Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome
Abstract
1. Introduction
1.1. The Idiopathic Nephrotic Syndrome
1.2. The Similarity of Neuronal Cells to Podocytes
1.3. Brain-Derived Neurotrophic Factor (BDNF)
1.4. Main Aims of the Study
2. Results
2.1. Descriptive Analysis and Comparison—INS Patients and Control Group
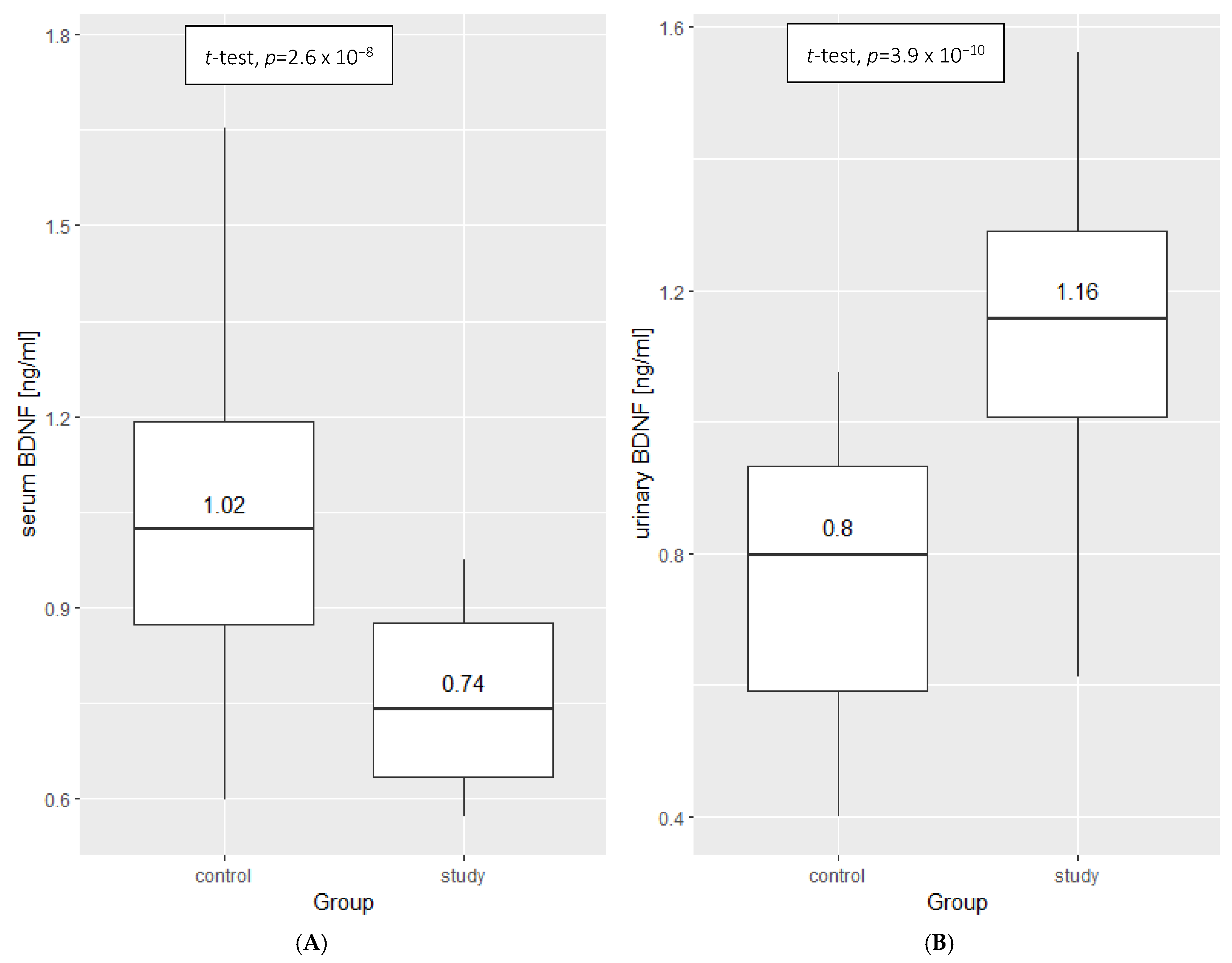
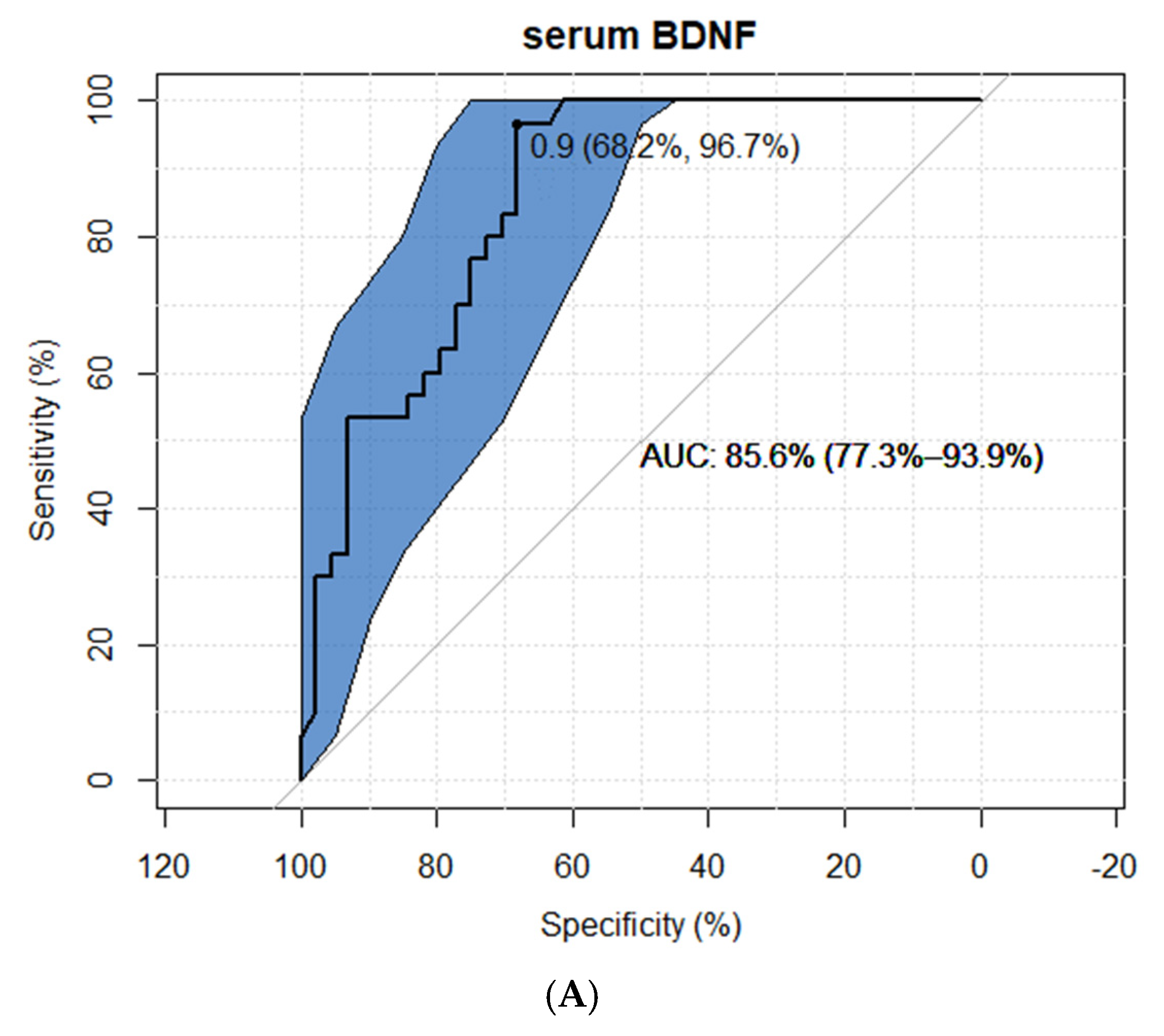
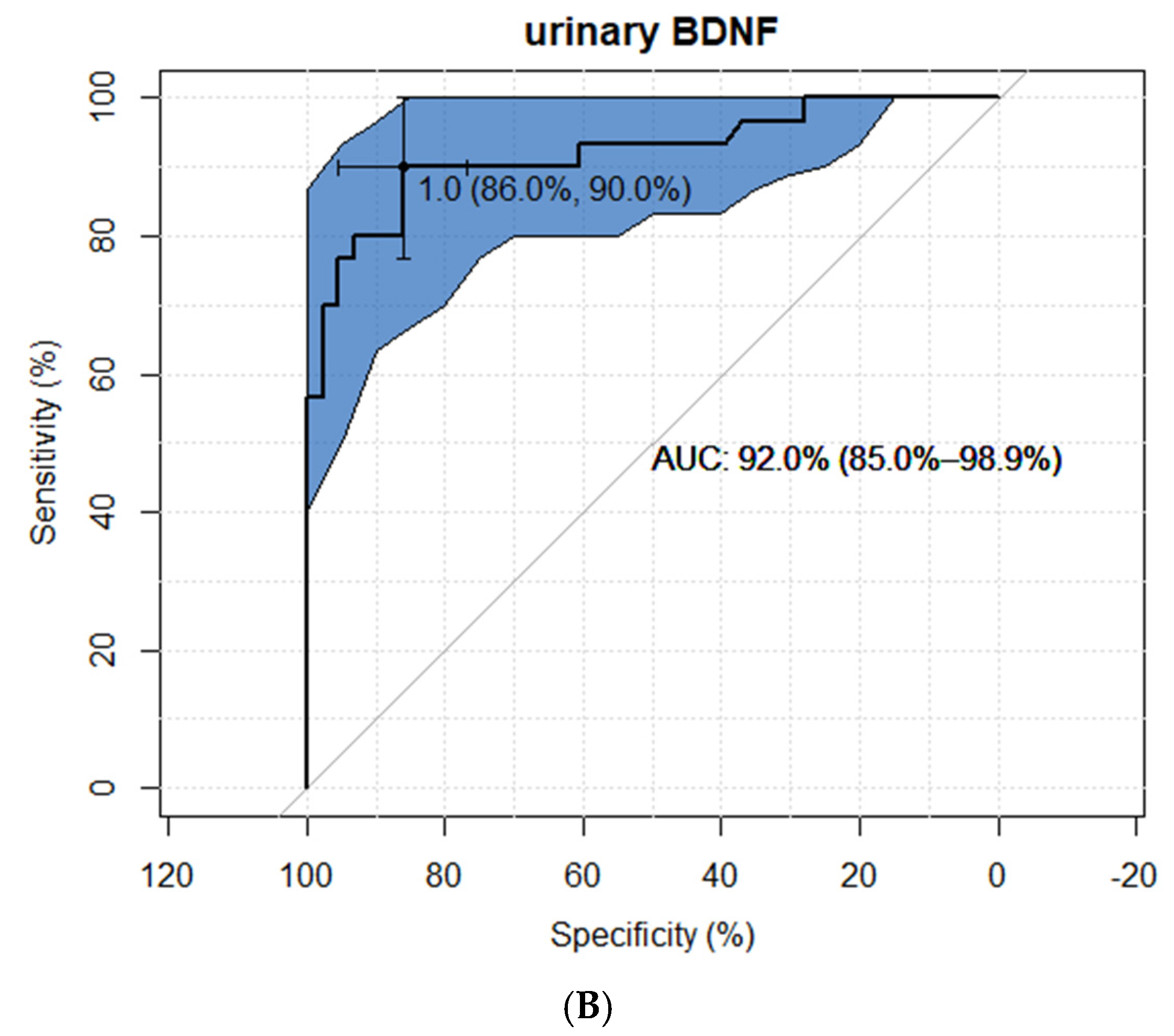
2.2. BDNF Results
3. Discussion
3.1. BDNF as an INS Marker
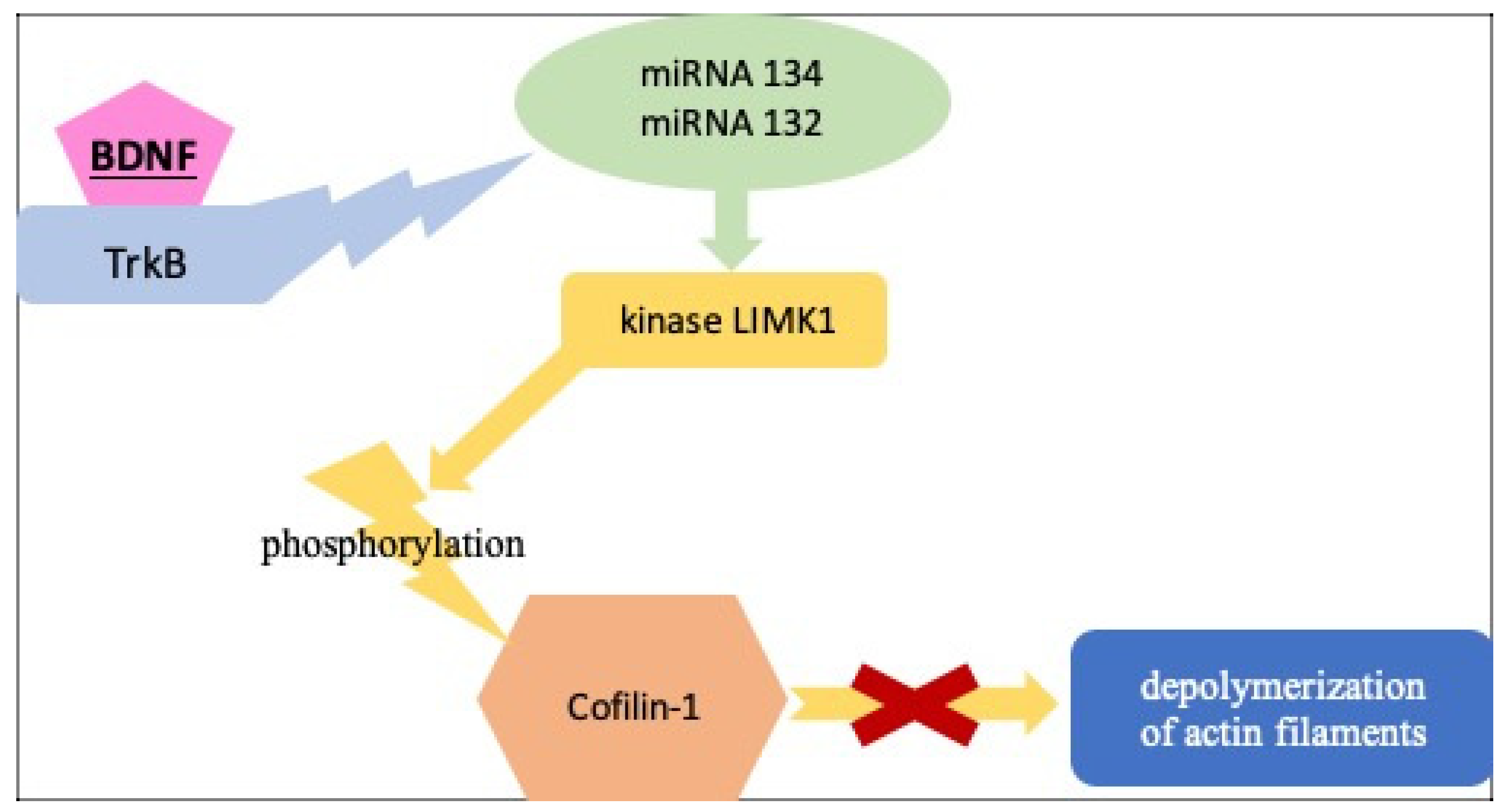
3.2. BDNF as a Potential Therapy for Damaged Podocytes
4. Materials and Methods
4.1. Studied Groups—Characteristics, Laboratory Outcome and Anthropometric Measures
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Downie, M.L.; Gallibois, C.; Parekh, R.S.; Noone, D.G. Nephrotic syndrome in infants and children: Pathophysiology and management. Paediatr. Int. Child Health 2017, 37, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, M.; Bałasz-Chmielewska, I.; Grenda, R.; Musiał, K.; Ogarek, I.; Pańczyk-Tomaszewska, M.; Zachwieja, J.; Żurowska, A. The Polish Society for Pediatric Nephrology (PTNFD) recommendations on the management of children with nephrotic syndrome. Ren. Dis. Transplant. Forum 2022, 15, 36–57. [Google Scholar]
- Koskimies, O.; Vilska, J.; Rapola, J.; Hallman, N. Long-term outcome of primary nephrotic syndrome. Arch. Dis. Child. 1982, 57, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Tarshish, P.; Tobin, J.N.; Bernstein, J.; Edelmann, C.M., Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J. Am. Soc. Nephrol. 1997, 8, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Ruth, E.M.; Kemper, M.J.; Leumann, E.P.; Laube, G.F.; Neuhaus, T.J. Children with steroid-sensitive nephrotic syndrome come of age: Long-term outcome. J. Pediatr. 2005, 147, 202–207. [Google Scholar] [CrossRef]
