The Tumorigenicity of Breast Cancer Cells Is Reduced upon Treatment with Small Extracellular Vesicles Isolated from Heparin Treated Cell Cultures
Abstract
:1. Introduction
2. Results
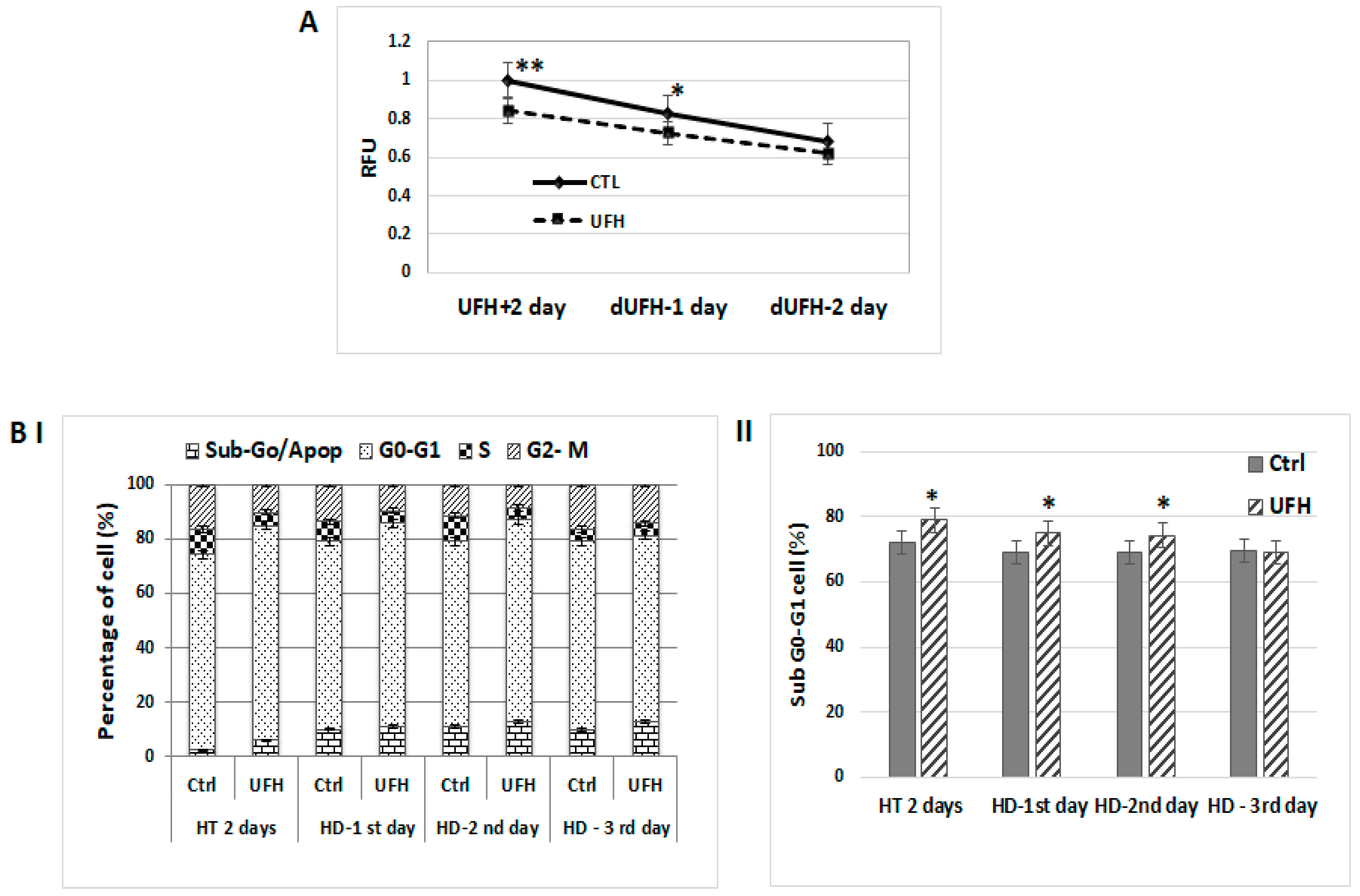
2.1. Antitumorigenic Effects Observed in Heparin-Treated Human Breast Cancer Cells Persist Temporarily after Heparin Has Been Discontinued
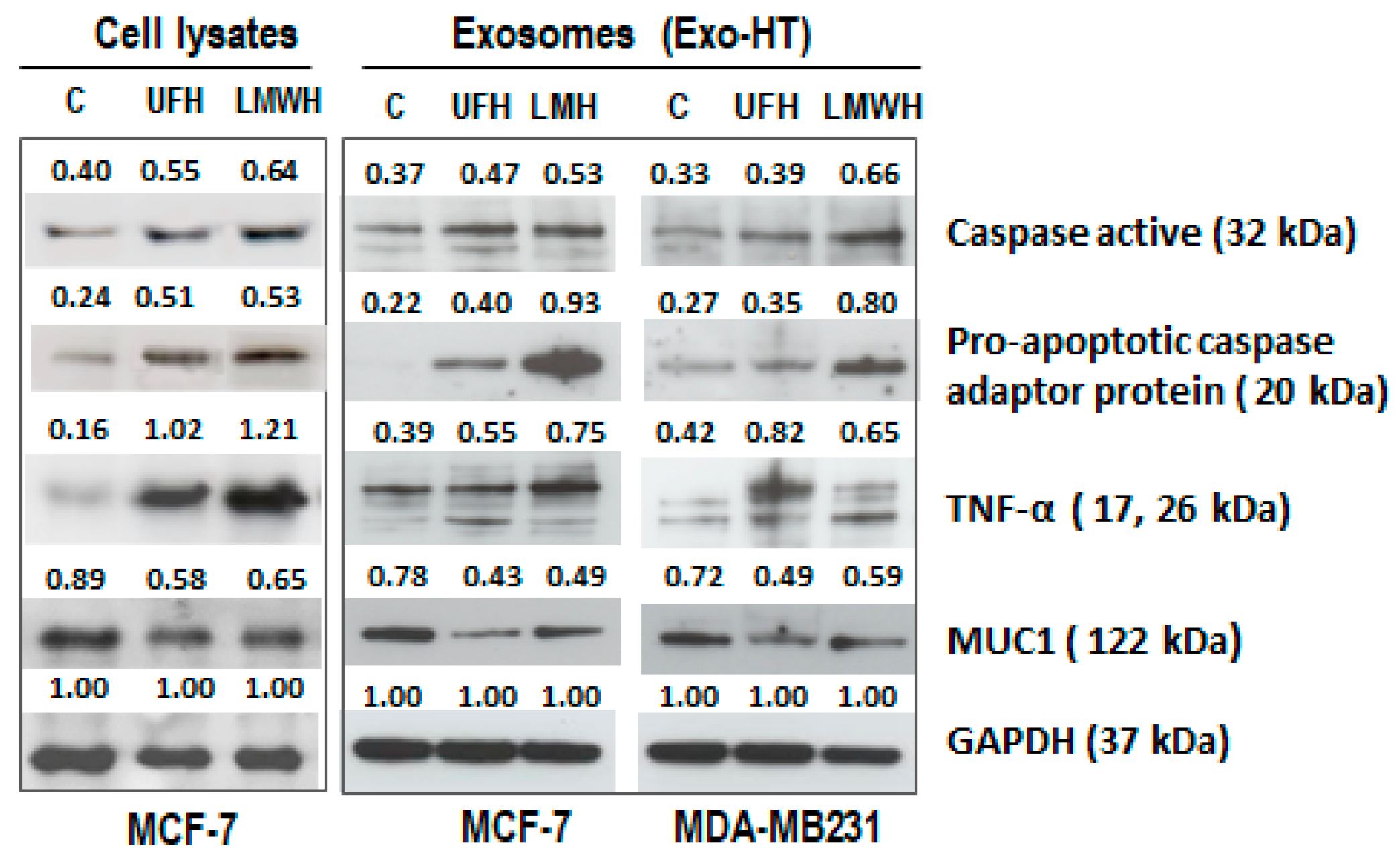
2.2. Similar Changes Are Observed in the Expression Profiles of Apoptotic and Pro-Tumorigenic Proteins following Heparin Treatment in the Cells and in the sEV Produced by the Cells (sEV-HT)
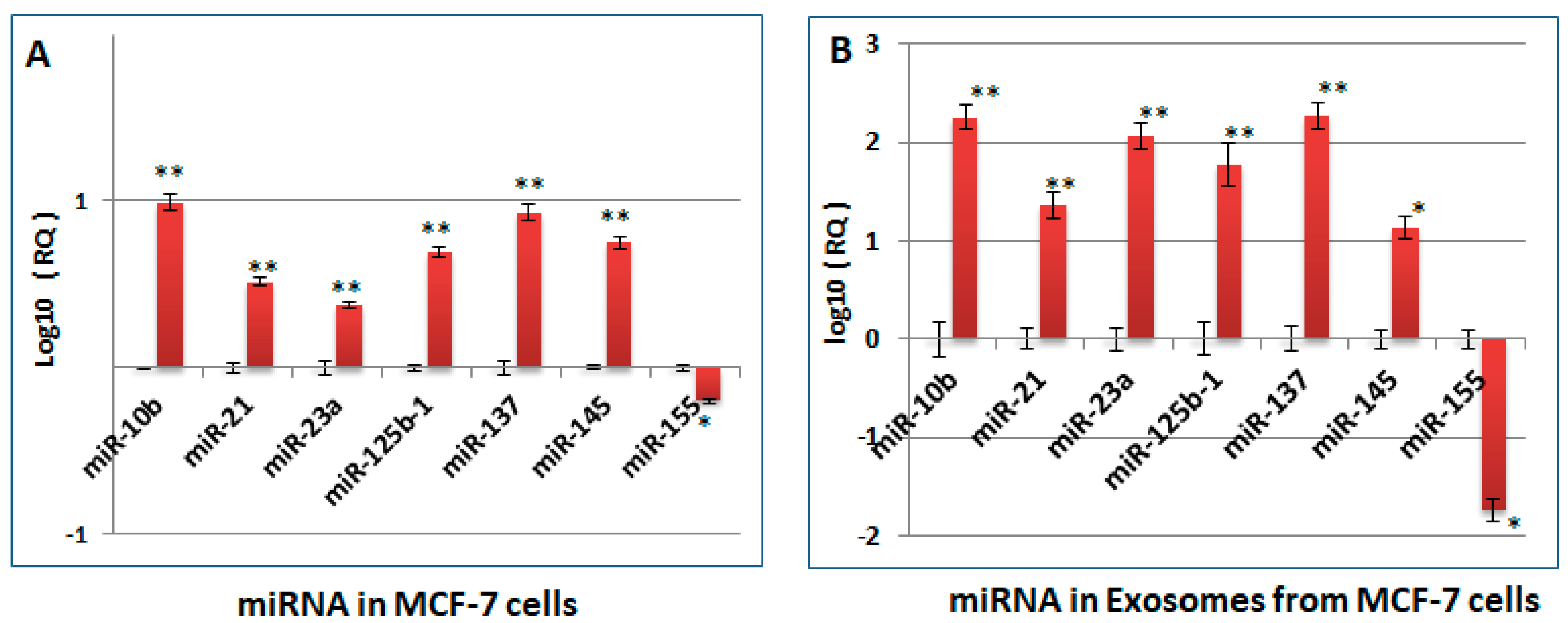
2.3. Changes Observed in the miRNA Profile of Heparin-Treated Host Cells, Which Are Pertinent to Tumorigenesis and Cell Cycle Regulation, Are Also Observed in the sEV Released by the Cells (sEV-HT)
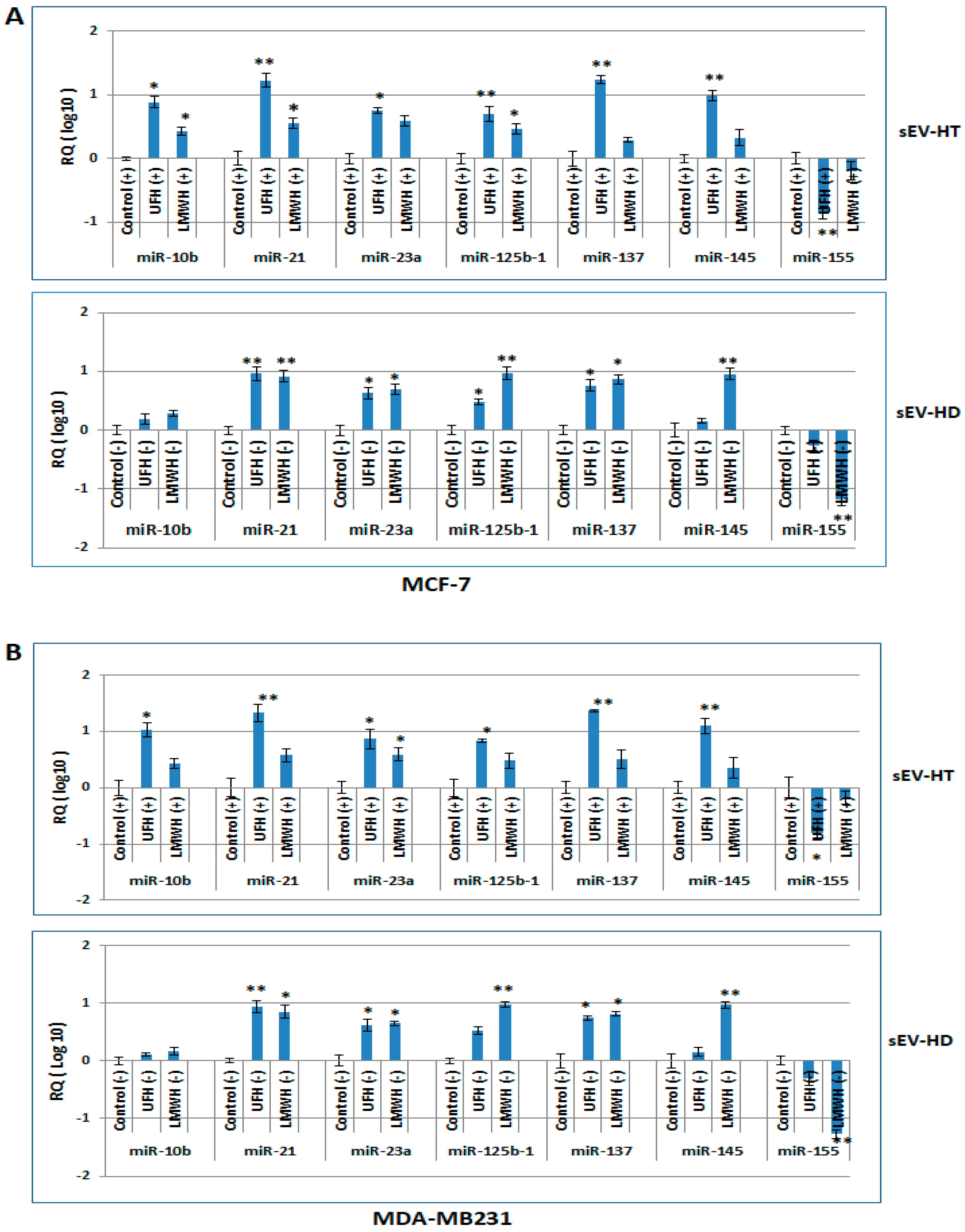
2.4. The miRNA Profile of Breast Cancer Cells Is Modulated following Treatment with sEV-HT and sEV-HD
2.5. Pro-Tumorigenic and Cell Cycle Regulatory Proteins as Well as Signalling Activities Are Modulated in Breast Cancer Cells Treated with sEV-HT and sEV-HD
2.6. The Cytological Profile Is Also Modulated upon Treatment of MCF-7 and MDA-MB231 Cells with SEV-HT and SEV-HD
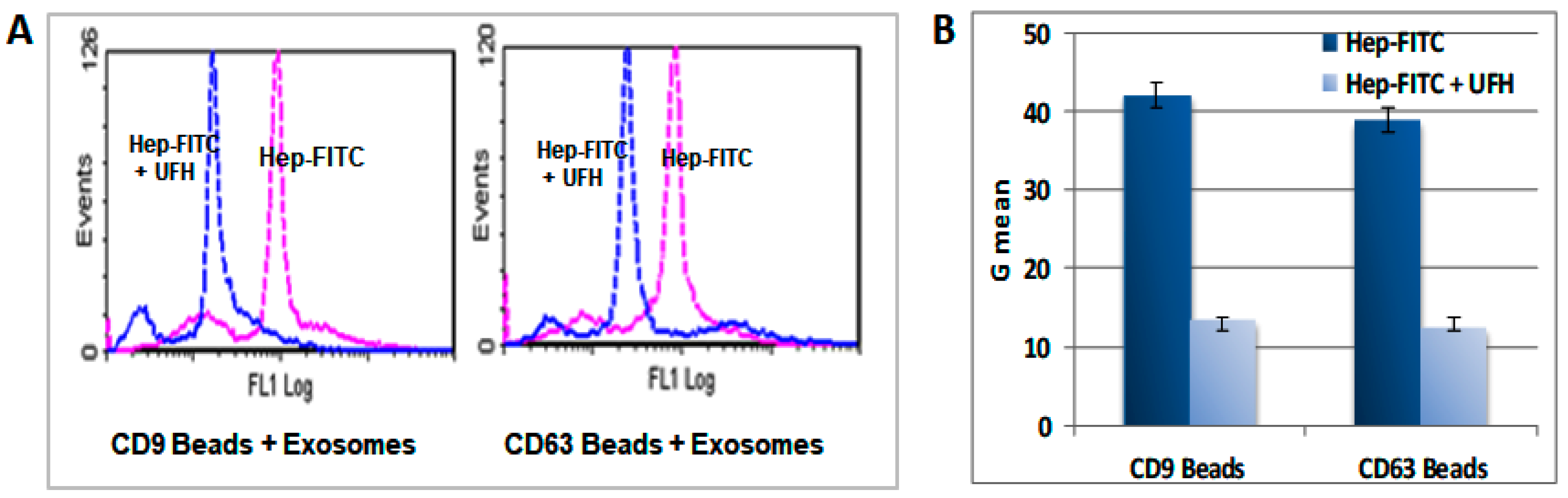
2.7. Heparin Binds to the Surface of the sEV
3. Discussion
4. Material and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. An. Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar]
