stimulates the expression of the NGF gene through JNK signaling (Table 2) [36].
Wong et al. [55] conducted a study on the effects of H. erinaceus extracts on the NG108-15 cell line, characterized by high proliferative activity and rapid neurite growth. In their research, they demonstrated that water extracts from lyophilized fruiting bodies of this fungus, cultivated in tropical conditions, showed the greatest stimulation of neurite growth in in vitro cultures. Hazekawa et al. [33] also showed that a 14-day treatment with dried, water-dissolved H. erinaceus fruiting bodies exhibited neuroprotective effects in mice with MCA occlusion-induced brain ischemia. Moreover, treatment with H. erinaceus at the same concentration in healthy mice also showed increased NGF levels in the examined brain regions, which confirms the results of previous studies.
In 2014, Kim et al. [136] conducted in vivo and in vitro experiments combining extracts from H. erinaceus and Allium sativum. In vitro studies were performed on PC12 cells, and it was shown that the HGE extract (H. erinaceus mycelium enriched with garlic extract) partially delayed the apoptosis of nerve cells, leading to an increase in neurite numbers and accelerating the growth of nerve cells. HGE was found to downregulate the expression of the protein p21, which plays a significant role in the cell cycle and can stimulate nerve cell growth. Overexpression of this protein in ischemic tissues leads to cell cycle arrest and cell death. These results were confirmed in in vivo studies conducted on Mongolian gerbils (Meriones unguiculatus). A comparison of changes in the CA1 region of the hippocampus in animals with induced forebrain ischemia before and after HGE administration showed that the extract increased cell survival by 60%. Additionally, increased endothelial cell proliferation and vessel numbers were observed. These findings indicate the neuroprotective effect of this extract and suggest that it may aid in recovery after ischemic brain damage (Table 2).
In a study conducted by Wong et al. [50,137], the impact of the aqueous extract of fresh H. erinaceus fruiting bodies on sciatic nerve regeneration following crush injury in adult female Sprague-Dawley rats was investigated. It was found that the free radicals generated after the injury played a dominant role in delaying functional regeneration. Better regenerative effects were achieved with therapies aimed at counteracting the damage resulting from ischemia and reperfusion, such as antioxidants, lipid peroxidation inhibitors, and anti-inflammatory drugs. Therefore, the use of medicinal mushrooms may be a potential alternative to neurotrophic factors in peripheral nerve repair processes. Although the effectiveness of mushrooms is lower than that of neurotrophins, the aqueous extract of fresh H. erinaceus fruiting bodies can be used as an adjunct to neurotrophin therapy to enhance axonal regeneration in the nervous system and reduce the dosage of neurotrophins to limit potential toxic effects. The study showed that daily oral administration of the aqueous extract of fresh H. erinaceus fruiting bodies accelerated the regeneration of damaged peripheral nerves in rats. Additionally, scientists pointed out that H. erinaceus could influence neuronal functions by regulating the activity of various signaling pathways, such as the activation of cAMP response element-binding (CREB). CREB signaling plays a significant role in hippocampal long-term potentiation, which is important for learning and memory processes, as well as in CREB hyperphosphorylation (Table 2).
Research conducted by Üstün and Ayhan [56] demonstrated that the aqueous extract of fresh H. erinaceus fruiting bodies (HE) has the ability to prevent neuronal death and promote axonal regeneration in an experimental axonal injury model. In that study, the effects of HE were compared with NGF. Both HE and NGF showed neuroprotective and regenerative effects on damaged peripheral sensory neurons, but the protective efficacy of HE treatment or the combination of HE with NGF showed higher protective activity. It was observed that the regenerative ability of the NGF + HE combination was stronger than that of the individual substances alone. Therefore, HE or its combination with NGF may represent an effective and safe therapeutic option for the treatment of peripheral nerve injuries.
Scientists suggest that NGF and HE may act through different protective and regenerative mechanisms while also exhibiting antioxidant and anti-inflammatory properties. It is worth noting that the neuroprotective activity of HE is higher than that of NGF, possibly due to the presence of additional antioxidant and anti-inflammatory properties. The results obtained indicate that HE may become a promising candidate for preventing neuronal death, improving nerve function, and treating peripheral nerve injuries. Zhang et al. [60] also conducted research on the impact of water extracts from H. erinaceus. The study showed that HE exhibits protective properties against L-Glu-induced neurotoxicity in DPC12 cells, mainly through the regulation of mitochondrial pathways. Additionally, experiments conducted on mice with Alzheimer’s disease induced by AlCl3 and D-gal confirmed the protective action of HE, which may also affect neurotransmitter modulation. Furthermore, a significant increase in the concentration of acetylcholine (ACh) and choline acetyltransferase (ChAT) activity was observed in the serum and hypothalamus of mice with Alzheimer’s disease (AD) after HE administration. Patients with AD show reduced ChAT activity and insufficient ACh content in the brain, leading to impaired learning and memory abilities. Based on these results, it can be inferred that HE may be a potential candidate as a neuroprotective substance in the treatment and prevention of neurodegenerative diseases (Table 2).
Travato et al. [61] also conducted research that demonstrated the neuroprotective properties of H. erinaceus. During three months of supplementing HE in rats, increased expression of the genes responsible for cell protection, especially HSP70, HO-1, and TRX, was observed, leading to increased synthesis of lipoxin A4 (LXA4) in various regions of the rat brain. The largest inductions of LXA4 were observed in the cerebral cortex, hippocampus, striatum, and cerebellum. This discovery is of significant importance for the pathogenesis of Alzheimer’s disease (AD) and Parkinson’s disease (PD), especially in the context of theories connecting aging, neuronal degeneration, and oxidative damage. The results also indicate that HE supplementation may modulate the nutritional regulations of key proteins responsible for brain tolerance to stress. There is a probability that HE supplementation increases the redox potential, leading to the induction of genes encoding protective proteins. This may strengthen neurons sensitive to stress and protect them from apoptosis-induced neurodegeneration. These results are consistent with the study conducted by Hsu et al. [125]. The scientists conducted research using the MPTP mouse model, which showed that HEM may increase dopamine levels in patients with Parkinson’s disease (PD).