- Lewis, M.A.; Baildom, E.M.; Davis, N.; Houston, I.B.; Postlethwaite, R.J. Nephrotic syndrome: From toddlers to twenties. Lancet 1989, 33, 255–259. [Google Scholar] [CrossRef]
- Fakhouri, F.; Bocquet, N.; Taupin, P.; Presne, C.; Gagnadoux, M.-F.; Landais, P.; Lesavre, P.; Chauveau, D.; Knebelmann, B.; Broyer, M.; et al. Steroid-sensitive nephrotic syndrome: From childhood to adulthood. Am. J. Kidney Dis. 2003, 41, 550–557. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Pańczyk-Tomaszewska, M.; Roszkowska-Blaim, M.; Wawer, Z.; Bieniaś, B.; Zajączkowska, M.; Pstrusińska, K.K.; Jakubowska, A.; Szczepaniak, M.; Pawlak-Bratkowska, M.; et al. Long-term outcomes in idiopathic nephrotic syndrome: From childhood to adulthood. Clin. Nephrol. 2014, 81, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, A.C.; Oliveira, E.A.; Froes, B.P.; Faria, L.D.C.; Pinto, J.S.; Nogueira, M.M.I.; Lima, G.O.; Resende, P.I.; Assis, N.S.; Silva, A.C.S.E.; et al. A predictive model of progressive chronic kidney disease in idiopathic nephrotic syndrome. Pediatr. Nephrol. 2015, 30, 2011–2020. [Google Scholar] [CrossRef]
- Gipson, D.S.; Chin, H.; Presler, T.P.; Jennette, C.; de Ferris, M.E.D.-G.; Massengill, S.; Gibson, K.; Thomas, D.B. Differential risk of remission and ESRD in childhood FSGS. Pediatr. Nephrol. 2006, 21, 344–349. [Google Scholar] [CrossRef]
