KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study
Abstract
:1. Introduction
2. Aim of Study
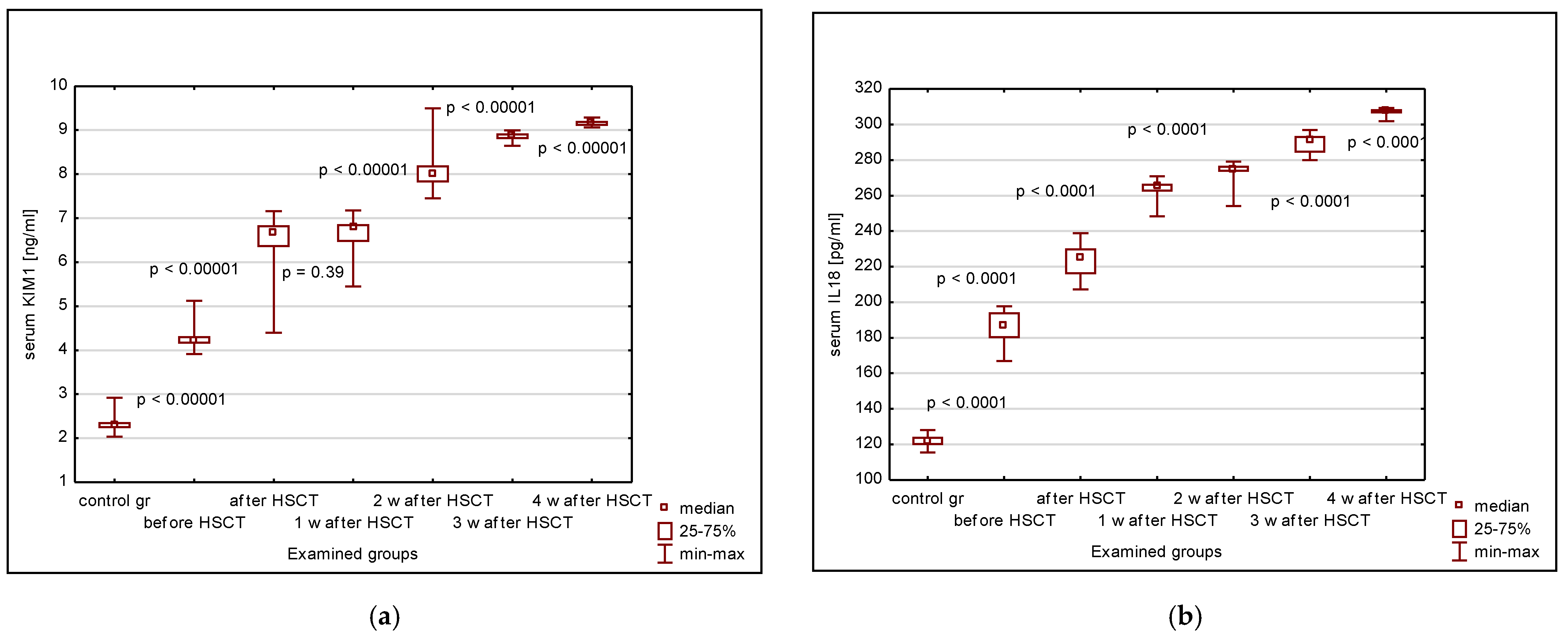
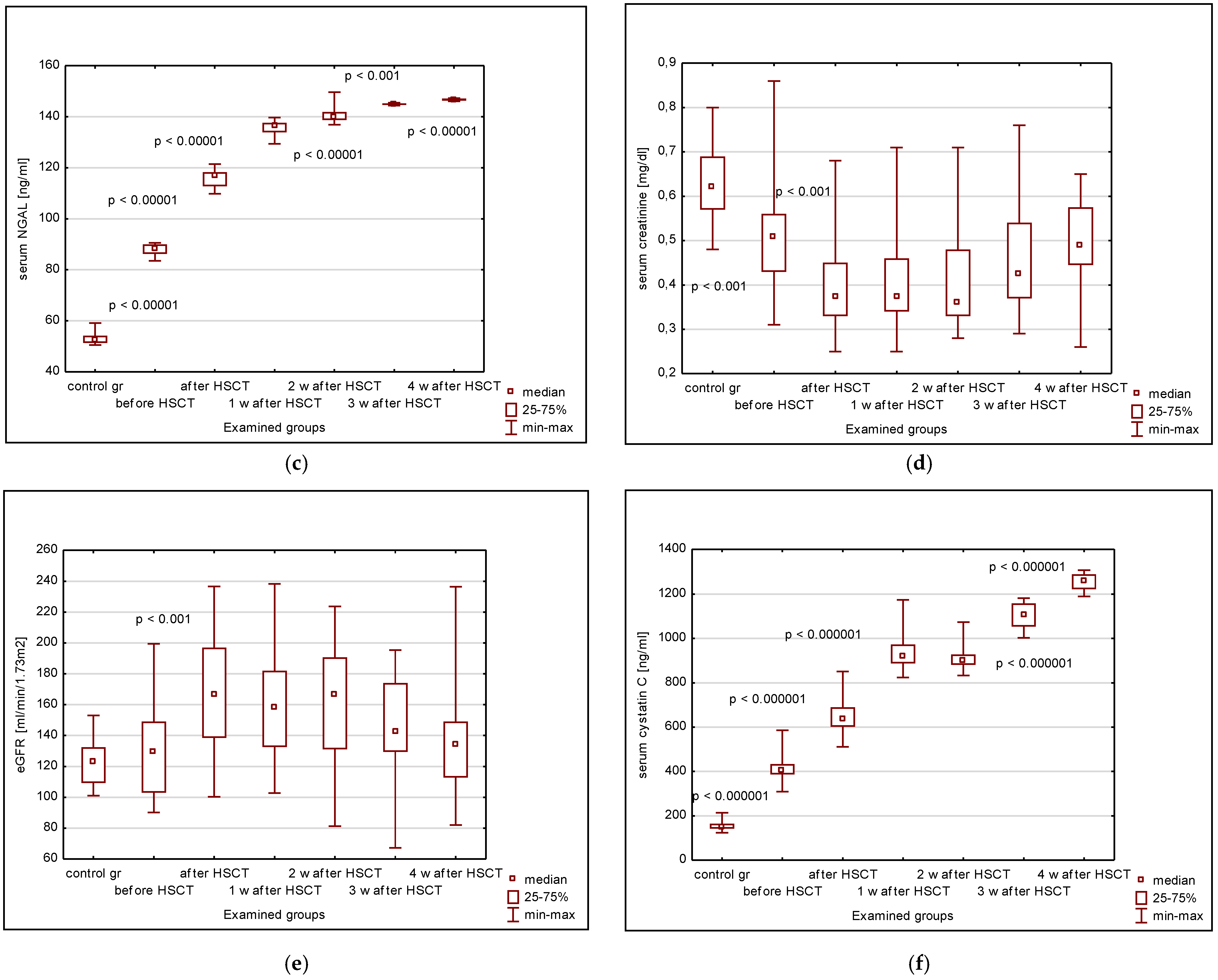
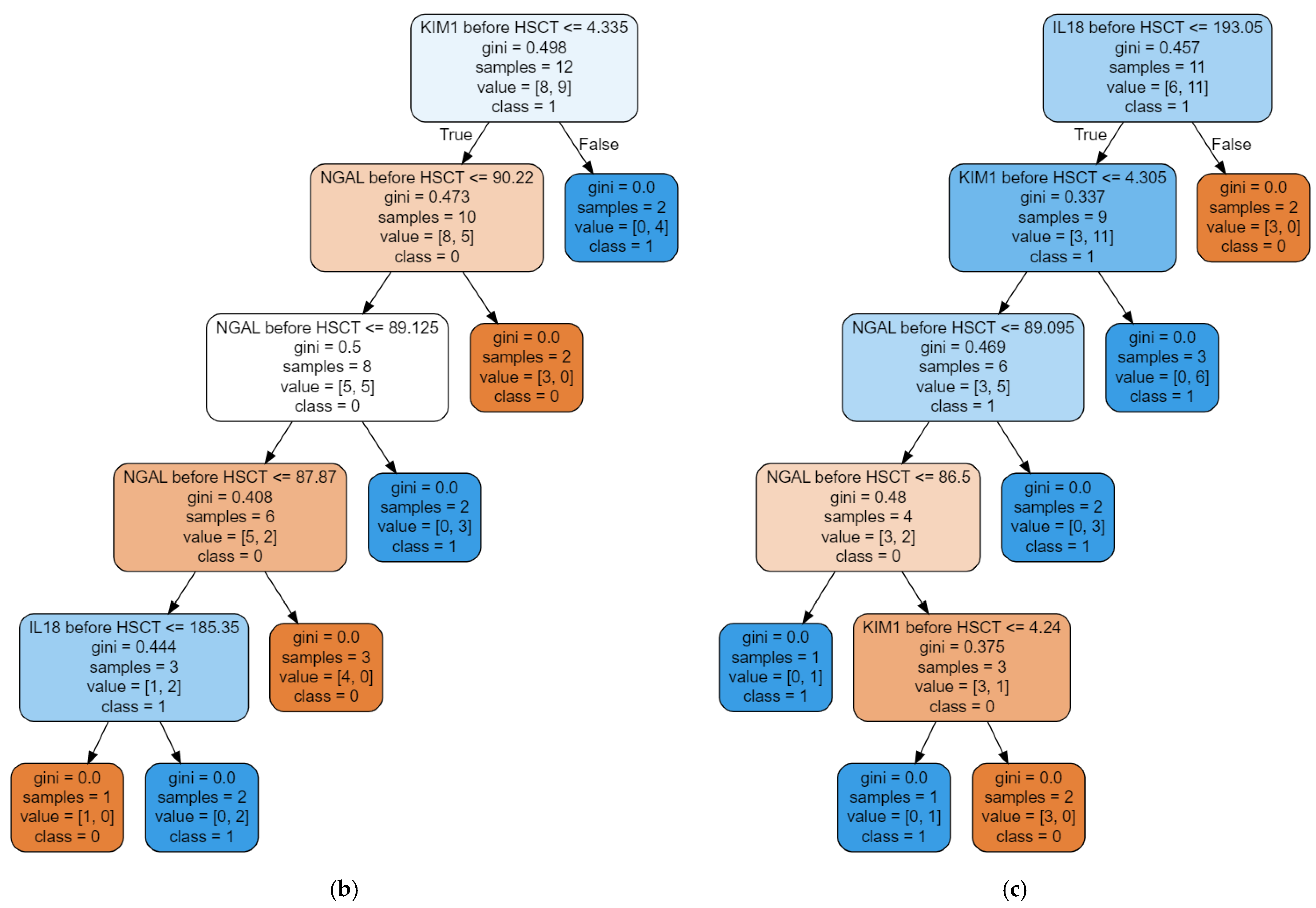
3. Results
3.1. Serum KIM-1, IL-18, NGAL, Cystatin C, Creatinine, eGFR
3.2. The Random Forest Classifier (RFC) Model
4. Discussion
5. Materials and Methods
5.1. Study Design and Settings
5.2. Methods
5.3. Statistical Analysis
5.4. Building the Random Forest Classifier Model
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didsbury, M.S.; Mackie, F.E.; Kennedy, S.E. A systematic review of acute kidney injury in pediatric allogeneic hemato-poietic stem cell recipients. Pediatr. Transplant. 2015, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Kizilbash, S.J.; Kashtan, C.E.; Chavers, B.M.; Cao, Q.; Smith, A.R. Acute Kidney Injury and the Risk of Mortality in Children Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Herrera, N.; Krishnappa, V.; Sethi, S.K.; Deep, A.; Kao, W.; Bunchman, T.; Abu-Arja, R. Hematopoietic stem cell transplantation and acute kidney injury in children: A comprehensive review. Pediatr. Transplant. 2017, 21. [Google Scholar] [CrossRef]
- Koh, K.-N.; Sunkara, A.; Kang, G.; Sooter, A.; Mulrooney, D.A.; Triplett, B.; Onder, A.M.; Bissler, J.; Cunningham, L.C. Acute Kidney Injury in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation: Incidence, Risk Factors, and Outcomes. Biol. Blood Marrow Transplant. 2018, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Shan, J.; Yi, L.; Xin, Y.; Zhong, Z.; Xu, H. Risk factors for acute kidney injury in pediatric patients after hematopoietic stem cell transplantation: A systematic review and meta-analysis. Pediatr. Nephrol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Kakegawa, D.; Miwa, S.; Umeda, C.; Takemasa, Y.; Tokunaga, A.; Kawakami, Y.; Ito, A. Independent risk factors and long-term outcomes for acute kidney injury in pediatric patients undergoing hematopoietic stem cell trans-plantation: A retrospective cohort study. BMC Nephrol. 2020, 21, 373. [Google Scholar] [CrossRef]