- Gallagher, J.T.; Lyon, M. Molecular structure of Heparan Sulfate and interactions with growth factors and morphogens. In Proteoglycans: Structure, Biology and Molecular Interactions; Iozzo, M.V., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 27–59. [Google Scholar]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan Sulfate Proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Ma, S.N.; Mao, Z.X.; Wu, Y.; Liang, M.X.; Wang, D.D.; Chen, X.; Chang, P.A.; Zhang, W.; Tang, J.H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adh Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Icli, F.; Akbulut, H.; Utkan, G.; Yalcin, B.; Dincol, D.; Isikdogan, A.; Demirkazik, A.; Onur, H.; Cay, F.; Büyükcelik, A. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J. Surg. Oncol. 2007, 95, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Carta, M.; Cogo, A.; Ruol, A.; Vigo, M.; Casara, D.; Lensing, A.W.A.; Büller, H.R.; Ten Cate, J.W. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 1992, 339, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Dembitzer, A.D.; Doyle, R.L.; Hastie, T.J.; Garber, A.M. Low-Molecular-Weight Heparins Compared with Unfractionated Heparin for Treatment of Acute Deep Venous Thrombosis. A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 1999, 130, 800–809. [Google Scholar] [CrossRef]
- Yoshitomi, Y.; Nakanishi, H.; Kusano, Y.; Munesue, S.; Oguri, K.; Tatematsu, M.; Yamashina, I.; Okayama, M. Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Lett. 2004, 207, 165–174. [Google Scholar] [CrossRef]