Jang et al. [124] conducted research to assess the impact of H. erinaceus supplementation (HE) on neuroprotection in pilocarpine-induced status epilepticus (SE) and to investigate the underlying mechanisms, with a focus on potential applications in the treatment of temporal lobe epilepsy (TLE). In patients with epilepsy, extensive neuronal damage, inflammation, and abnormal neurogenesis are considered contributing factors to chronic seizure occurrences. Therefore, numerous studies are being conducted to identify potential dietary supplements and functional foods that could be used in TLE treatment. Researchers found that administering doses of 60 and 120 mg/kg HE resulted in reduced neuronal death in the hippocampus 7 days after pilocarpine-induced seizures. However, high doses of HE did not show neuroprotective effects, suggesting the existence of an optimal dose range of HE that provides protection against seizure activity. Under the influence of HE treatment, reduced cyclooxygenase-2 (COX2) expression, a pro-inflammatory factor, was observed with doses of 60 and 120 mg/kg HE. These findings indicate that HE supplementation may contribute to neuroprotection in cases of SE and represent a novel candidate as a nutritional substance for treating TLE. These results confirm the previously mentioned studies cited above (Table 2).
It has been demonstrated that compounds known as 3-hydroxyhericenone F [85] and dilinoleoyl phosphatidylethanolamine [38] that are purified from the extract of dried H. erinaceus fruiting bodies have the ability to reduce cell death induced by endoplasmic reticulum stress. Similar properties were observed for hericenone C and regioisomers of hericenones B-D [107]. This may contribute to reducing the risk associated with cell death induced by neurodegenerative diseases (Table 1).
4.2. Cellular Aging Inhibition
Noh et al. [117] evaluated the inhibitory effects of six compounds isolated from H. erinaceus on cell aging induced by adriamycin in human skin fibroblasts (HDF) and human umbilical vein endothelial cells (HUVEC). One of these compounds, ergosterol peroxide, showed decreased senescence-associated β-galactosidase (SA-β-gal) activity, which was elevated in HUVEC cells treated with adriamycin. This suggests that one or more pure compounds may have potential in the treatment or prevention of age-related diseases in humans (Table 2).
4.3. Improvement of Cognitive Function
Adult hippocampal neurogenesis is one of the most interesting aspects of neurogenic zones in the adult brain due to its role in higher cognitive functions, especially in memory processes and some affective behaviors. In particular, neurogenesis in the dentate gyrus of the hippocampus leads to the generation of new granule cells in the adult brain and significantly contributes to lifelong plasticity. Newly formed neurons in the dentate gyrus have also been shown to be essential for mediating the beneficial effects of antidepressant treatment [138].
The first in vivo and in vitro studies conducted on wild-type mice by Brandalise et al. [126] demonstrated that oral supplementation of H. erinaceus significantly improved recognition memory in a behavioral test and increased spontaneous and induced synaptic activity in mossy fiber-CA3 synapses in mouse hippocampal sections. This suggests that H. erinaceus exerts a reinforcing effect on neuronal functions, even under non-pathological conditions.
Ratto et al. [31] conducted a study on the same animal model using aging mice. To best generalize the results and translate them to humans, researchers decided to use extracts from mycelium and fruiting bodies that mimic the supplements used by humans. It was confirmed that He1 supplementation is able to improve cognitive abilities in older mice and reverse cognitive decline in mice with signs of weakening. A restored proliferation of granule cells in the dentate gyrus and pyramidal neurons in the CA3 layer of the hippocampus was observed. Additionally, the presence of progenitor cells in the granule cells of the DG region was observed, supporting the hypothesis that the H. erinaceus extract promoted neurogenesis in the hippocampus of adult mice. These results are consistent with a study conducted by Ryu et al. [86], where it was demonstrated that a 28-day administration of an ethanol extract of HE significantly increases the proliferation and survival of precursor cells in the hippocampus without affecting the proportions of differentiated neurons. Scientists suggest that the molecular mechanism underlying this phenomenon may be related to the production of NGF, which regulates the proliferation and differentiation of neural stem cells. The findings of Ratto et al. [31] are also interesting, as based on observations of increased cell proliferation in the cerebellum, they suggest the presence of newly formed, immature neurons in the cerebellar cortex of mice treated with H. erinaceus, despite the cerebellum being considered a “non-neurogenic” area.
Mori et al. [21] conducted a double-blind trial on Japanese men and women with diagnosed mild cognitive impairment. The study aimed to investigate the effectiveness of oral administration of H. erinaceus in improving cognitive functioning, measured with the Revised Hasegawa Dementia Scale (HDS-R). Cognitive function scores increased with a longer duration of administration. Laboratory research showed no adverse effects of H. erinaceus, and the substance proved effective in improving mild cognitive impairment.
Mori et al. [34] also conducted a study to investigate the impact of oral administration of H. erinaceus fruiting body powder for 23 days in mice with cognitive and learning deficits induced by Aβ(25-35) peptide administration. Researchers assessed learning function and demonstrated that H. erinaceus supplementation improved cognitive deficits induced by the amyloid peptide. These results suggest a promising effect of this supplement in treating cognitive function disorders. These findings are consistent with the results of a pilot study conducted by Vigna et al. [37] on 77 overweight patients (62 women, 15 men), which found that H. erinaceus supplementation contributed to improving depression-anxiety mood disorders and the quality of nocturnal rest. These effects were still present eight weeks after the end of H. erinaceus supplementation, suggesting that it may affect neuronal plasticity. Improvement in mood disorders was associated with changes in pro-BDNF levels and the pro-BDNF/BDNF ratio in peripheral blood.
Similar results were obtained by Lee et al. [53], who conducted a study on the long-term intake of EAHEM and demonstrated improved learning ability by improving memory behavior. Similar results were also obtained by Tzeng et al. [133], who conducted a study on transgenic APP/PS1 mice. Chiu et al. [26] observed that H. erinaceus supplementation led to an increase in the levels of neurotransmitters such as dopamine, serotonin, and norepinephrine in the hippocampus of mice deprived of mobility. These results suggest the antidepressant effects of H. erinaceus, which is consistent with the findings of Nagano et al. [130], who showed that H. erinaceus supplementation led to a reduction in depression and anxiety in 30 women after 4 weeks of supplementation. Saitsu et al. [128] conducted a comparative study in which the consumption of cookies containing H. erinaceus for 12 weeks showed alleviation of symptoms of short-term memory impairment and improved cognitive function in 31 participants.
Li et al. [51] confirmed the effectiveness of H. erinaceus in alleviating neurodegenerative disorders in patients with mild Alzheimer’s disease. Meanwhile, Rodriguez and Lippi [127] conducted a study on the rTg4510 mouse model, which exhibits tau protein pathology and serves as a model for Alzheimer’s disease. Researchers did not confirm an improvement in cognitive function following H. erinaceus supplementation but did observe an anxiolytic effect. Rossi et al. [57] demonstrated a positive impact of H. erinaceus on spatial memory in mice (Table 2)
4.4. Anti-Neuroinflammatory and Antioxidant Effects
The occurrence of neurodegenerative diseases is closely related to neuroinflammation. Studies have shown that microglial cell activation can be induced by lipopolysaccharide (LPS), leading to the generation of significant amounts of ROS, nitric oxide (NO), and pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These factors are responsible for neuronal damage and ultimately contribute to the development of neurodegenerative diseases. Brain neurons and endothelial cells are responsible for the production of NO through the transformation of arginine involving the NO synthase enzyme, which is activated by calcium ions (Ca2+). This process also requires the involvement of NADPH and O2.
