Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates
Abstract
:1. Introduction
2. Results and Discussion
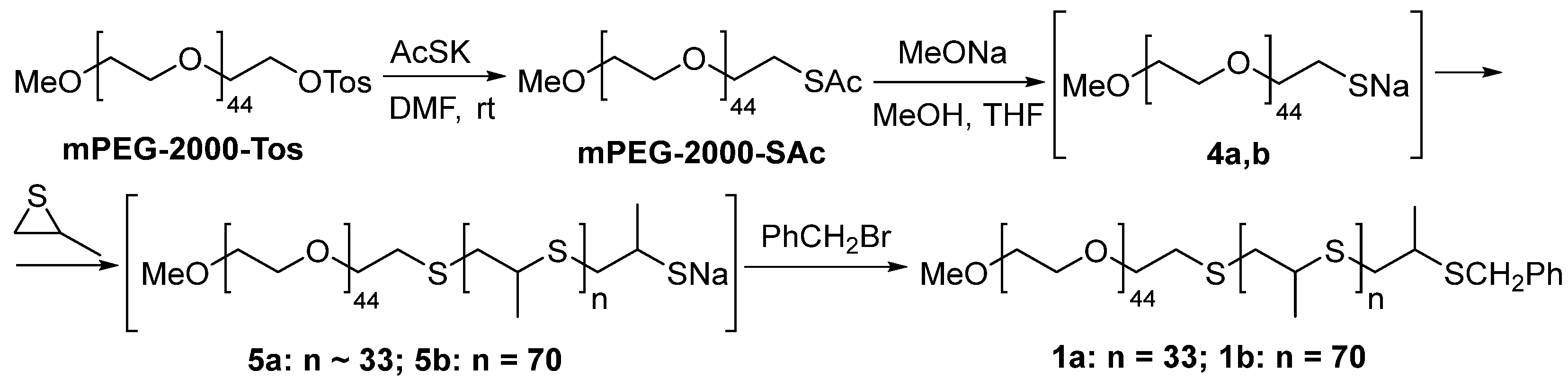
2.1. Synthesis of Amphiphilic Block Copolymers for Construction of Nanoreactors
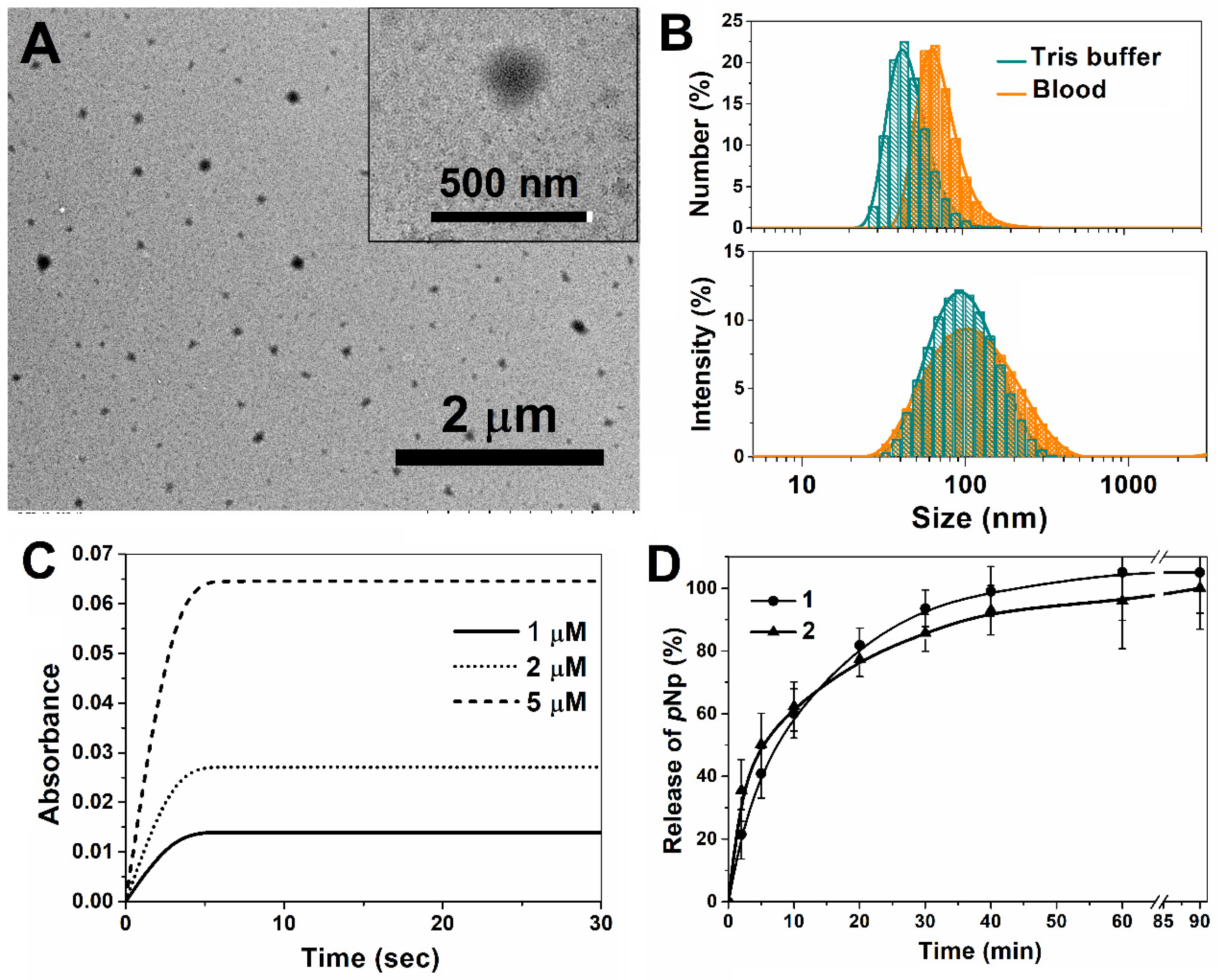
2.2. Preparation and Characterization of Nanoreactors
2.3. Enzyme-Loaded Nanoreactor Based on 1b Polymersomes
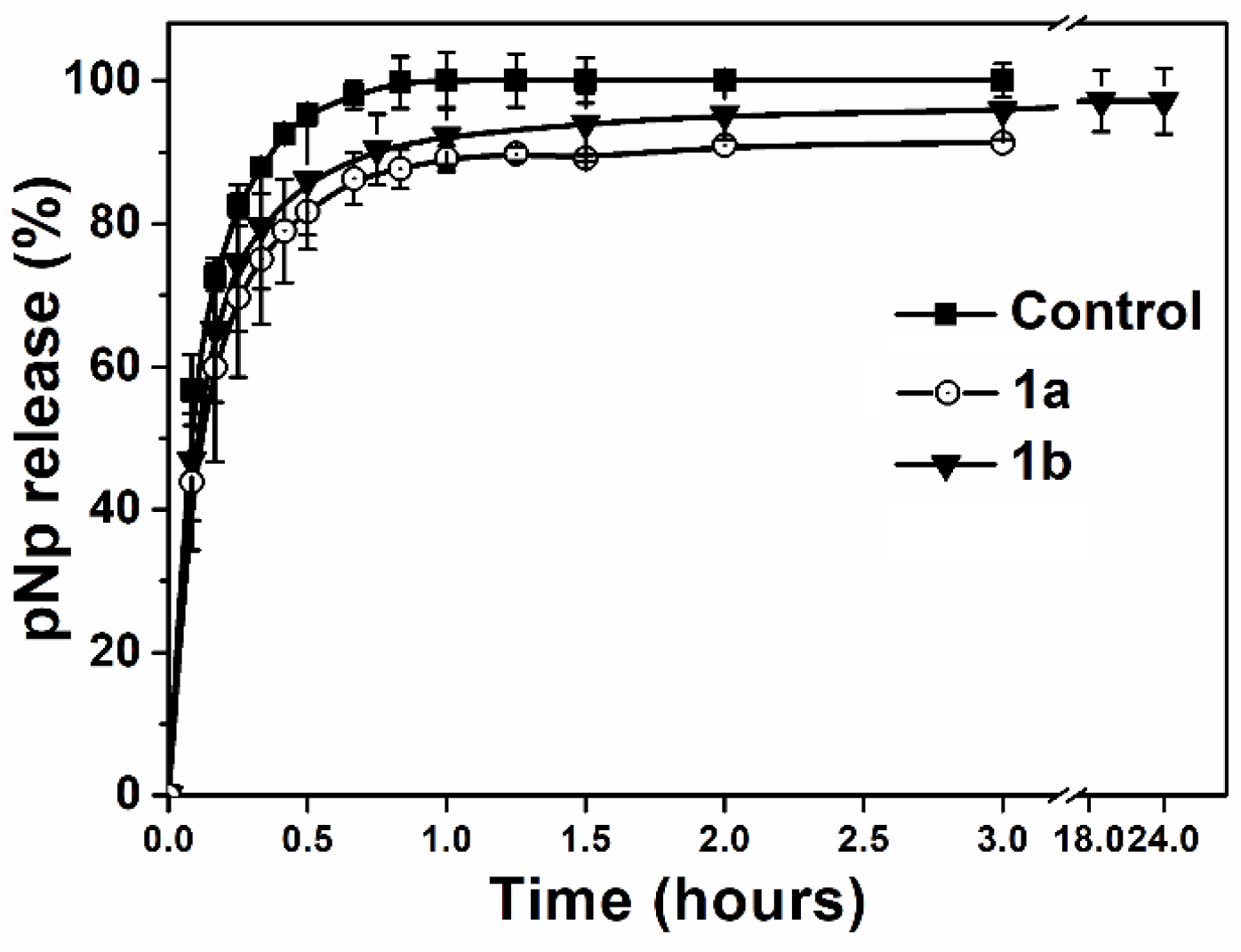
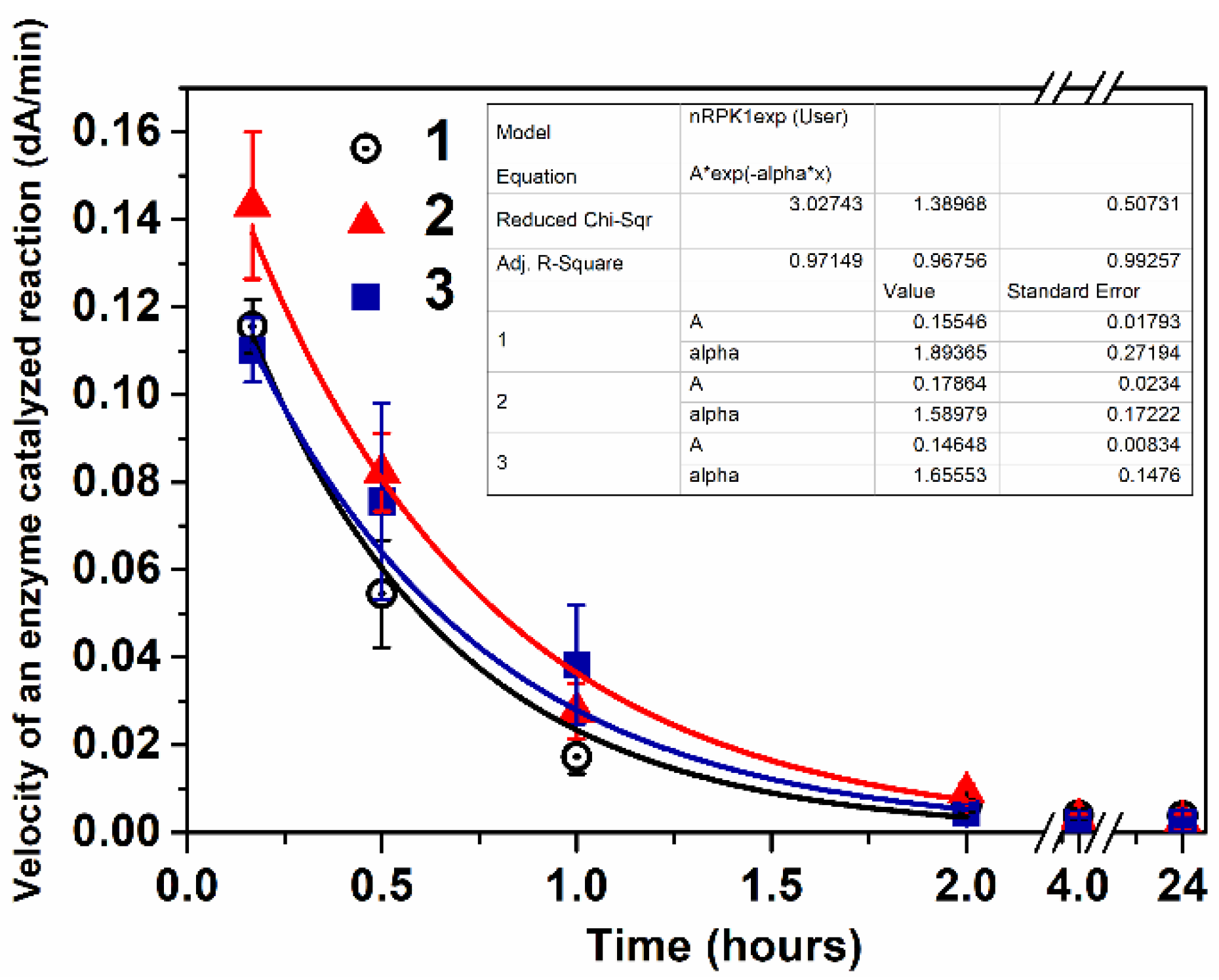
2.4. In Vitro Study of Enzyme Nanoreactors
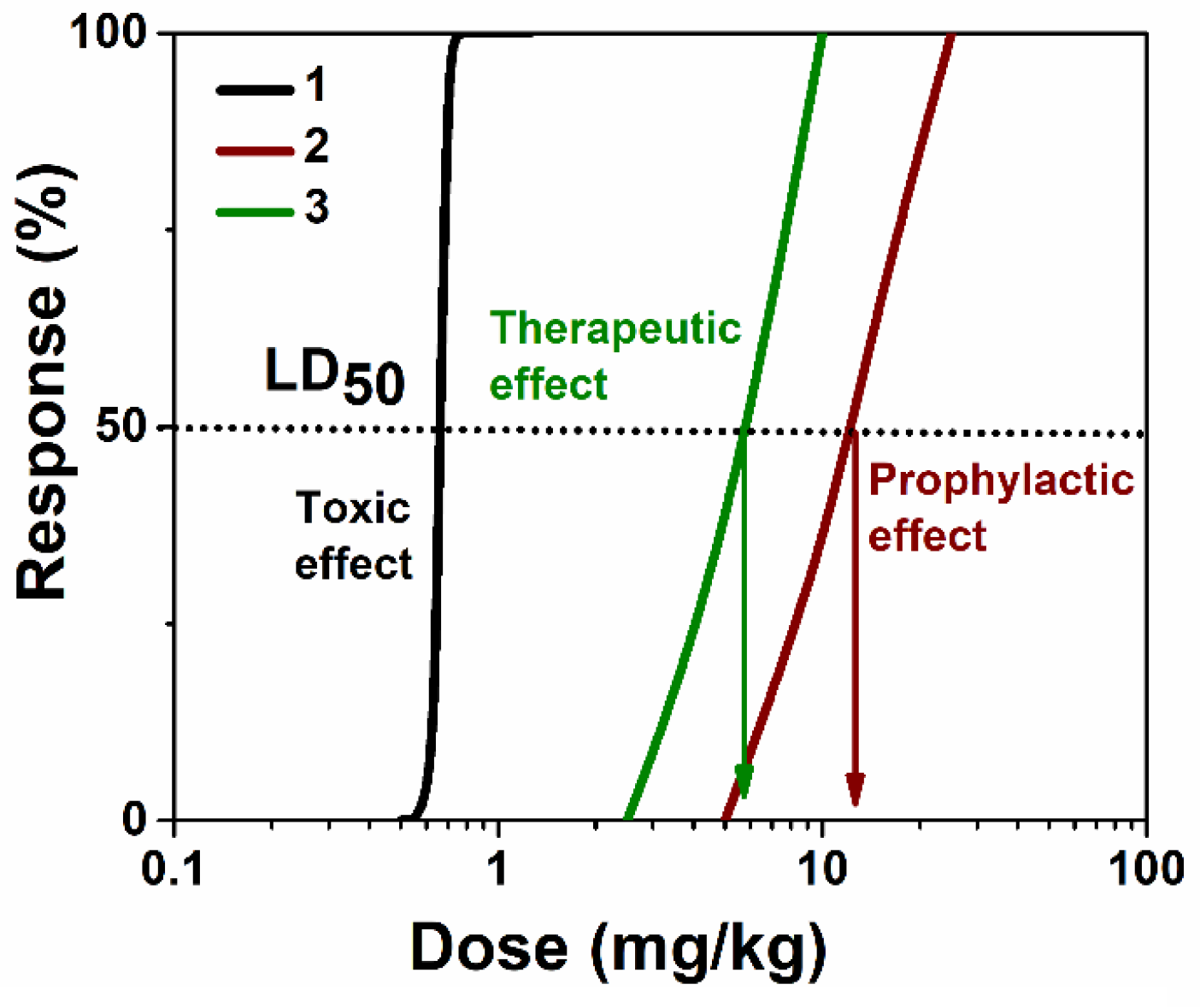
2.5. Prophylaxis and Post-Exposure Treatment Trials of Nanoreactors In Vivo
2.5.1. LD50 Shifts
2.5.2. Behavioral Test
2.6. Pharmacokinetics Study in Mice
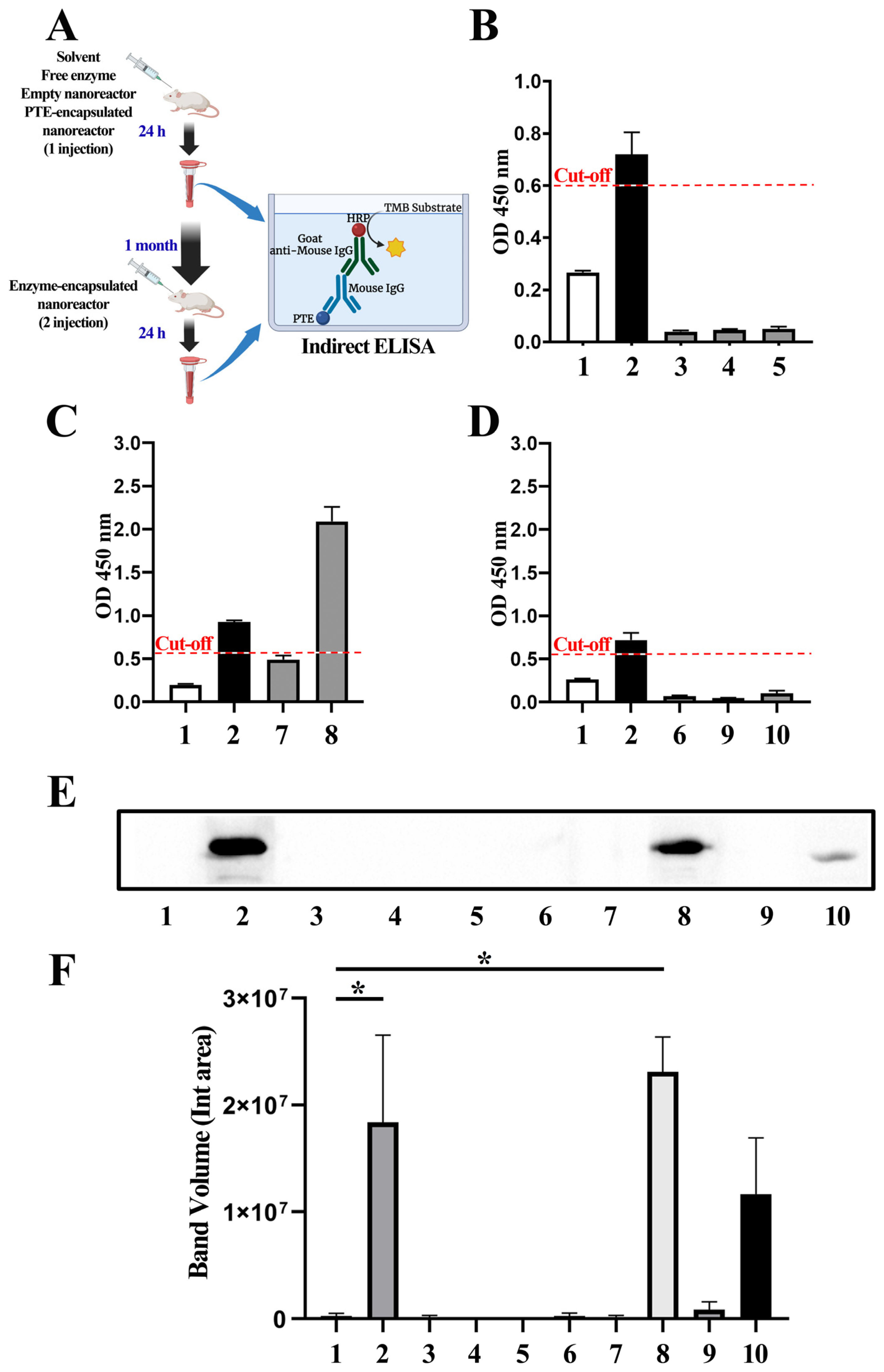
2.7. Immunological Analyses
2.7.1. ELISA
2.7.2. Western Blot
3. Materials and Methods
3.1. Subsection
Chemicals
3.2. Enzyme Source and Catalytic Activity against POX
3.3. Synthesis Polymers for Nanoreactor Construction
3.4. Procedure of Nanoreactor Preparation
3.5. Characterization of Nanoreactors
3.5.1. The Size, Surface Charge, and Morphology
3.5.2. Encapsulation Efficiency (EE, %) and Loading Capacity (LC, %)
3.5.3. In Vitro Stability of Nanoreactors in Blood
3.5.4. Purification of Enzyme-Loaded Nanoreactors
3.5.5. In Vitro the Nanoreactor Permeability
3.6. In Vitro Simulations of Enzyme-Loaded Nanoreactor Reactions against POX in Spectrophotometer Cuvette
3.7. In Vivo Study of Free Enzyme and Enzyme-Loaded Nanoreactors
3.7.1. Animals
3.7.2. POX LD50 Shifts at Pre- and Post-Exposure Treatments in Mice
3.7.3. Elevated plus Maze Test
3.8. Pharmacokinetics in Mice
3.8.1. Assay of Enzyme Activity in Serum for Pharmacokinetic Analysis
3.8.2. Statistical Analysis
3.9. Immune Response to Injected Enzyme-Loaded Nanoreactors
3.9.1. Enzyme-Linked Immunosorbent Assay (ELISA)
3.9.2. Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salinas, G.; Beladi-Mousavi, S.M.; Kuhn, A. Recent progress in enzyme-driven micro/nanoswimmers: From fundamentals to potential applications. Curr. Opin. Electrochem. 2022, 32, 100887. [Google Scholar] [CrossRef]