- Borsig, L.; Wong, R.; Hynes, R.O.; Varki, N.M.; Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl. Acad. Sci. USA 2002, 99, 2193–2198. [Google Scholar] [CrossRef]
- Green, D.; Hull, R.; Brant, R.; Pineo, G. Lower mortality in cancer patients treated with low-molecular-weight versus standard heparin. Lancet 1992, 339, 1476. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, B.; Chastang, C.; Brechot, J.-M.; Capron, F.; Dautzenberg, B.; Delaisements, C.; Mornet, M.; Brun, J.; Hurdebourcq, J.-P.; Lemarie, E. Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer 1994, 74, 38–45. [Google Scholar] [CrossRef]
- Chen, Y.; Scully, M.; Dawson, G.; Goodwin, C.; Xia, M.; Lu, X.; Kakkar, A. Perturbation of the heparin/heparan-sulfate interactome of human breast cancer cells modulates pro-tumorigenic effects associated with PI3K/Akt and MAPK/ERK signalling. Thromb. Haemost. 2013, 109, 1148–1157. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013, 13, 3354–3364. [Google Scholar]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [PubMed]
- Qazi, K.R.; Torregrosa Paredes, P.; Dahlberg, B.; Grunewald, J.; Eklund, A.; Gabrielsson, S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 2010, 65, 1016–1024. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar]