There are three isoforms of NO synthase: endothelial NO synthase (eNOS), neuronal NO synthase (nNOS), and inducible NO synthase (iNOS). Higher levels of iNOS can be observed in many cell types, such as glial cells, macrophages, skeletal muscles, neurons, platelets, and leukocytes. iNOS expression is usually elevated in response to inflammatory conditions and oxidative stress [53,139]. iNOS activation in cells can be stimulated by LPS, TNF-α, and IL-1 [42]. It has been shown that hericenone C may inhibit iNOS expression, leading to the inhibition of LPS-induced NO production [42]. These results are consistent with the findings of Lee et al. [53], who demonstrated that EAHEM administration to mice resulted in reduced iNOS expression in the brain. These results suggest that the protective action of EAHEM may be a result of reduced oxidative stress and inflammatory state, which is consistent with a previous study by Lee et al. [20], conducted in vivo, which showed that EAHEM reduced the levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α. Additionally, reduced iNOS expression and proteins containing nitrotyrosine were observed, as well as inhibition of the p38 MAPK and CHOP signaling pathways. These results suggest that the iNOS/p38 MAPK signaling pathway may be involved in neuron survival, which can be mediated by erinacine A after brain ischemic injury.
NO also acts as a mediator in the nervous system, influencing learning and memory. The action of reducing IL-6 and TNF-α levels was also confirmed by Chiu et al. in 2018 [26]. Moreover, researchers demonstrated reduced NF-κB and IκB protein expression in the cytosolic fraction of the hippocampus in restraint-stressed (RS) animal models, which was normalized in mice supplemented with HE. NF-κB is a key transcription factor that translocates to the cell nucleus and activates the transcription of many important genes, such as pro-inflammatory cytokines and induced enzymes like inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2. Therefore, targeting the NF-κB pathway may be an interesting therapeutic strategy for the treatment of depression, as inflammatory states play a significant role in the development of this disorder. Reduced NF-κB protein expression and phosphorylation of IκBα (p-IκBα) were also confirmed by Wang et al. in 2019 [42]. In their study, they demonstrated that hericenone C can inhibit Kelch-like ECH-associated protein 1 (Keap1) while increasing nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor activity and heme oxygenase-1 (HO-1) expression. Based on this data, it can be inferred that the mechanism of action of hericenone C involves inhibiting the expression of IκB, p-IκBα, and iNOS while activating the Nrf2/HO-1 pathway.
Tsai et al. [54] conducted an experiment on 15-month-old mice fed a high-fat and high-sucrose diet (HFSD), also showing that both HEM and EA act as anti-inflammatory agents by reducing mRNA expression of TNF-α and IL-1β in the mouse hippocampus. Mice treated with HEM showed increased mRNA expression of NGF and NeuN. It was also shown that EA and HEM can reverse spatial learning deficits. They also demonstrated that both HEM and EA effected reductions in body weight, abdominal fat tissue, blood glucose levels, total serum cholesterol, and liver triglycerides. Based on these results, it can be concluded that HEM may be a potential health-promoting supplement that minimizes aging progression and neurodegeneration induced by obesity by reducing metabolic disorders and neuroinflammatory cytokines, as well as by increasing neurogenesis factors.
The antioxidant activity of H. erinaceus has also been identified as another significant aspect alongside its anti-inflammatory activity.
Mainly, the antioxidant activity of H. erinaceus is due to the presence of polysaccharides (β-glucans) and diterpenoids (hericenones, erinacines) [76]. However, several researchers have stated that there is a close link between the antioxidant activity of H. erinaceus and the presence of flavonoids and polyphenols [140,141].
Oxidative stress is a significant component in the pathogenesis of neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases. At the core of these molecular mechanisms lies the accumulation of ROS and nitrogen species (RNS), resulting in damage to lipids, proteins, and organelles, disruption of mitochondrial membranes, and apoptosis, ultimately leading to neuronal death [115,142]. A growing body of evidence suggests that inflammatory reactions and the release of pro-inflammatory cytokines also lead to oxidative stress and disrupt redox homeostasis associated with mitochondrial dysfunction [139].
Research has demonstrated that the administration of hot water extracts from HE leads to improved scavenging of free radicals and inhibition of lipid peroxidation [143]. Polysaccharide extracts from H. erinaceus have also been found to reduce peroxidation levels, increase antioxidant enzyme activity, and enhance free radical scavenging activity [125,136,144]. Previous studies conducted on an MPTP-induced mouse model showed that administration of EAHEM counteracted oxidative stress. It was observed that EAHEM administration led to a decrease in nitrotyrosine and 4-HNE expression while restoring motor function in the rotarod test in mice. These findings suggest that H. erinaceus may exhibit protective potential against brain oxidative damage through its antioxidant action. Protection against oxidative stress may play a significant role in preventing and alleviating neurodegenerative diseases.
These findings were corroborated by Amara et al. [121], who investigated the in vitro neuroprotective role of HE biomass preparation against DEHP-induced neurotoxicity. The scientists demonstrated that pretreatment with HE significantly attenuated cell death induced by DEHP. This protective effect may be attributed to HEs ability to reduce intracellular reactive oxygen species levels, preserve the activity of respiratory complexes, and stabilize the mitochondrial membrane potential. Kushairi et al. 2019 [115] also evaluated the protective and anti-inflammatory effects of aqueous (HE-HWA) and ethanol (HE-ETH) extracts from H. erinaceus fruiting bodies. In their in vitro studies, the scientists found that HE-ETH exhibited more potent neuroprotection and more effective anti-inflammatory activity than HE-HWA, inhibiting NO production in HT22 mouse hippocampal cells and LPS-activated BV2 microglia. The mechanisms of neuroprotection included enhancing antioxidant activity, mitochondrial function, and anti-apoptotic effects.