- Hierlmeier, S.; Eyrich, M.; Wölfl, M.; Schlegel, P.-G.; Wiegering, V. Early and late complications following hematopoietic stem cell transplantation in pediatric patients – A retrospective analysis over 11 years. PLoS ONE 2018, 13, e0204914. [Google Scholar] [CrossRef]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef]
- Daraskevicius, J.; Azukaitis, K.; Dziugeviciute-Tupko, J.; Peciulyte, M.; Planciunaite, R.; Vaitkeviciene, G.; Rascon, J.; Jankauskiene, A. Phenotypes and Baseline Risk Factors of Acute Kidney Injury in Children After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Kwatra, N.S.; Meany, H.J.; Ghelani, S.J.; Zahavi, D.; Pandya, N.; Majd, M. Glomerular hyperfiltration in children with cancer: Prevalence and a hypothesis. Pediatr. Radiol. 2016, 47, 221–226. [Google Scholar] [CrossRef]
- Cortinovis, M.; Perico, R.; Ruggenenti, P.; Remuzzi, A.; Remuzzi, G. Glomerular hyperfiltration. Nat. Rev. Nephrol. 2022, 18, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, F.R.; Rodrigues, K.E.; Belisario, A.R.; Simoes-e-Silva, A.C. Glomerular hyperfiltration and beta-2 microglobulin as biomarkers of incipient renal dysfunction in cancer survivors. Future Sci. OA 2018, 4, FSO333. [Google Scholar] [CrossRef]
- Filler, G.; Lee, M. Educational review: Measurement of GFR in special populations. Pediatr. Nephrol. 2017, 33, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI—An emerging syndrome with important consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Kellum, J.A.; Haase, M. Subclinical AKI is still AKI. Crit. Care 2012, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.K.; Wong, H.R.; Krawczeski, C.D.; Wheeler, D.S.; Manning, P.B.; Chawla, L.S.; Devarajan, P.; Goldstein, S.L. Combining Functional and Tubular Damage Biomarkers Improves Diagnostic Precision for Acute Kidney Injury After Cardiac Surgery. J. Am. Coll. Cardiol. 2014, 64, 2753–2762. [Google Scholar] [CrossRef]
- Benoit, S.W.; Dixon, B.P.; Goldstein, S.L.; Bennett, M.R.; Lane, A.; Lounder, D.T.; Rotz, S.J.; Gloude, N.J.; Lake, K.E.; Litts, B.; et al. A novel strategy for identifying early acute kidney injury in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019, 54, 1453–1461. [Google Scholar] [CrossRef]