- Arqué, X.; Patiño, T.; Sánchez, S. Enzyme-powered micro- and nano-motors: Key parameters for an application-oriented design. Chem. Sci. 2022, 13, 9128–9146. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Xu, L.; Hong, W.; She, Y.; Yang, G. Enzyme-driven micro/nanomotors: Recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 167, 457–469. [Google Scholar] [CrossRef]
- Mathesh, M.; Sun, J.; Wilson, D.A. Enzyme catalysis powered micro/nanomotors for biomedical applications. J. Mater. Chem. B 2020, 8, 7319–7334. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hou, Y. Micro/Nanorobots as Active Delivery Systems for Biomedicine: From Self-Propulsion to Controllable Navigation. Adv. Ther. 2022, 5, 2100228. [Google Scholar] [CrossRef]
- Kwon, K.; Jung, J.; Sahu, A.; Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Release 2022, 344, 160–172. [Google Scholar] [CrossRef]
- Geng, X.; Du, X.; Wang, W.; Zhang, C.; Liu, X.; Qu, Y.; Zhao, M.; Li, W.; Zhang, M.; Tu, K.; et al. Confined Cascade Metabolic Reprogramming Nanoreactor for Targeted Alcohol Detoxification and Alcoholic Liver Injury Management. ACS Nano 2023, 17, 7443–7455. [Google Scholar] [CrossRef]
- Forster, V.; Leroux, J.-C. Nano-antidotes for drug overdose and poisoning. Sci. Transl. Med. 2015, 7, 290ps14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Lu, Y. Enzyme therapeutics for systemic detoxification. Adv. Drug Deliv. Rev. 2015, 90, 24–39. [Google Scholar] [CrossRef]
- Muhammad, F.; Nguyen, T.D.T.; Raza, A.; Akhtar, B.; Aryal, S. A review on nanoparticle-based technologies for biodetoxification. Drug Chem. Toxicol. 2017, 40, 489–497. [Google Scholar] [CrossRef]
- Pratsinis, A.; Zuercher, S.; Forster, V.; Fischer, E.J.; Luciani, P.; Leroux, J.-C. Liposome-supported enzymatic peritoneal dialysis. Biomaterials 2017, 145, 128–137. [Google Scholar] [CrossRef]
- Xu, D.; Han, H.; He, Y.; Lee, H.; Wu, D.; Liu, F.; Liu, X.; Liu, Y.; Lu, Y.; Ji, C. A Hepatocyte-Mimicking Antidote for Alcohol Intoxication. Adv. Mater. 2018, 30, 1707443. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xing, R.; Guan, J.; Yang, B.; Zhao, X.; Yang, J.; Zhan, C.; Zhang, S. A Nanoantidote Alleviates Glioblastoma Chemotoxicity without Efficacy Compromise. Nano Lett. 2021, 21, 5158–5166. [Google Scholar] [CrossRef]