Studies suggest that treatment with erinacine A acts by protecting against endoplasmic reticulum (ER) stress, which is associated with increased neurotoxicity and neuronal apoptosis. The main mechanism of action involves the activation of the RE1α/TRAF2, JNK1/2, and p38 MAPK pathways, leading to the regulation of various factors such as CHOP, IKB-β, NF-κB, Fas, and Bax. These factors can influence the process of apoptosis and protect neuronal cells. Activation of the RE1α/TRAF2 pathway is linked to the regulation of the ER stress response and may play a role in protecting neurons from oxidative stress and other factors. The JNK1/2 and p38 MAPK pathways are also involved in the ER stress response and the regulation of apoptotic and cell survival processes in neuronal cells. CHOP expression may impact the process of apoptosis, while IKB-β and NF-κB may be involved in regulating inflammation. Fas and Bax expression can affect the course of apoptosis by activating appropriate pathways. In summary, erinacine A acts on various signaling pathways that may play a crucial role in protecting neuronal cells from stressful factors such as ER stress. The protective action of erinacine A may contribute to reducing neurotoxicity and apoptosis, which could be significant in the prevention and treatment of neurodegenerative diseases (Table 2).
5. Toxicology Studies
Many of the studies mentioned above have confirmed the significant therapeutic properties of H. erinaceus. However, it is necessary to consider its safety and the associated risks of its use. Experimental analyses based on the action of H. erinaceus mycelium components suggest that they are potentially safe and do not induce side effects. In the study by Lai et al. (2013) [2], no cytotoxic effects were observed with aqueous solutions of H. erinaceus on NG108-15 cells and human lung fibroblasts. This is consistent with the results of Lew et al. (2020) [118], who also did not observe such effects in studies conducted on PC-12 cells. Additionally, in the research conducted by Li et al. (2018) [96], it was shown that all rats treated with EAHE mycelia (at a dose of 5 g/kg body weight) did not exhibit any signs of treatment-related toxicity or mortality. This suggests the low toxicity of EAHE mycelia in the context of their effect on animal growth. Additional studies have shown that EAHE did not cause developmental defects in these animals, even at doses of up to 2625 mg/kg. Previous studies in rats also demonstrated that oral administration of EAHE at doses of up to 3 g/kg for 28 days did not induce any signs of toxicity, morbidity, or mortality in rats of both sexes [145]. A subchronic toxicity study was also conducted in rats using water extracts of H. erinaceus at doses up to 1000 mg/kg body weight for 90 days. No toxicity, morbidity, or mortality signs were observed [146]. Chen et al. (2022) also conducted subchronic toxicity and genotoxicity studies of H. erinaceus β-glucan extract preparation [147]. The study was conducted in Sprague-Dawley rats, and based on the results, the No Observed Adverse Effect Level (NOAEL) for the β-glucan extract preparation from H. erinaceus was determined to be 2000 mg/kg body weight/day, which was the highest tested dose. These results suggest that it is unlikely for the β-glucan extract from H. erinaceus to induce any genotoxic effects. These findings are consistent with studies by Li et al. (2014) [148], which showed that H. erinaceus mycelia were not mutagenic in the bacterial reverse mutation test (Ames test), in vitro chromosomal aberration test, and in vivo micronucleus test on erythrocytes, both with and without metabolic activation.
6. Conclusions
Research on H. erinaceus and its neuroprotective properties has shown promising results and provides an excellent starting point for further studies to gain a more in-depth understanding of this species and prepare potential drugs/dietary supplements. Fungi show immense potential as polypharmaceutical drugs due to their rich and complex chemistry and diverse forms of bioactivity. They contain many chemical compounds, such as polysaccharides, triterpenes, alkaloids, flavonoids, and other components that exhibit potential therapeutic effects. However, standardization of dietary supplements based on medicinal mushrooms is still in its early stages of development. There are no uniform standards and protocols regarding the quality and composition of mushroom supplements. Difficulties in standardization and isolation of specific substances arise from the fact that secondary metabolites, widely analyzed in H. erinaceus, are often produced in response to various stress conditions. This makes it challenging to ensure consumers receive products of appropriate quality with expected health benefits. To guarantee the quality and effectiveness of supplements with medicinal mushrooms, further scientific research is necessary, along with the development of appropriate standards and protocols for the production, extraction, standardization, and quality control of these products. It is also essential to monitor the chemical composition and biological activity of fungi and develop suitable tests and methodologies to evaluate the effectiveness and safety of dietary supplements with mushrooms. All these efforts will contribute to the development of more advanced and personalized therapies based on medicinal mushrooms and enable consumers to use high-quality products with potential health benefits.
Further research and standardization work on dietary supplements based on H. erinaceus is essential to ensuring their effectiveness and safety in the context of neuroprotection in humans. Increased availability of pure analytical standards and the development of techniques for isolating and characterizing active ingredients will be crucial in the standardization and production of high-quality dietary supplements based on H. erinaceus. Furthermore, to assess the effectiveness and safety of using mushrooms for neuroprotection in humans, further research, including clinical trials, is necessary. Clinical trials will evaluate the impact of mushrooms on cognitive functions, memory, and other neuroprotective indicators in patients with neurodegenerative diseases. Understanding the mechanisms of action of active mushroom ingredients at the molecular and cellular levels may lead to the further development of mushroom-based therapies and the identification of specific compounds with potential neuroprotective effects.