- Pavenstädt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Mundel, P.; Kriz, W. Structure and function of podocytes: An update. Anat. Embryol. 1995, 192, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Armelloni, S.; Zennaro, C.; Wei, C.; Corbelli, A.; Ikehata, M.; Berra, S.; Giardino, L.; Mattinzoli, D.; Watanabe, S.; et al. BDNF repairs podocyte damage by microRNA-mediated increase of actin polymerization. J. Pathol. 2015, 235, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Putaala, H.; Soininen, R.; Kilpeläinen, P.; Wartiovaara, J.; Tryggvason, K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum. Mol. Genet. 2001, 10, 1–8. [Google Scholar] [CrossRef]
- Deller, T.; Mundel, P.; Frotscher, M. Potential role of synaptopodin in spine motility by coupling actin to the spine apparatus. Hippocampus 2000, 10, 569–581. [Google Scholar] [CrossRef]
- Sala, C.; Segal, M. Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 2014, 94, 141–188. [Google Scholar] [CrossRef]
- Reiser, J.; Sever, S. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 2013, 64, 357–366. [Google Scholar] [CrossRef]
- Greka, A.; Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012, 74, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sobue, G.; Yamamoto, K.; Terao, S.; Mitsuma, T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, TrkA, TrkB, and TrkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem. Res. 1996, 21, 929–938. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, P.; Arcamone, N.; Lucini, C.; Simeoli, M.P.; Castaldo, L.; Gargiulo, G. TRK neurotrophin receptor-like proteins in the kidney of frog (Rana esculenta) and lizard (Podarcis sicula): An immunohistochemical study. Anat. Embryol. 2004, 207, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, E.; Koizumi, H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am. J. Pathol. 1996, 148, 1807–1818. [Google Scholar]