- Musiał, K.; Augustynowicz, M.; Miśkiewicz-Migoń, I.; Kałwak, K.; Ussowicz, M.; Zwolińska, D. Clusterin as a New Marker of Kidney Injury in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation—A Pilot Study. J. Clin. Med. 2020, 9, 2599. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, B.; Tang, F.; Li, X.; Xiao, D. Predictive value of neutrophil gelatinase-associated lipocalin in children with acute kidney injury: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1147033. [Google Scholar] [CrossRef]
- Yousefifard, M.; Ahmadzadeh, K.; Toloui, A.; Ahmadzadeh, H.; Neishaboori, A.M.; Alavi, S.N.R.; Ghelichkhani, P.; Tavallaei, M.J.; Safari, S.; Ataei, N.; et al. Assessing the value of serum and urinary interleukins for diagnosis of acute kidney injury in children and adolescents: A systematic review and meta-analysis. Pract. Lab. Med. 2022, 28, e00262. [Google Scholar] [CrossRef] [PubMed]
- Fazel, M.; Sarveazad, A.; Ali, K.M.; Yousefifard, M.; Hosseini, M. Accuracy of Urine Kidney Injury Molecule-1 in Predicting Acute Kidney Injury in Children; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e44. [Google Scholar] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, Y.; Huang, M.; Feng, Z. Machine learning for acute kidney injury: Changing the traditional disease prediction mode. Front. Med. 2023, 10, 1050255. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Pattharanitima, P.; Kattah, A.G.; Mao, M.A.; Keddis, M.T.; Dillon, J.J.; Kaewput, W.; Tangpanithandee, S.; Krisanapan, P.; Qureshi, F.; et al. Explainable Preoperative Automated Machine Learning Prediction Model for Cardiac Surgery-Associated Acute Kidney Injury. J. Clin. Med. 2022, 11, 6264. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, Y.; Li, S.; Guo, F.; Xue, H.; Jiang, L.; Wang, Z.; Zhang, C.; Xie, W.; Zhu, F. Predicting renal function recovery and short-term reversibility among acute kidney injury patients in the ICU: Comparison of machine learning methods and conventional regression. Ren. Fail. 2022, 44, 1327–1338. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sutherland, S.M.; Byrnes, J.J.; Kothari, M.; Longhurst, C.A.; Dutta, S.; Garcia, P.; Goldstein, S.L. AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin. J. Am. Soc. Nephrol. 2015, 10, 554–561. [Google Scholar] [CrossRef]
- Zou, C.; Wang, C.; Lu, L. Advances in the study of subclinical AKI biomarkers. Front. Physiol. 2022, 13, 960059. [Google Scholar] [CrossRef]