Author Contributions
Discussed the concept of the manuscript, I.S.-K. and A.T-R.; contributed to the literature collection and writing of the manuscript, I.S.-K.; critically reviewed and revised the manuscript, I.S.-K., A.T.-R. and P.K.; supervision and funding acquisition, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry at Pomeranian Medical University in Szczecin, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattson, M.P. Neuroprotective signalling and the ageing brain: Take away my food and let me run. Brain Res. 2000, 886, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.L.; Naidu, M.; Sabaratnam, V.; Wong, K.H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N. Neurotrophic properties of the Lion’s Mane medicinal mushroom, Hericium erinaceus (higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Sokół, S.; Golak-Siwulska, I.; Sobieralski, K.; Siwulski, M.; Górka, K. Biology, cultivation, and medicinal functions of the mushroom Hericium erinaceum. Acta Mycol. 2016, 50, 1069. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sarma, M.K.; Deb, U.; Steinhauser, G.; Walther, C.; Gupta, D.K. Mushrooms: From nutrition to mycoremediation. Environ. Sci. Pollut. Res. 2017, 24, 19480–19493. [Google Scholar] [CrossRef]
- Sabaratnam, V.; Phan, C.-W. Neuroactive Components of Culinary and Medicinal Mushrooms with Potential to Mitigate Age-Related Neurodegenerative Diseases. In Discovery and Development of Neuroprotective Agents from Natural Products; Nature Reviews Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–413. [Google Scholar]
- Su, W.T.; Shih, Y.A. Nanofiber containing carbon nanotubes enhanced PC12 cell proliferation and neuritogenesis by electrical stimulation. Biomed. Mater. Eng. 2015, 26, 189–195. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Inhibitory effect on in vitro LDL oxidation and HMG Co-a reductase activity of the liquid-liquid partitioned fractions of Hericium erinaceus (Bull.) persoon (Lion’s Mane Mushroom). BioMed Res. Int. 2014, 2014, 828149. [Google Scholar] [CrossRef]
- Mori, K.; Ouchi, K.; Hirasawa, N. The Anti-Inflammatory Effects of Lion’s Mane Culinary-Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes) in a Coculture System of 3T3-L1 Adipocytes and RAW264 Macrophages. Int. J. Med. Mushrooms 2015, 17, 609–618. [Google Scholar] [CrossRef]
- Yang, B.K.; Park, J.B.; Song, C.H. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci. Biotechnol. Biochem. 2003, 67, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kikuchi, H.; Obara, Y.; Iwashita, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Oshima, Y.; Nakahata, N. Inhibitory effect of hericenone B from Hericium erinaceus on collagen-induced platelet aggregation. Phytomedicine 2010, 17, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, R.N.; Tang, Q.J.; Zhang, J.S.; Yang, Y.; Shang, X.-D. A new diterpene from the fungal mycelia of Hericium erinaceus. Phytochem. Lett. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Kawagishi, H.; Zhuang, C. Compounds for dementia from Hericium erinaceum. Drugs Future 2008, 33, 149–155. [Google Scholar] [CrossRef]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.-l.; Wang, A.-h.; Sun, Z.-c.; Zhao, Y.-f.; Xu, Y.-t.; He, Y.-l. Protective effect of ethanol extracts of Hericium erinaceus on alloxan-induced diabetic neuropathic pain in rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 595480. [Google Scholar]
- Wang, J.C.; Hu, S.H.; Su, C.H.; Lee, T.M. Antitumor and immunoenhancing activities of polysaccharide from culture broth of Hericium spp. Kaohsiung J. Med. Sci. 2001, 17, 461–467. [Google Scholar]
- Zhang, F.; Lu, H.; Zhang, X. Erinacerins, Novel Glioma Inhibitors from Hericium erinaceus, Induce Apoptosis of U87 Cells through Bax/Capase-2 Pathway. Anticancer Agents Med. Chem. 2020, 20, 2082–2088. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective Effects of Hericium erinaceus Mycelium and Its Isolated Erinacine A against Ischemia-Injury-Induced Neuronal Cell Death via the Inhibition of iNOS/p38 MAPK and Nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Tzeng, T.-T.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Lu, C.-K.; Shen, C.-C.; Huang, F.C.-Y.; Chen, C.-C.; Young-Ji, S. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar]
- Lee, K.F.; Tung, S.Y.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Post-Treatment with Erinacine A, a Derived Diterpenoid of H. erinaceus, Attenuates Neurotoxicity in MPTP Model of Parkinson’s Disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P. Active Substances for Preventing Hearing Deterioration, the Composition Containing the Active Substances, and the Preparation Method Thereof. U.S. Patent 10,405,504, 10 September 2019. [Google Scholar]
- Yao, W.; Zhang, J.C.; Dong, C.; Zhuang, C.; Hirota, S.; Inanaga, K.; Hashimoto, K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015, 136, 7–12. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mong, M.C. Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.O. Hericium erinaceus Extract Reduces Anxiety and Depressive Behaviors by Promoting Hippocampal Neurogenesis in the Adult Mouse Brain. J. Med. Food 2018, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2019, 21, 163. [Google Scholar] [CrossRef]
- Martínez-Mármol, R.; Chai, Y.; Conroy, J.N.; Khan, Z.; Hong, S.M.; Kim, S.B.; Gormal, R.S.; Lee, D.H.; Lee, J.K.; Coulson, E.J.; et al. Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signalling enhancing spatial memory. J. Neurochem. 2023, 165, 791–808. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Wong, K.H.; Naidu, M.; David, R.P.; Bakar, R.; Sabaratnam, V. Neuroregenerative potential of lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (higher Basidiomycetes), in the treatment of peripheral nerve injury (review). Int. J. Med. Mushrooms 2012, 14, 427–446. [Google Scholar] [CrossRef]
- Hazekawa, M.; Kataoka, A.; Hayakawa, K.; Uchimasu, T.; Furuta, R.; Irie, K.; Akitake, Y.; Yoshida, M.; Fujioka, T.; Egashira, N.; et al. Neuroprotective Effect of Repeated Treatment with Hericium erinaceum in Mice Subjected to Middle Cerebral Artery Occlusion. J. Health Sci. 2010, 56, 296–303. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid β(25–35) peptideinduced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef]
- Phan, C.W.; Lee, G.S.; Hong, S.L.; Wong, Y.T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signalling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F.; et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid.-Based Complement. Altern. Med. 2019, 2019, 7861297. [Google Scholar] [CrossRef]