- Kim, J.-K.; Uchiyama, S.; Gong, H.; Stream, A.; Zhang, L.; Nizet, V. Engineered Biomimetic Platelet Membrane-Coated Nanoparticles Block Staphylococcus aureus Cytotoxicity and Protect Against Lethal Systemic Infection. Engineering 2021, 7, 1149–1156. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wei, T.; Gayet, O.; Loncle, C.; Borge, L.; Dusetti, N.; Ma, X.; Marson, D.; Laurini, E.; et al. Dendrimeric nanosystem consistently circumvents heterogeneous drug response and resistance in pancreatic cancer. Exploration 2021, 1, 21–34. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, W.; Chen, J.; Zhou, J. Mono-functionalized pillar[n]arenes: Syntheses, host–guest properties and applications†. Chin. Chem. Lett. 2023, 108740. [Google Scholar] [CrossRef]
- Wu, S.; Yan, M.-M.; Zhou, J. Supramolecular polymeric materials based on macrocyclic hosts for cancer therapy. Biomed. Eng. Commun. 2023, 2, 15. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, M.; Rong, Q.; Zhang, M.; Zhang, X. Nucleic acid sensors in vivo: Challenges and opportunities. VIEW 2023, 4, 20220064. [Google Scholar] [CrossRef]
- Li, J.; de Ávila, B.E.-F.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Liu, F.; Anton, N.; Niko, Y.; Klymchenko, A.S. Controlled Release and Capture of Aldehydes by Dynamic Imine Chemistry in Nanoemulsions: From Delivery to Detoxification. ACS Appl. Bio Mater. 2023, 6, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Prchalova, E.; Kohoutova, Z.; Knittelova, K.; Malinak, D.; Musilek, K. Strategies for enhanced bioavailability of oxime reactivators in the central nervous system. Arch. Toxicol. 2023, 97, 2839–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadieux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11, eaau7091. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Gao, W.; Wang, F.; Chuluun, E.; Angsantikul, P.; Thamphiwatana, S.; Lu, W.; et al. Detoxification of Organophosphate Poisoning Using Nanoparticle Bioscavengers. ACS Nano 2015, 9, 6450–6458. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wang, B.; Wang, Q.; Liu, G.; Song, J.; Zhang, F.; Li, J.; Wang, F.; He, Q.; Zhu, Y.; et al. Dual-Modal Nanoscavenger for Detoxification of Organophosphorus Compounds. ACS Appl. Mater. Interfaces 2022, 14, 42454–42467. [Google Scholar] [CrossRef]