- Sachdeva, M.; Mo, Y.Y. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010, 70, 378–387. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS ONE 2011, 6, e19139. [Google Scholar]
- Shi, L.; Zhang, J.; Pan, T.; Zhou, J.; Gong, W.; Liu, N.; Fu, Z.; You, Y. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010, 1312, 120–126. [Google Scholar]
- Huang, L.; Luo, J.; Cai, Q.; Pan, Q.; Zeng, H.; Guo, Z.; Dong, W.; Huang, J.; Lin, T. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int. J. Cancer 2011, 128, 1758–1769. [Google Scholar]
- Banzhaf-Strathmann, J.; Edbauer, D. Good guy or bad guy: The opposing roles of microRNA 125b in cancer. Cell Commun. Signal. 2014, 12, 30. [Google Scholar] [PubMed]
- Zhu, X.; Li, Y.; Shen, H.; Li, H.; Long, L.; Hui, L.; Xu, W. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013, 587, 73–81. [Google Scholar]
- Qiu, T.; Zhou, X.; Wang, J.; Du, Y.; Xu, J.; Huang, Z.; Zhu, W.; Shu, Y.; Liu, P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014, 588, 1168–1177. [Google Scholar]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar]
- Engeland, K. Cell cycle regulation: p53-p21-RB signalling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Niers, T.M.; Klerk, C.P.; DiNisio, M.; Van Noorden, C.J.; Büller, H.R.; Reitsma, P.H.; Richel, D.J. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit. Rev. Oncol. Hematol. 2007, 61, 195–207. [Google Scholar] [PubMed]
- Zacharski, L.R.; Lee, A.Y. Heparin as an anticancer therapeutic. Expert Opin. Investig. Drugs 2008, 17, 1029–1037. [Google Scholar] [CrossRef]
