Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics
Abstract
:1. Introduction
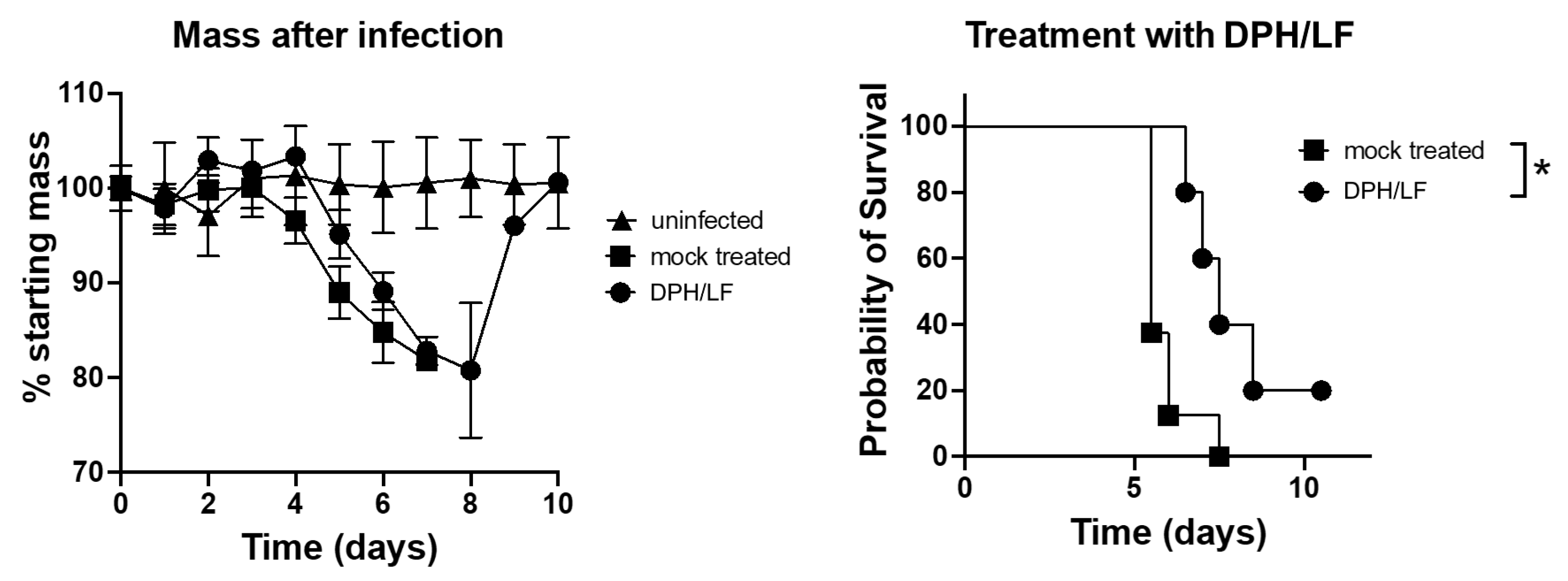
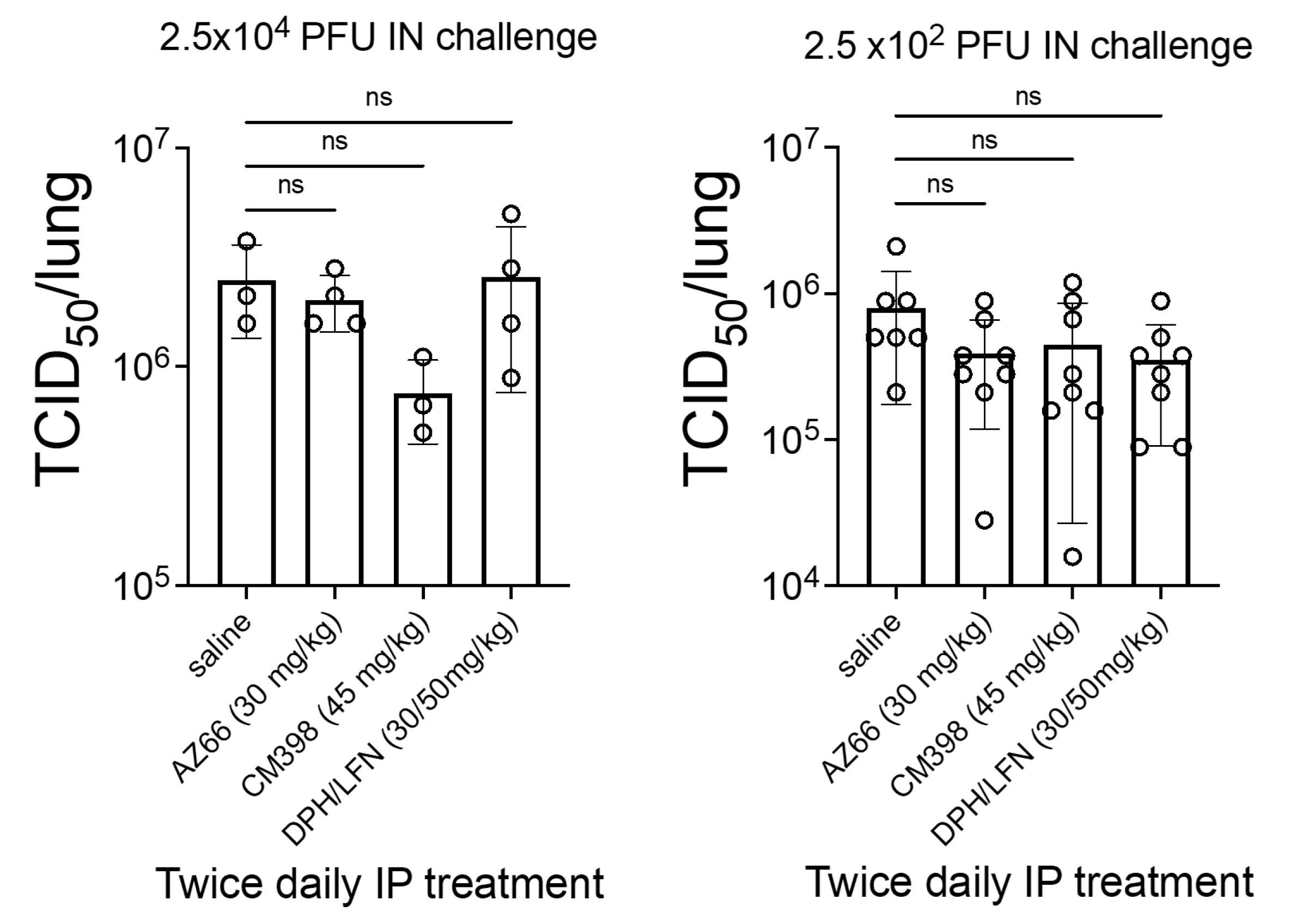
2. Results
3. Discussion