- García-Suárez, O.; González-Martínez, T.; Germana, A.; Monjil, D.F.; Torrecilla, J.R.; Laurà, R.; Silos-Santiago, I.; Guate, J.L.; Vega, J.A. Expression of TrkB in the murine kidney. Microsc. Res. Tech. 2006, 69, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Clarke, D.B.; Kittlerova, P.; Bray, G.M.; Aguayo, A.J. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J. Neurosci. 1996, 16, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Horch, H.W.; Katz, L.C. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat. Neurosci. 2002, 5, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Asanuma, K.; Yanagida-Asanuma, E.; Kim, K.; Mundel, P. Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007, 17, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Theriot, J.A. Regulation of the actin cytoskeleton in living cells. Semin. Cell Dev. Biol. 1994, 5, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013, 25, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Verma, R.; Cook, L.; Soofi, A.; Venkatareddy, M.; George, B.; Mizuno, K.; Gurniak, C.; Witke, W.; Holzman, L. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J. Biol. Chem. 2010, 285, 22676–22688. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.; Olson, M.F. LIM kinases: Function, regulation and association with human disease. J. Mol. Med. 2007, 85, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Suh, J.S.; Cho, B.S. Linkage and association study of neurotrophins and their receptors as novel susceptibility genes for childhood IgA nephropathy. Pediatr. Res. 2011, 69, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Holtzman, D.M. BDNF protects the neonatal brain from hypoxic–ischemic injury in vivo via the ERK pathway. J. Neurosci. 