- Mousa, S.A.; Petersen, L.J. Anti-cancer properties of low-molecular-weight heparin: Preclinical evidence. Thromb. Haemost. 2009, 102, 258–267. [Google Scholar] [PubMed]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J. Biol. Chem. 2011, 286, 19892–19904. [Google Scholar]
- Hu, G.; Drescher, K.M.; Chen, X.M. Exosomal miRNAs: Biological Properties and Therapeutic Potential. Front. Genet. 2012, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar]
- Zhang, J.; Li, S.; Li, L.; Li MGuo, C.; Yao, J.; Mi, S. Exosome and Exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinfoatics 2015, 13, 17–24. [Google Scholar]
- Iorio, M.V.; Ferracin, M.; Liu, C.G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [PubMed]
- Rajabi, H.; Jin, C.; Ahmad, R.; McClary, C.; Joshi, M.D.; Kufe, D. Mucin 1 Oncoprotein Expression Is Suppressed by the miR-125b Oncomir. Genes Cancer 2010, 1, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar]
- Li, T.; Yang, J.; Lv, X.; Liu, K.; Gao, C.; Xing, Y.; Xi, T. miR-155 regulates the proliferation and cell cycle of colorectal carcinoma cells by targeting E2F2. Biotechnol. Lett. 2014, 36, 1743–1752. [Google Scholar] [CrossRef]
- Jay, C.; Nemunaitis, J.; Chen, P.; Fulgham, P.; Tong, A.W. MiRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol. 2007, 26, 293–300. [Google Scholar] [CrossRef]