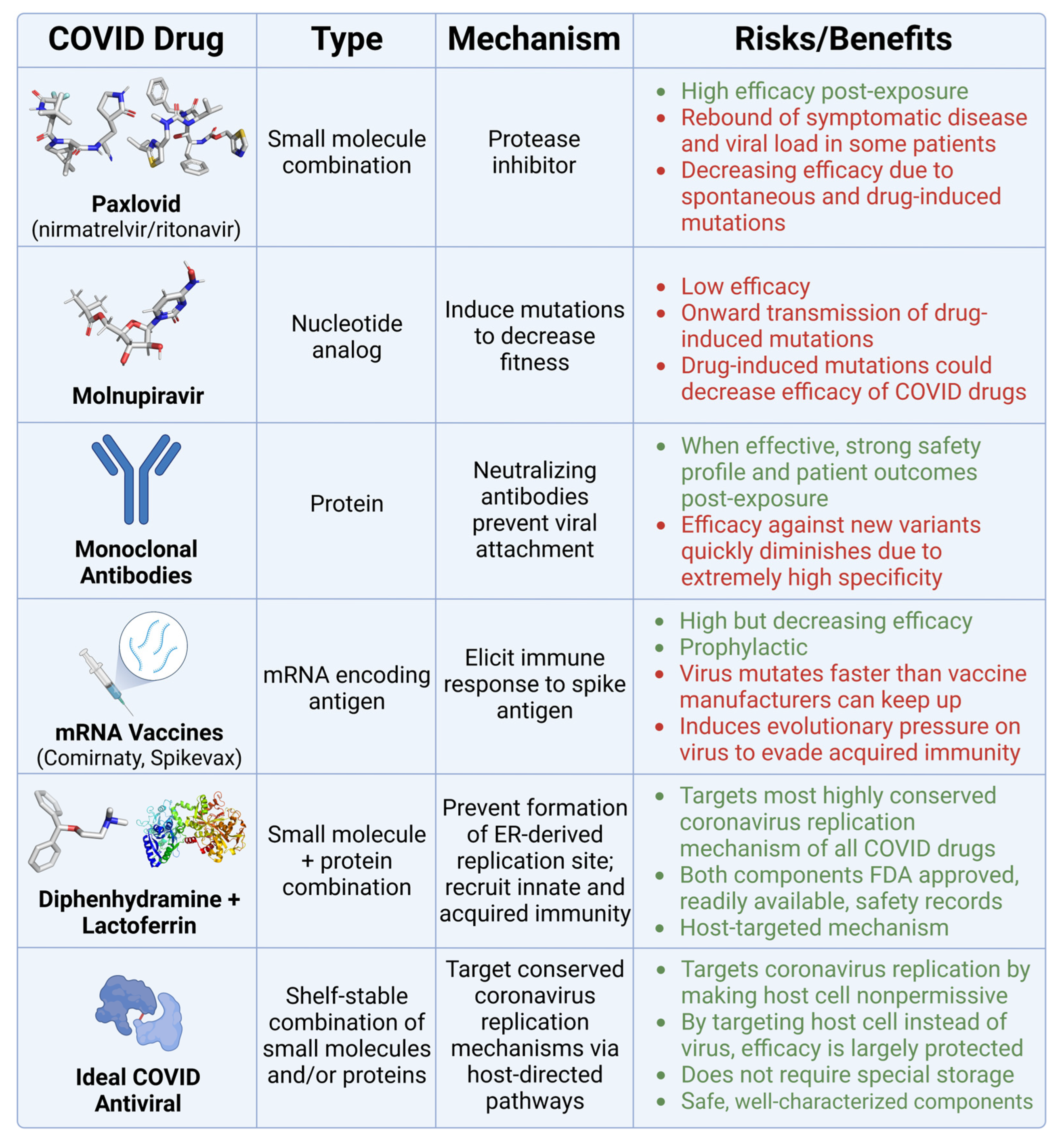
3.1. Future of Coronavirus and COVID Drugs
3.2. Current Tools against COVID, and