2000, 20, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Silhol, M.; Moulière, F.; Meffre, J.; Höllinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Thiele, C.J.; Li, Z.; McKee, A.E. On Trk—The TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin. Cancer Res. 2009, 15, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, H.; Bałasz-Chmielewska, I.; Grenda, R.; Musiał, K.; Ogarek, I.; Szczepańska, M.; Zachwieja, J.; Żurowska, A. Recommendations of Polish Society Paediatric Nephrology for the management of the child with nephrotic syndrome. Forum Nefrol. 2015, 8, 238–256. Available online: https://journals.viamedica.pl/renal_disease_and_transplant/article/view/44383/34544 (accessed on 11 October 2022).
- Kułaga, Z.; Różdżyńska, A.; Palczewska, I.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Litwin, M. Percentile charts of height, body mass and body mass index in children and adolescents in Poland—Results of the OLAF study. Stand. Med. Pediatr. 2010, 7, 690–700. [Google Scholar]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Kułaga, K. Distribution of blood pressure in school-aged children and adolescents reference population. Stand. Med. Pediatr. 2010, 7, 100–111. [Google Scholar]


| Parameter | Children with Idiopathic Nephrotic Syndrome (n = 30) | Control Group (n = 44) | ||||
|---|---|---|---|---|---|---|
| Whole Group | Female | Male | Whole Group | Female | Male | |
| Age (year) | 7.50 ± 4.14 | 6.14 ± 3.35 | 9.00 ± 4.40 | 7.75 ± 4.10 | 8.33 ± 3.95 | 9.85 ± 4.17 |
| (2.00–17.00) | (2.00–13.00) | (2.00–17.00) | (2.00–17.50) | (2.00–17.00) | (4.50–17.50) | |