- Nagai, K.; Chiba, A.; Nishino, T.; Kubota, T.; Kawagishi, H. Dilinoleoyl-phosphatidylethanolamine from Hericium erinaceum protects against ER stress-dependent Neuro2a cell death via protein kinase C pathway. J. Nutr. Biochem. 2006, 17, 525–530. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B, and C, strong stimulators of nerve growth factor (NGF)–synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, C.S.; Chen, Y.H.; Chen, C.C.; Chen, C.C.; Chuang, C.H. Anti-Inflammatory Effect of Erinacine C on NO Production through Down-Regulation of NF-kappaB and Activation of Nrf2-Mediated HO-1 in BV2 Microglial Cells Treated with LPS. Molecules 2019, 24, 3317. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shizuki, K.; Ojima, F.; Mori, H.; Okamoto, K.; Sakamoto, H.; Furukawa, S. Erinacine D, a stimulator of NGF-synthesis, from the mycelia of Hericium erinaceum. Heterocycl. Commun. 1996, 2, 51–54. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996, 37, 7399–7402. [Google Scholar] [CrossRef]
- Lee, E.W.; Shizuki, K.; Hosokawa, S.; Suzuki, M.; Suganuma, H.; Inakuma, T.; Li, J.; Ohnishi-Kameyama, M.; Nagata, T.; Furukawa, S.; et al. Two novel diterpenoids, Erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000, 64, 2402–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Bao, L.; Yang, Y.; Ma, K.; Liu, H. Three new cyathane diterpenes with neurotrophic activity from the liquid cultures of Hericium erinaceus. J. Antibiot. 2018, 71, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, Y.; Guo, C.; Yang, Y.; Han, J.; Yu, B.; Yin, W.; Liu, H. Reconstitution of biosynthetic pathway for mushroom-derived cyathane diterpenes in yeast and generation of new “non-natural” analogues. Acta Pharm. Sin. B 2021, 11, 2945–2956. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Lee, D.G.; Kang, H.-W.; Park, C.-G.; Ahn, Y.-S.; Shin, Y. Isolation and identification of phytochemicals and biological activities of Hericium ernaceus and their contents in Hericium strains using HPLC/UV analysis. J. Ethnopharmacol. 2016, 184, 219–225. [Google Scholar] [CrossRef]
- Wong, K.H.; Naidu, M.; David, P.; Abdulla, M.A.; Abdullah, N.; Kuppusamy, U.R.; Sabaratnam, V. Peripheral Nerve Regeneration Following Crush Injury to Rat Peroneal Nerve by Aqueous Extract of Medicinal Mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae). Evid.-Based Complement. Altern. Med. 2011, 2011, 580752. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P. Prevention of early Alzheimer’s disease by erinacine a-enriched Hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Lee, L.Y.; Chou, W.; Chen, W.P.; Wang, M.F.; Chen, Y.J.; Chen, C.C.; Tung, K.C. Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Lin, Y.C.; Huang, C.C.; Villaflores, O.B.; Wu, T.Y.; Huang, S.M.; Chin, T.Y. Hericium erinaceus Mycelium and Its Isolated Compound, Erinacine A, Ameliorate High-Fat High-Sucrose Diet-Induced Metabolic Dysfunction and Spatial Learning Deficits in Aging Mice. J. Med. Food 2019, 22, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Sabaratnam, V.; Abdullah, N.; Naidu, M.; Keynes, R. Activity of aqueous extracts of lion’s mane mushroom Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) on the neural cell line NG108-15. Int. J. Med. Mushrooms 2007, 9, 57–65. [Google Scholar] [CrossRef]
- Üstün, R.; Ayhan, P. Regenerative activity of Hericium erinaceus on axonal injury model using in vitro laser microdissection technique. Neurol. Res. 2019, 41, 265–274. [Google Scholar] [CrossRef]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Dietary Supplementation of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), and Spatial Memory in Wild-Type Mice. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, H.S.; Won, M.H.; Lee, J.H.; Lee, S.Y.; Lee, H.Y. Effect of an exo-polysaccharide from the culture broth of Hericium erinaceus on enhancement of growth and differentiation of rat adrenal nerve cells. Cytotechnology 2002, 39, 155–162. [Google Scholar] [CrossRef]
- Cheng, J.H.; Tsai, C.L.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170. [Google Scholar] [CrossRef]
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The Neuroprotective Properties of Hericium erinaceus in Glutamate-Damaged Differentiated PC12 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 9, 13–23. [Google Scholar] [CrossRef]
- Howard, L.; Wyatt, S.; Nagappan, G.; Davies, A.M. ProNGF promotes neurite growth from a subset of NGF dependent neurons by a p75NTR-dependent mechanism. Development 2013, 140, 2108–2117. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Role of Nerve Growth Factor in Plasticity of Forebrain Cholinergic Neurons. Biochemistry 2017, 82, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative Therapy for Alzheimer’s Disease-with Focus on Biodelivery of NGF. Front. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Crutcher, K.A.; Collins, F. In Vitro Evidence for Two Distinct Hippocampal Growth Factors: Basis of Neuronal Plasticity? Science 1982, 217, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF Is Essential for Hippocampal Plasticity and Learning. J. Neurosci. 2009, 29, 10883–10889. [Google Scholar] [CrossRef]
- Birch, A.M.; Kelly, Á.M. Chronic intracerebroventricular infusion of nerve growth factor improves recognition memory in the rat. Neuropharmacology 2013, 75, 255–261. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Chao, M.V.; Hempstead, B.L. p75 and Trk: A tworeceptor system. Trends Neurosci. 1995, 18, 321–326. [Google Scholar] [CrossRef]
- Esposito, D.; Patel, P.; Stephens, R.M.; Perez, P.; Chao, M.V.; Kaplan, D.R.; Hempstead, B.L. The Cytoplasmic and Transmembrane Domains of the p75 and Trk A Receptors Regulate High Affinity Binding to Nerve Growth Factor. J. Biol. Chem. 2001, 276, 32687–32695. [Google Scholar] [CrossRef]
- Roux, P.P.; Barker, P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002, 67, 203–233. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Morcillo, M.A.; Berger, J.; Sánchez-Prieto, R.; Saada, S.; Naves, T.; Guillaudeau, A.; Perraud, A.; Sindou, P.; Lacroix, A.; Descazeaud, A.; et al. p75 neurotrophin receptor and pro-BDNF promote cell survival and migration in clear cell renal cell carcinoma. Oncotarget 2016, 7, 34480–34497. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.L.; Lin, H.C.; Ma, J.T.; Song, S.F. Non-volatile taste components of several speciality mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef]
- Eisenhut, R.; Fritz, D.; Tiefel, P. Investigations on nutritionally valuable constituents (mineral substances, amino acids, aromatic substances) of Hericium erinaceus (Bull.: Fr.) Pers. Eur. J. Hortic. Sci. 1995, 60, 212–218. [Google Scholar]
- Yang, Y.; Zhou, C.Y.; Zhang, J.S.; Tang, Q.J. Comparison of chemical component and biological activity of Hericium erinaceus fruit body and mycelial extracts. Junwu Yanjiu 2006, 4, 15–19. [Google Scholar]