- Novikov, B.N.; Grimsley, J.K.; Kern, R.J.; Wild, J.R.; Wales, M.E. Improved pharmacokinetics and immunogenicity profile of organophosphorus hydrolase by chemical modification with polyethylene glycol. J. Control. Release 2010, 146, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R.; Szebeni, J.; Webster, T.J.; Audette, G.F. (Eds.) Immune Aspects of Biopharmaceuticals and Nanomedicines; Jenny Stanford Publishing: Singapore, 2019; ISBN 9780203731536. [Google Scholar]
- Meunier, S.; de Bourayne, M.; Hamze, M.; Azam, A.; Correia, E.; Menier, C.; Maillère, B. Specificity of the T Cell Response to Protein Biopharmaceuticals. Front. Immunol. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Chauhan, K.; Zárate-Romero, A.; Sengar, P.; Medrano, C.; Vazquez-Duhalt, R. Catalytic Kinetics Considerations and Molecular Tools for the Design of Multienzymatic Cascade Nanoreactors. ChemCatChem 2021, 13, 3732–3748. [Google Scholar] [CrossRef]
- Shajhutdinova, Z.; Pashirova, T.; Masson, P. Kinetic Processes in Enzymatic Nanoreactors for In Vivo Detoxification. Biomedicines 2022, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.; Shaihutdinova, Z.; Mansurova, M.; Kazakova, R.; Shambazova, D.; Bogdanov, A.; Tatarinov, D.; Daudé, D.; Jacquet, P.; Chabrière, E.; et al. Enzyme Nanoreactor for In Vivo Detoxification of Organophosphates. ACS Appl. Mater. Interfaces 2022, 14, 19241–19252. [Google Scholar] [CrossRef]
- Jacquet, P.; Rémy, B.; Bross, R.P.T.; van Grol, M.; Gaucher, F.; Chabrière, E.; de Koning, M.C.; Daudé, D. Enzymatic Decontamination of G-Type, V-Type and Novichok Nerve Agents. Int. J. Mol. Sci. 2021, 22, 8152. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Billot, R.; Shimon, A.; Hoekstra, N.; Bergonzi, C.; Jenks, A.; Chabrière, E.; Daudé, D.; Mikael, H. Elias Changes in Active Site Loop Conformation Relate to the Transition toward a Novel Enzymatic Activity. bioRxiv 2023, 2023.05.22.541809. [Google Scholar] [CrossRef]
- Merone, L.; Mandrich, L.; Porzio, E.; Rossi, M.; Müller, S.; Reiter, G.; Worek, F.; Manco, G. Improving the promiscuous nerve agent hydrolase activity of a thermostable archaeal lactonase. Bioresour. Technol. 2010, 101, 9204–9212. [Google Scholar] [CrossRef]
- Suzumoto, Y.; Dym, O.; Roviello, G.N.; Worek, F.; Sussman, J.L.; Manco, G. Structural and Functional Characterization of New SsoPox Variant Points to the Dimer Interface as a Driver for the Increase in Promiscuous Paraoxonase Activity. Int. J. Mol. Sci. 2020, 21, 1683. [Google Scholar] [CrossRef]