| Height (cm) | 125 ± 25.00 | 115.46 ± 19.24 | 134.41 ± 26.70 | 127 ± 24.90 | 127.50 ± 21.59 | 142.17 ± 25.60 |
| (92.00–179.00) | (92.00–158.00) | (92.00–179.00) | (82.00–197.00) | (82.00–170.00) | (109.00–197.00) | |
| SDS for height | 0.06 ± 1.36 | −00.7 ± 1.20 | 0.08 ± 1.53 | 0.09 ± 1.10 | −0.14 ± 1.08 | 0.35 ± 1.08 |
| (−2.80–2.29) | (−2.80–2.20) | (−2.80–2.29) | (1.53–3.00) | (−1.53–2.35) | (−1.34–3.00) | |
| BW (kg) | 29.80 ± 21.60 | 26.54 ± 13.55 | 43.26 ± 24.49 | 27.10 ± 18.80 | 30.35 ± 15.03 | 38.38 ± 20.68 |
| (12.10–88.90) | (12.10–59.80) | (14.50–88.90) | (9.70–87.50) | (9.70–71.00) | (15.00–87.50) | |
| SDS for BW | 1.15 ± 2.1 | 1.12 ± 1.58 | 1.75 ± 2.49 | 0.07 ± 1.11 | 0.08 ± 1.23 | 0.04 ± 1.04 |
| (−1.83–9.58) | (−1.83–3.80) | (−1.63–9.58) | (−2.34–2.12) | (−1.87–2.11) | (−2.34–2.12) | |
| BMI (kg/m2) | 18.90 ± 4.07 | 18.41 ± 3.20 | 20.92 ± 4.48 | 16.60 ± 3.45 | 17.52 ± 3.12 | 17.52 ± 3.72 |
| (13.50–30.90) | (13.82–23.98) | (13.50–30.90) | (12.40–26.50) | (13.90–24.60) | (12.40–26.50) | |
| SDS for BMI | 0.83 ± 2.10 | 0.07 ± 1.18 | 2.20 ± 2.26 | −0.06 ± 1.18 | 0.25 ± 1.05 | −0.25 ± 1.25 |
| (−2.18–8.17) | (−1.47–2.35) | (−2.18–8.17) | (−2.97–2.29) | (−1.38–2.29) | (−2.97–2.02) | |
| SYS (mmHg) | 115 ± 14.6 | 108.21 ± 14.87 | 117.88 ± 13.17 | 110.00 ± 11.30 | 107.17 ± 8.54 | 113.96 ± 12.25 |
| (81.00–152.00) | (81.00–138.00) | (102.00–152.00) | (85.00–134.00) | (89.00–122.00) | (85.00–134.00) | |
| DIA (mmHg) | 67.50 ± 10.90 | 64.71 ± 9.51 | 73.44 ± 10.66 | 70.00 ± 12.00 | 66.83 ± 11.45 | 69.96 ± 12.44 |
| (50.00–90.00) | (50.00–88.00) | (56.00–90.00) | (40.00–107.00) | (45.00–96.00) | (40.00–107.00) | |
| MAP (mmHg) | 82.50 ± 11.30 | 79.21 ± 9.97 | 88.25 ± 11.03 | 82.50 ± 10.70 | 80.28 ± 9.54 | 84.63 ± 11.23 |