- Meena, J.; Thomas, C.C.; Kumar, J.; Mathew, G.; Bagga, A. Biomarkers for prediction of acute kidney injury in pediatric patients: A systematic review and meta-analysis of diagnostic test accuracy studies. Pediatr. Nephrol. 2023, 38, 3241–3251. [Google Scholar] [CrossRef]
- Albert, C.; Zapf, A.; Haase, M.; Röver, C.; Pickering, J.W.; Albert, A.; Bellomo, R.; Breidthardt, T.; Camou, F.; Chen, Z.; et al. Neutrophil Gelatinase-Associated Lipocalin Measured on Clinical Laboratory Platforms for the Prediction of Acute Kidney Injury and the Associated Need for Dialysis Therapy: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Elger, A.; Elitok, S.; Kettritz, R.; Nickolas, T.L.; Barasch, J.; Luft, F.C.; Schmidt-Ott, K.M. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011, 80, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Washburn, K.K.; Zappitelli, M.; Arikan, A.A.; Loftis, L.; Yalavarthy, R.; Parikh, C.R.; Edelstein, C.L.; Goldstein, S.L. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol. Dial. Transplant. 2007, 23, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, H.; Jiao, P.; Jiang, L.; Geng, J.; Yang, Q.; Liao, R.; Su, B. The value of urinary interleukin-18 in predicting acute kidney injury: A systematic review and meta-analysis. Ren. Fail. 2022, 44, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, J.; Zhan, J.; Yi, F.; Fan, X.; Wei, Y.; Zhang, W. The value of serum cystatin C in early evaluation of renal insuf-ficiency in patients undergoing chemotherapy: A systematic review and meta-analysis. Cancer Chemother. Pharmacol. 2019, 83, 561–571. [Google Scholar] [CrossRef]
- Fu, R.; Tajima, S.; Suetsugu, K.; Watanabe, H.; Egashira, N.; Masuda, S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharmacol. Sin. 2018, 40, 151–159. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Chen, Y.; Wu, H.; Zhang, S.; Chen, X. Prediction Value of Serum NGAL in the Diagnosis and Prognosis of Experimental Acute and Chronic Kidney Injuries. Biomolecules 2020, 10, 981. [Google Scholar] [CrossRef]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.H.; Kellum, J.A.; Chawla, L.S.; Cruz, D.N.; Ince, C.; Okusa, M.D. ADQI 10 workgroup Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014, 85, 513–521. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.-J.; Cheng, C.-T.; Chang, C.-H. Does Artificial Intelligence Make Clinical Decision Better? A Review of Artificial Intelligence and Machine Learning in Acute Kidney Injury Prediction. Healthcare 2021, 9, 1662. [Google Scholar] [CrossRef]