- Owensiii, D.; Peppas, N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Haroon, H.B.; Hunter, A.C.; Farhangrazi, Z.S.; Moghimi, S.M. A brief history of long circulating nanoparticles. Adv. Drug Deliv. Rev. 2022, 188, 114396. [Google Scholar] [CrossRef]
- Vincent, M.P.; Karabin, N.B.; Allen, S.D.; Bobbala, S.; Frey, M.A.; Yi, S.; Yang, Y.; Scott, E.A. The Combination of Morphology and Surface Chemistry Defines the Immunological Identity of Nanocarriers in Human Blood. Adv. Ther. 2021, 4, 2100062. [Google Scholar] [CrossRef]
- Najer, A.; Rifaie-Graham, O.; Yeow, J.; Adrianus, C.; Chami, M.; Stevens, M.M. Differences in Human Plasma Protein Interactions between Various Polymersomes and Stealth Liposomes as Observed by Fluorescence Correlation Spectroscopy. Macromol. Biosci. 2022, 23, 2200424. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Allen, S.D.; Liu, Y.-G.; Ouyang, B.Z.; Li, X.; Augsornworawat, P.; Thorp, E.B.; Scott, E.A. Tailoring Nanostructure Morphology for Enhanced Targeting of Dendritic Cells in Atherosclerosis. ACS Nano 2016, 10, 11290–11303. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J.; et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef]
- Allen, S.D.; Liu, Y.-G.; Bobbala, S.; Cai, L.; Hecker, P.I.; Temel, R.; Scott, E.A. Polymersomes scalably fabricated via flash nanoprecipitation are non-toxic in non-human primates and associate with leukocytes in the spleen and kidney following intravenous administration. Nano Res. 2018, 11, 5689–5703. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef]
- Velluto, D.; Demurtas, D.; Hubbell, J.A. PEG- b -PPS Diblock Copolymer Aggregates for Hydrophobic Drug Solubilization and Release: Cyclosporin A as an Example. Mol. Pharm. 2008, 5, 632–642. [Google Scholar] [CrossRef]
- Cerritelli, S.; Velluto, D.; Hubbell, J.A. PEG-SS-PPS: Reduction-Sensitive Disulfide Block Copolymer Vesicles for Intracellular Drug Delivery. Biomacromolecules 2007, 8, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.A.; Stano, A.; Gillard, M.; Maio-Liu, A.C.; Swartz, M.A.; Hubbell, J.A. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 2012, 33, 6211–6219. [Google Scholar] [CrossRef]