- Yang, F.; Wang, H.; Feng, G.; Zhang, S.; Wang, J.; Cui, L. Rapid Identification of Chemical Constituents in Hericium erinaceus Based on LC-MS/MS Metabolomics. J. Food Qual. 2021, 2021, 5560626. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, I.C.; Lin, T.W.; Chen, W.P.; Lee, L.Y.; Chen, C.C.; Kuo, C.F. Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules 2019, 24, 1624. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Ratto, D.; Priori, E.C.; Savino, E.; Bottone, M.G.; Rossi, P. Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology 2023, 12, 196. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Y.J.; Hsu, C.H.; Lin, Y.H.; Chen, P.T.; Kuo, T.H.; Ho, C.T.; Chen, H.H.; Huang, S.J.; Chiu, H.C.; et al. Erinacine S from Hericium erinaceus mycelium promotes neuronal regeneration by inducing neurosteroids accumulation. J. Food Drug Anal. 2023, 31, 32–54. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Mizuno, T. Hericenone A and B as cytotoxic principles from the mushroom Hericium erinaceum. Tetrahedron Lett. 1990, 31, 373–376. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Shinba, K.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry 1992, 32, 175–178. [Google Scholar] [CrossRef]
- Ueda, K.; Tsujimori, M.; Kodani, S.; Chiba, A.; Kubo, M.; Masuno, K.; Sekiya, A.; Nagai, K.; Kawagishi, H. An endoplasmic reticulum (ER) stress-suppressive compound and its analogues from the mushroom Hericium erinaceum. Bioorg. Med Chem. 2008, 16, 9467–9470. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Hong, S.M.; Khan, Z.; Lee, S.K.; Vishwanath, M.; Turk, A.; Yeon, S.W.; Jo, Y.H.; Lee, D.H.; Lee, J.K.; et al. Neurotrophic isoindolinones from the fruiting bodies of Hericium erinaceus. Bioorg. Med. Chem. Lett. 2021, 31, 127714. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Song, S.B.; Shim, S.H.; Kim, Y.H. Sterol fatty acid esters from the mushroom Hericium erinaceum and their PPAR transactivational effects. J. Nat. Prod. 2014, 77, 2611–2618. [Google Scholar] [CrossRef]
- Mizuno, T. Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. and its medicinal utilization. Int. J. Med. Mushrooms 1999, 1, 105–119. [Google Scholar] [CrossRef]
- Jia, L.M.; Liu, L.; Dong, Q.; Fang, J.N. Structural investigation of a novel rhamnoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Carbohydr. Res. 2004, 339, 2667–2671. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Zhang, J.S.; Tang, Q.J.; Jia, W.; Yang, Y.; Liu, Y.F.; Fan, J.M.; Pan, Y.J. Structural elucidation of a novel fucogalactan that contains 3-O-methyl rhamnose isolated from the fruiting bodies of the fungus, Hericium erinaceus. Carbohydr. Res. 2006, 341, 645–649. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Keong, C.Y.; Amini Abdul Rashid, B.; Swee Ing, Y.; Ismail, Z. Quantification and identification of polysaccharide contents in Hericium erinaceus. Nutr. Food Sci. 2007, 37, 260–271. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Hong, Q.; Zhang, X.; Liu, Z.; Zhang, T. Polysaccharides from Hericium erinaceus Fruiting Bodies: Structural Characterization, Immunomodulatory Activity and Mechanism. Nutrients 2022, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ding, X.; Hou, W. Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus. Mol. Med. Rep. 2015, 11, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Lee, L.Y.; Tzeng, T.T.; Chen, W.P.; Chen, Y.P.; Shiao, Y.J.; Chen, C.C. Neurohealth Properties of Hericium erinaceus Mycelia Enriched with Erinacines. Behav. Neurol. 2018, 2018, 5802634. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, W.P.; Chen, Y.P.; Lee, L.Y.; Tsai, Y.T.; Chen, C.C. Acute and developmental toxicity assessment of erincine A-enriched Hericium erinaceus mycelia in Sprague-Dawley rats. Drug Chem. Toxicol. 2018, 41, 459–464. [Google Scholar] [CrossRef]
- Ma, B.J.; Zhou, Y.; Li, L.Z.; Li, H.M.; Gao, Z.M.; Ruan, Y. A new cyathanexyloside from the mycelia of Hericium erinaceum. Z. Naturforschung B 2008, 63, 1241–1242. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of Metabolites in Italian Hericium erinaceus Mycelium, Primordium, and Sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef]
- Kawagishi, H.; Masui, A.; Tokuyama, S.; Nakamura, T. Erinacines J and K from the mycelia of Hericium erinaceum. Tetrahedron 2006, 62, 8463–8466. [Google Scholar] [CrossRef]
- Kenmoku, H.; Sassa, T.; Kato, N. Isolation of erinacine P, a new parental metabolite of cyathane-xylosides, from Hericium erinaceum and its biomimetic conversion into erinacines A and B. Tetrahedron Lett. 2000, 41, 4389–4393. [Google Scholar] [CrossRef]
- Kenmoku, H.; Shimai, T.; Toyomasu, T.; Kato, N.; Sassa, T. Erinacine Q, a new erinacine from Hericium erinaceum and its biosynthetic route to erinacine C in the Basidiomycete. Biosci. Biotechnol. Biochem. 2002, 66, 571–575. [Google Scholar] [CrossRef]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2019, 106, 691–697. [Google Scholar] [CrossRef]
- Williamson, R.D.; McCarthy, F.P.; Manna, S.; Groarke, E.; Kell, D.B.; Kenny, L.C.; McCarthy, C.M. L-(+)-Ergothioneine Significantly Improves the Clinical Characteristics of Preeclampsia in the Reduced Uterine Perfusion Pressure Rat Model. Hypertension 2020, 75, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-Y.; Chen, C.-L.; Liao, J.-W.; Ou, H.-C.; Tsai, M. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem. Toxicol. 2010, 48, 3492–3499. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-Y.; Lin, H.-C.; Chen, C.-L.; Wu, J.-H.; Liao, J.-W.; Hu, M.-L. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free Radic. Res. 2014, 48, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Hamada, Y.; Yasumoto, T.; Hashino, Y.; Masuyama, A.; Nagai, K. Total syntheses and endoplasmic reticulum stress suppressive activities of hericenes A-C and their derivatives. Tetrahedron Lett. 2018, 59, 1733–1736. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tamura, T.; Koshishiba, M.; Yasumoto, T.; Shimizu, S.; Kintaka, T.; Nagai, K. Total Synthesis, Structure Revision, and Neuroprotective Effect of Hericenones C-H and Their Derivatives. J. Org. Chem. 2021, 86, 2602–2620. [Google Scholar] [CrossRef]
- Olariu, A.; Yamada, K.; Nabeshima, T. Amyloid pathology and protein kinase C (PKC): Possible therapeutics effects of PKC activators. J. Pharmacol. Sci. 2005, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, B.K.; Park, H.S.; Shim, J.K.; Kim, S.U.; Lee, S.H.; Baik, E.J.; Moon, C.H. Activation of protein kinase C-y attenuates kinate-induced cell death of cortical neurons. Neuroreport 2005, 16, 741–744. [Google Scholar] [CrossRef]