- Yanaihara, N.; Caplen, N.; Bowman, E.; Seike, M.; Kumamoto, K.; Yi, M.; Stephens, R.M.; Okamoto, A.; Yokota, J.; Tanaka, T.; et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.; Khandelwal, S.; Cines, D.B.; Poncz, M.; Rauova, L.; Arepally, G.M. Heparin enhances uptake of platelet factor 4/heparin complexes by monocytes and macrophages. J. Thromb Haemost. 2015, 13, 1416–1427. [Google Scholar] [CrossRef]
- Letourneur, D.; Caleb, B.L.; Castellot, J.J., Jr. Heparin binding internalization metabolism in vascular smooth muscle cells: I. Upregulation of heparin binding correlates with antiproliferative activity. J. Cell. Physiol. 1995, 165, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.; Mencio, C.; Desai, U.R.; Kuberan, B. Sulfation patterns determine cellular internalization of heparin-like polysaccharides. Mol. Pharm. 2013, 10, 1442–1449. [Google Scholar] [CrossRef]
- Berry, D.; Lynn, D.M.; Sasisekharan, R.; Langer, R. Poly (beta-amino ester) s promote cellular uptake of heparin and cancer cell death. J. Chem. Biol. 2004, 11, 487–498. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef]
- Barnes, B.; Caws, T.; Thomas, S.; Shephard, A.P.; Corteling, R.; Hole, P.; Bracewell, D.G. Investigating heparin affinity chromatography for extracellular vesicle purification and fractionation. J. Chromatogr. A 2022, 1670, 462987. [Google Scholar] [CrossRef]
- Carrier, M.; Le Gal, G.; Cho, R.; Tierney, S.; Rodger, M.; Lee, A.Y. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J. Thromb. Haemost. 2009, 7, 760–765. [Google Scholar] [CrossRef]