- O’Neil, C.P.; Suzuki, T.; Demurtas, D.; Finka, A.; Hubbell, J.A. A Novel Method for the Encapsulation of Biomolecules into Polymersomes via Direct Hydration. Langmuir 2009, 25, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.N.; Shaihutdinova, Z.M.; Mironov, V.F.; Masson, P. Biomedical Nanosystems for in vivo Detoxification: From Passive Delivery Systems to Functional Nanodevices and Nanorobots. Acta Nat. 2023, 15, 4–12. [Google Scholar] [CrossRef]
- Eyer, F.; Eyer, P. Enzyme-based assay for quantification of paraoxon in blood of parathion poisoned patients. Hum. Exp. Toxicol. 1998, 17, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Lushchekina, S.V. Emergence of catalytic bioscavengers against organophosphorus agents. Chem. Biol. Interact. 2016, 259, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Lee, C.; Rodgers, R.J. Antinociceptive effects of elevated plus-maze exposure: Influence of opiate receptor manipulations. Psychopharmacology 1990, 102, 507–513. [Google Scholar] [CrossRef]
- Wang, J.; Lozier, J.; Johnson, G.; Kirshner, S.; Verthelyi, D.; Pariser, A.; Shores, E.; Rosenberg, A. Neutralizing antibodies to therapeutic enzymes: Considerations for testing, prevention and treatment. Nat. Biotechnol. 2008, 26, 901–908. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Nicolai, O.; Pötschke, C.; Schmoeckel, K.; Darisipudi, M.N.; van der Linde, J.; Raafat, D.; Bröker, B.M. Antibody Production in Murine Polymicrobial Sepsis—Kinetics and Key Players. Front. Immunol. 2020, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, M.F.; Solano-Gallego, L.; Vullo, A.; Masucci, M.; Marty, P.; Delaunay, P.; Vitale, F.; Pennisi, M.G. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit. Vectors 2017, 10, 119. [Google Scholar] [CrossRef]
- Nulty, C.; Barber, G.; Flis, E.; Creagh, E.M. Evaluating Host Responses to Helicobacter pylori Using ELISA and Western Blot; Humana: New York, NY, USA, 2021; pp. 175–190. [Google Scholar]
- Poirier, L.; Pinault, L.; Armstrong, N.; Ghigo, E.; Daudé, D.; Chabrière, E. Evaluation of a robust engineered enzyme towards organophosphorus insecticide bioremediation using planarians as biosensors. Chem. Biol. Interact. 2019, 306, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Plener, L.; Poirier, L.; Elias, M.; Daudé, D.; Chabrière, E. Harnessing hyperthermostable lactonase from Sulfolobus solfataricus for biotechnological applications. Sci. Rep. 2016, 6, 37780. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Tirelli, N.; Kilcher, G.; Hubbell, A. New Synthetic Methodologies for Amphiphilic Multiblock Copolymers of Ethylene Glycol and Propylene Sulfide. Macromolecules 2001, 34, 8913–8917. [Google Scholar] [CrossRef]
- Mahou, R.; Wandrey, C. Versatile Route to Synthesize Heterobifunctional Poly(ethylene glycol) of Variable Functionality for Subsequent Pegylation. Polymers 2012, 4, 561–589. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Shargel, L.; Wu-Pong, S.; Yu, A. (Eds.) One-Compartment Open Model: Intravenous Bolus Administration; McGraw Hill: New York, NY, USA, 2012. [Google Scholar]

| Polymer | C (%, w/w) | Size (nm) | Z-Average (nm) | PDI | ξ (mV) | |
|---|---|---|---|---|---|---|
| Intensity | Number | |||||
| 1a | 0.5 | 38 ± 6 | 21 ± 5 | 37 ± 1 | 0.14 ± 0.01 | −5.0 ± 1 |
| 1 | 44 ± 7 | 24 ± 6 | 41 ± 0.2 | 0.12 ± 0.01 | −5.5 ± 1 | |
| 2 | 38 ± 6 | 21 ± 5 | 38 ± 1 | 0.15 ±0.02 | −5.0 ± 1 | |
| 1b | 0.1 | 91 ± 11 | 44 ± 10 | 84 ± 0.3 | 0.17 ± 0.01 | −6 ± 0.3 |
| 0.2 | 79 ± 11 | 44 ± 11 | 88 ± 0.1 | 0.14 ± 0.01 | −7 ± 0.4 | |
| 0.5 | 106 ± 13 | 44 ± 10 | 94 ± 2 | 0.17 ± 0.01 | −6.5 ± 0.3 | |
| 0.75 | 122 ± 13 | 51 ± 11 | 112 ± 1 | 0.2 ± 0.01 | −5 ± 1 | |
| 1 | 106 ± 13 | 51 ± 12 | 106 ± 1 | 0.18 ± 0.01 | −4 ± 1 | |
| Polymer | C (%, w/w) | Size (nm) | Z-Aver (nm) | PDI | ξ (mV) | EE, % | LC, % | |
|---|---|---|---|---|---|---|---|---|
| Intensity | Number | |||||||
| 1a | 0.5 | 38 ± 5 | 21 ± 5 | 37 ± 0.5 | 0.1 ± 0.01 | −8 ± 1 | 83 ± 4 | 16.7 ± 0.7 |
| 1 | 34 ± 9 | 23 ± 4 | 33 ± 0.5 | 0.1± 0.01 | - | 82 ± 4 | 8.2 ± 0.4 | |
| 2 | 36 ± 12 | 23 ± 4 | 33 ± 0.5 | 0.12± 0.01 | −9 ± 2 | 96.8 ± 0.2 | 4.8 ± 0.0.01 | |
| 1b | 0.2 | 106 ± 11 | 38 ± 8 | 96 ± 0.6 | 0.22 ± 0.01 | −5.5 ± 0.6 | 99.9 ± 0.01 | 49.98 ± 0.1 |
| 0.5 | 190 ± 21 | 44 ± 3 | 145 ± 1 | 0.23 ± 0.01 | −5.2 ± 0.3 | 99.8 ± 0.1 | 19.98 ± 0.1 | |
| No. | C (%, w/w) | Cenzyme (µM) | Size (nm) | Z-Average (nm) | PDI | ξ (mV) | EE, % | LC, % | |
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Number | ||||||||
| 1 | 0.1 | 2.5 | 106 ± 12 | 44 ± 8 | 95 ± 1 | 0.18 ± 0.01 | −5 ± 0.7 | 89 ± 7 | 16 ± 1.5 |