- Martelli, A.M.; Mazzotti, G.; Capitani, S. Nuclear protein kinase C isoforms and apoptosis. Eur. J. Histochem. 2004, 48, 89–94. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Zhou, W.; Shim, S.H.; Kim, Y.H. Erinacene D, a new aromatic compound from Hericium erinaceum. J. Antibiot. 2014, 67, 727–729. [Google Scholar] [CrossRef]
- Zan, X.; Cui, F.; Li, Y.; Yang, Y.; Wu, D.; Sun, W.; Ping, L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2015, 76, 242–253. [Google Scholar] [CrossRef]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Chen, S.-C.; Chang, J.-C.; Lin, W.-Y.; Chen, C.-C.; Li, C.-C.; Hsieh, M.; Chen, H.-W.; Chang, T.-Y.; Liu, C.-S.; et al. The protective effect of erinacine A–enriched Hericium erinaceus mycelium ethanol extract on oxidative Stress–Induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type 3. Free Radic. Biol. Med. 2023, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-Induced Oxidative Damage and LPS-Induced Inflammation in HT22 Hippocampal Neurons and BV2 Microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Kolotushkina, E.V.; Moldavan, M.G.; Voronin, K.Y.; Skibo, G.G. The influence of Hericium erinaceus extract on myelination process in vitro. Fiziol. Zhurnal 2003, 49, 38–45. [Google Scholar]
- Noh, H.J.; Yang, H.H.; Kim, G.S.; Lee, S.E.; Lee, D.Y.; Choi, J.H.; Kim, S.Y.; Lee, E.S.; Ji, S.H.; Kang, K.S.; et al. Chemical constituents of Hericium erinaceum associated with the inhibitory activity against cellular senescence in human umbilical vascular endothelial cells. J. Enzym. Inhib. Med. Chem. 2015, 30, 934–940. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, W.Y.; Lee, H.Y. Enhancement of the neuroprotective activity of Hericium erinaceus mycelium co-cultivated with Allium sativum extract. Arch. Physiol. Biochem. 2015, 121, 19–25. [Google Scholar] [CrossRef]
- Raman, J.; Lakshmanan, H.; John, P.A.; Zhijian, C.; Periasamy, V.; David, P.; Naidu, M.; Sabaratnam, V. Neurite outgrowth stimulatory effects of myco synthesized AuNPs from Hericium erinaceus (bull.: Fr.) Pers. on pheochromocytoma (PC-12) cells. Int. J. Nanomed. 2015, 10, 5853–5863. [Google Scholar]
- Amara, I.; Scuto, M.; Zappalà, A.; Ontario, M.L.; Petralia, A.; Abid-Essefi, S.; Maiolino, L.; Signorile, A.; Trovato Salinaro, A.; Calabrese, V. Hericium erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2138. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, E.K.; Hwang, J.W.; Kim, C.G.; Choi, D.K.; Lim, B.O.; Moon, S.H.; Jeon, B.T.; Park, P.J. Neuroprotective effect of Hericium erinaceum against oxidative stress on PC12 cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 283–289. [Google Scholar] [CrossRef]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, J.E.; Jeong, K.H.; Lim, S.C.; Kim, S.Y.; Cho, K.O. The Neuroprotective Effect of Hericium erinaceus Extracts in Mouse Hippocampus after Pilocarpine-Induced Status Epilepticus. Int. J. Mol. Sci. 2019, 20, 859. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Liao, E.C.; Chiang, W.C.; Wang, K.L. Antioxidative Activities of Micronized Solid-State Cultivated Hericium erinaceus Rich in Erinacine A against MPTP-Induced Damages. Molecules 2023, 28, 3386. [Google Scholar] [CrossRef] [PubMed]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef]
- Rodriguez, M.N.; Lippi, S.L.P. Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model. Behav. Sci. 2022, 12, 235. [Google Scholar] [CrossRef]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef]
- Kasahara, K.; Kaneko, N.; Shimizu, K. Effects of Hericium erinaceum on aged patients with impairment. Gunma Med. Suppl. 2001, 76, 77–78. (In Japanese) [Google Scholar]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef]
- Inanaga, K. Marked improvement of neurocognitive impairment after treatment with compounds from Hericium erinaceum: A case study of recurrent depressive disorder. Pers. Med. Universe 2014, 3, 46–48. [Google Scholar] [CrossRef]
- Okamura, H.; Anno, N.; Tsuda, A.; Inokuchi, T.; Uchimura, N.; Inanaga, K. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Pers. Med. Universe 2015, 4, 76–78. [Google Scholar] [CrossRef]
- Tzeng, T.-T.; Chen, C.-C.; Chen, C.-C.; Tsay, H.-J.; Lee, L.-Y.; Chen, W.-P.; Shen, C.-C.; Shiao, Y.-J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Grygansky, A.P.; Moldavan, M.G.; Kolotushkina, O.; Kirchhoff, B.; Skibo, G.G. Hericium erinaceus (Bull.: Fr.) Pers. extract effect on nerve cells. Int. J. Med. Mushrooms 2001, 3, 152. [Google Scholar] [CrossRef]
- Kempermann, G. Adult neurogenesis: An evolutionary perspective. Cold Spring Harb. Perspect. Biol. 2016, 8, a018986. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. Correction to Hericium erinaceus (Lion’s Mane) Mushroom Extracts Inhibit Metastasis of Cancer Cells to the Lung in CT-26 Colon Cancer-Transplanted Mice. J. Agric. Food Chem. 2013, 61, 4898–4904. [Google Scholar] [CrossRef]
- Wong, K.H.; Naidu, M.; David, R.P.; Abdulla, M.A.; Kuppusamy, U.R. Functional recovery enhancement following Injury to rodent peroneal nerve by lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) . Int. J. Med. Mushrooms 2009, 11, 225–236. [Google Scholar] [CrossRef]
- Samuels, B.A.; Hen, R. Neurogenesis and affective disorders. Eur. J. Neurosci. 2011, 33, 1152–1159. [Google Scholar] [CrossRef]
- Malinski, T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimer’s Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. Phenolic and flavonoid content in Hericium erinaceus, Ganoderma lucidum, and Agrocybe aegerita under selenium addition. Acta Aliment. 2016, 45, 300–308. [Google Scholar] [CrossRef]
- Lew, S.Y.; Yow, Y.Y.; Lim, L.W.; Wong, K.H. Antioxidant-mediated protective role of Hericium erinaceus (Bull.: Fr.) Pers. against oxidative damage in fibroblasts from Friedreich’s ataxia patient. Food Sci. Technol. 2020, 40, 264–272. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of ageing. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 464238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-C.; Chen, Y.-L.; Lee, L.-Y.; Chen, W.-P.; Tsai, Y.-T.; Chen, C.-S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, H.; Raman, J.; David, P.; Wong, K.-H.; Naidu, M.; Sabaratnam, V. Haematological, biochemical and histopatho-logical aspects of Hericium erinaceus ingestion in a rodent model: A sub-chronic toxi-cological assessment. J. Ethnopharmacol. 2016, 194, 1051–1059. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, C.S.; Yang, M.F.; Chen, S.; Soni, M.; Mahadevan, B. Subchronic toxicity and genotoxicity studies of Hericium erinaceus β-glucan extract preparation. Curr. Res. Toxicol. 2022, 3, 100068. [Google Scholar] [CrossRef]
- Li, I.C.; Chen, Y.L.; Chen, W.P.; Lee, L.-Y.; Tsai, Y.-T.; Chen, C.-C.; Chen, C.-S. Genotoxicity profile of erinacine A-enriched Hericium erinaceus mycelium. Toxicol. Rep. 2014, 1, 1195–1201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).