- von Tempelhoff, G.F.; Harenberg, J.; Niemann, F.; Hommel, G.; Kirkpatrick, C.J.; Heilmann, L. Effect of low molecular weight heparin (Certoparin) versus unfractionated heparin on cancer survival following breast and pelvic cancer surgery: A prospective randomized double-blind trial. Clin. Trial Int. J. Oncol. 2000, 16, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Scully, M. The Tumorigenicity of Breast Cancer Cells Is Reduced upon Treatment with Small Extracellular Vesicles Isolated from Heparin Treated Cell Cultures. Int. J. Mol. Sci. 2023, 24, 15736. https://doi.org/10.3390/ijms242115736
Chen Y, Scully M. The Tumorigenicity of Breast Cancer Cells Is Reduced upon Treatment with Small Extracellular Vesicles Isolated from Heparin Treated Cell Cultures. International Journal of Molecular Sciences. 2023; 24(21):15736. https://doi.org/10.3390/ijms242115736
Chicago/Turabian StyleChen, Yunliang, and Michael Scully. 2023. "The Tumorigenicity of Breast Cancer Cells Is Reduced upon Treatment with Small Extracellular Vesicles Isolated from Heparin Treated Cell Cultures" International Journal of Molecular Sciences 24, no. 21: 15736. https://doi.org/10.3390/ijms242115736
APA StyleChen, Y., & Scully, M. (2023). The Tumorigenicity of Breast Cancer Cells Is Reduced upon Treatment with Small Extracellular Vesicles Isolated from Heparin Treated Cell Cultures. International Journal of Molecular Sciences, 24(21), 15736. https://doi.org/10.3390/ijms242115736