- Kellum, J.A.; Bihorac, A. Artificial intelligence to predict AKI: Is it a breakthrough? Nat. Rev. Nephrol. 2019, 15, 663–664. [Google Scholar] [CrossRef]
- Low, S.; Vathsala, A.; Murali, T.M.; Pang, L.; MacLaren, G.; Ng, W.-Y.; Haroon, S.; Mukhopadhyay, A.; Lim, S.-L.; Tan, B.-H.; et al. Electronic health records accurately predict renal replacement therapy in acute kidney injury. BMC Nephrol. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomašev, N.; Glorot, X.; Rae, J.W.; Zielinski, M.; Askham, H.; Saraiva, A.; Mottram, A.; Meyer, C.; Ravuri, S.; Protsyuk, I.; et al. A clinically ap-plicable approach to continuous prediction of future acute kidney injury. Nature 2019, 572, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feng, T.; Thapa-Chhetry, B.; Cho, B.G.; Shum, T.; Inwald, D.P.; Newth, C.J.L.; Vaidya, V.U. Machine learning model for early prediction of acute kidney injury (AKI) in pediatric critical care. Crit. Care 2021, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ishwaran, H. Random forests for genomic data analysis. Genomics 2012, 99, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Cabrera, J. Enriched Random Forest for High Dimensional Genomic Data. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 2817–2828. [Google Scholar] [CrossRef]
- Acharjee, A.; Larkman, J.; Xu, Y.; Cardoso, V.R.; Gkoutos, G.V. A random forest based biomarker discovery and power analysis framework for diagnostics research. BMC Med Genom. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Tilde]Oz, A.M.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New Equations to Estimate GFR in Children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Cachat, F.; Combescure, C.; Cauderay, M.; Girardin, E.; Chehade, H. A Systematic Review of Glomerular Hyperfiltration Assessment and Definition in the Medical Literature. Clin. J. Am. Soc. Nephrol. 2015, 10, 382–389. [Google Scholar] [CrossRef]