| 2 | 5 | 106 ± 12 | 51 ± 11 | 113 ± 1 | 0.23 ± 0.01 | −7 ± 0.6 | 88 ± 4 | 32 ± 1 | |
| 3 | 0.2 | 2.5 | 122 ± 12 | 44 ± 9 | 117 ± 4 | 0.26 ± 0.01 | −5 ± 0.7 | 90 ± 2 | 8.2 ± 0.2 |
| 4 | 5 | 106 ± 13 | 44 ± 9 | 96 ± 1 | 0.17 ± 0.01 | −6.2 ± 0.1 | 90 ± 3 | 16 ± 0.5 | |
| 5 | 12.5 | 122 ± 14 | 51 ± 11 | 114 ± 1 | 0.16 ± 0.02 | −5.5 ± 0.2 | 92 ± 2 | 41 ± 1 | |
| 6 | 0.5 | 2.5 | 122 ± 15 | 44 ± 9 | 108 ± 1 | 0.17 ± 0.01 | −4.5 ± 0.3 | 89 ± 2 | 3.2 ± 0.1 |
| 7 | 5 | 106 ± 12 | 44 ± 10 | 100 ± 1 | 0.2 ± 0.01 | −9 ± 0.5 | 89 ± 2 | 6.5 ± 0.1 | |
| 8 | 12.5 | 106 ± 14 | 59 ± 12 | 106 ± 1 | 0.17 ± 0.01 | −8.3 ± 0.3 | 82 ± 7 | 15 ± 2 | |
| Sample | Number of Injections | α (min−1) | t1/2α 1 (min) |
|---|---|---|---|
| Free enzyme | 1, 1 day | 0.0315 ± 0.0045 | 22.00 ± 3.15 |
| Enzyme-loaded nanoreactors | 1, 1 day | 0.0265 ± 0.0028 | 26.16 ± 2.83 |
| Enzyme-loaded nanoreactors | 2, 30 days | 0.0277 ± 0.0025 | 25.02 ± 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashirova, T.; Shaihutdinova, Z.; Tatarinov, D.; Mansurova, M.; Kazakova, R.; Bogdanov, A.; Chabrière, E.; Jacquet, P.; Daudé, D.; Akhunzianov, A.A.; et al. Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates. Int. J. Mol. Sci. 2023, 24, 15756. https://doi.org/10.3390/ijms242115756
Pashirova T, Shaihutdinova Z, Tatarinov D, Mansurova M, Kazakova R, Bogdanov A, Chabrière E, Jacquet P, Daudé D, Akhunzianov AA, et al. Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates. International Journal of Molecular Sciences. 2023; 24(21):15756. https://doi.org/10.3390/ijms242115756
Chicago/Turabian StylePashirova, Tatiana, Zukhra Shaihutdinova, Dmitry Tatarinov, Milana Mansurova, Renata Kazakova, Andrei Bogdanov, Eric Chabrière, Pauline Jacquet, David Daudé, Almaz A. Akhunzianov, and et al. 2023. "Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates" International Journal of Molecular Sciences 24, no. 21: 15756. https://doi.org/10.3390/ijms242115756
APA StylePashirova, T., Shaihutdinova, Z., Tatarinov, D., Mansurova, M., Kazakova, R., Bogdanov, A., Chabrière, E., Jacquet, P., Daudé, D., Akhunzianov, A. A., Miftakhova, R. R., & Masson, P. (2023). Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates. International Journal of Molecular Sciences, 24(21), 15756. https://doi.org/10.3390/ijms242115756