| (67.00–111.00) | (67.00–100) | (71.33–111.00) | (58.70–116.00) | (62.33–102.33) | (58.67–115.70) | |
| Urine protein (g/L) | 4.45 ± 8.78 | 6.04 ± 6.15 | 10.28 ± 10.35 | X | X | X |
| (0.67–35.60) | (0.88–23.70) | (0.67–35.60) | ||||
| Parameter | Control Group (n= 44) | Study Group (n = 30) |
|---|---|---|
| Serum BDNF/serum creatinine | 0.02 ± 0.01 (0.01–0.04) | 0.03 ± 0.01 (0.01–0.06) |
| β | SE | t | p | |
|---|---|---|---|---|
| (Intercept) | −18.86 | 9.73 | −1.94 | 0.06 |
| BDNF serum level | 26.66 | 11.55 | 2.31 | 0.03 |
| Gender | 2.98 | 3.08 | 0.97 | 0.34 |
| Age | 0.69 | 0.38 | 1.82 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badeński, A.; Badeńska, M.; Świętochowska, E.; Didyk, A.; Morawiec-Knysak, A.; Szczepańska, M. Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome. Int. J. Mol. Sci. 2022, 23, 12312. https://doi.org/10.3390/ijms232012312
Badeński A, Badeńska M, Świętochowska E, Didyk A, Morawiec-Knysak A, Szczepańska M. Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome. International Journal of Molecular Sciences. 2022; 23(20):12312. https://doi.org/10.3390/ijms232012312
Chicago/Turabian StyleBadeński, Andrzej, Marta Badeńska, Elżbieta Świętochowska, Agnieszka Didyk, Aurelia Morawiec-Knysak, and Maria Szczepańska. 2022. "Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome" International Journal of Molecular Sciences 23, no. 20: 12312. https://doi.org/10.3390/ijms232012312
APA StyleBadeński, A., Badeńska, M., Świętochowska, E., Didyk, A., Morawiec-Knysak, A., & Szczepańska, M. (2022). Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome. International Journal of Molecular Sciences, 23(20), 12312. https://doi.org/10.3390/ijms232012312