- Iduoriyekemwen, N.; Ibadin, M.; Aikhionbare, H.; Idogun, S.; Abiodun, M. Glomerular hyperfiltration in excess weight adolescents. Niger. J. Clin. Pract. 2019, 22, 842–848. [Google Scholar] [CrossRef]
- Menze, B.H.; Kelm, B.M.; Masuch, R.; Himmelreich, U.; Bachert, P.; Petrich, W.; Hamprecht, F.A. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinform. 2009, 10, 213. [Google Scholar] [CrossRef]


| Type of Renal Dysfunction | Endurance of Dysfunction | Stages of AKI/AKD | Serum Creatinine | Markers of Damage |
|---|---|---|---|---|
| AKI | ≤7 days | Subclinical AKI | Normal value | Biomarker positive |
| Stage 1 | 1.5-fold increase | Biomarker positive or Biomarker negative ± | ||
| Stage 2 | 2-fold increase | |||
| Stage 3 | 3-fold increase | |||
| AKD | >7 days, but <3 months | Stage 0A | Return to baseline values | No evidence of injury Risk of long-term events |
| Stage 0B | Return to baseline values | Ongoing kidney damage/injury Loss of renal reserve | ||
| Stage 0C | Increase < 1.5-fold | Ongoing kidney damage/injury | ||
| Stage 1 | 1.5-fold increase | Ongoing kidney damage/injury | ||
| Stage 2 | 2-fold increase | Ongoing kidney damage/injury | ||
| Stage 3 | 3-fold increase | Ongoing kidney damage/injury |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musiał, K.; Stojanowski, J.; Miśkiewicz-Bujna, J.; Kałwak, K.; Ussowicz, M. KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 15791. https://doi.org/10.3390/ijms242115791
Musiał K, Stojanowski J, Miśkiewicz-Bujna J, Kałwak K, Ussowicz M. KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study. International Journal of Molecular Sciences. 2023; 24(21):15791. https://doi.org/10.3390/ijms242115791
Chicago/Turabian StyleMusiał, Kinga, Jakub Stojanowski, Justyna Miśkiewicz-Bujna, Krzysztof Kałwak, and Marek Ussowicz. 2023. "KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study" International Journal of Molecular Sciences 24, no. 21: 15791. https://doi.org/10.3390/ijms242115791
APA StyleMusiał, K., Stojanowski, J., Miśkiewicz-Bujna, J., Kałwak, K., & Ussowicz, M. (2023). KIM-1, IL-18, and NGAL, in the Machine Learning Prediction of Kidney Injury among Children Undergoing Hematopoietic Stem Cell Transplantation—A Pilot Study. International Journal of Molecular Sciences, 24(21), 15791. https://doi.org/10.3390/ijms242115791