Their Pitfalls
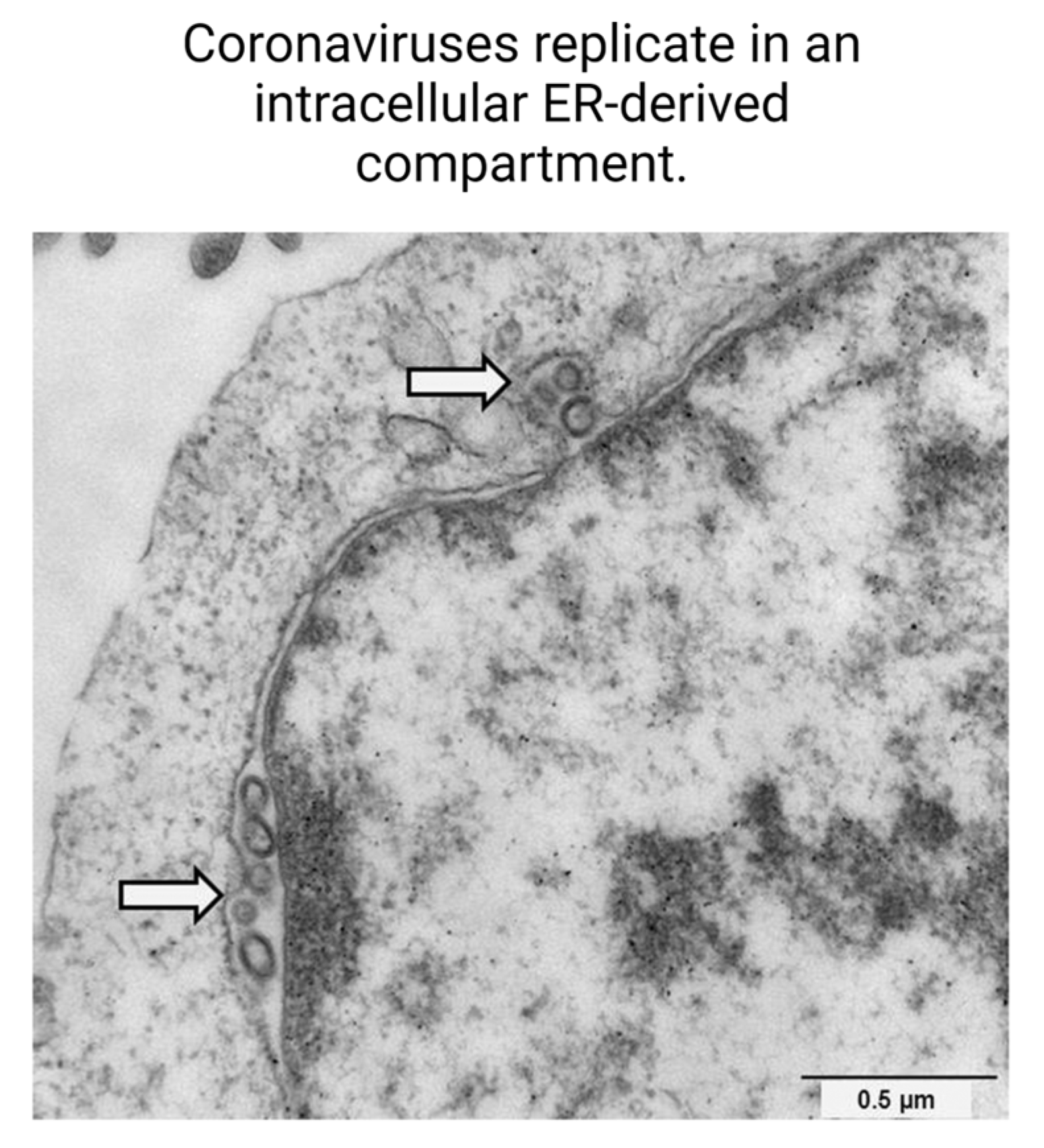
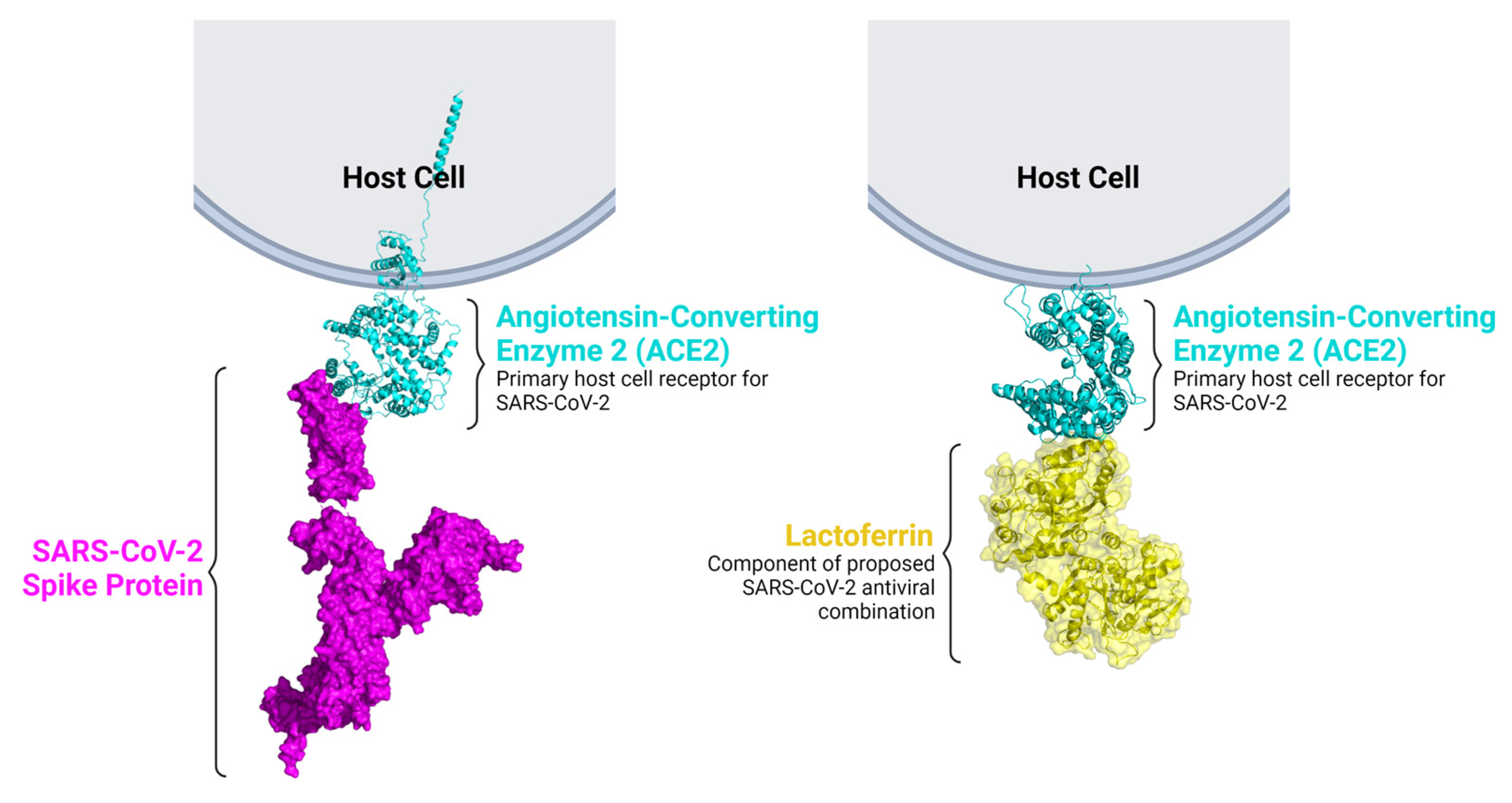
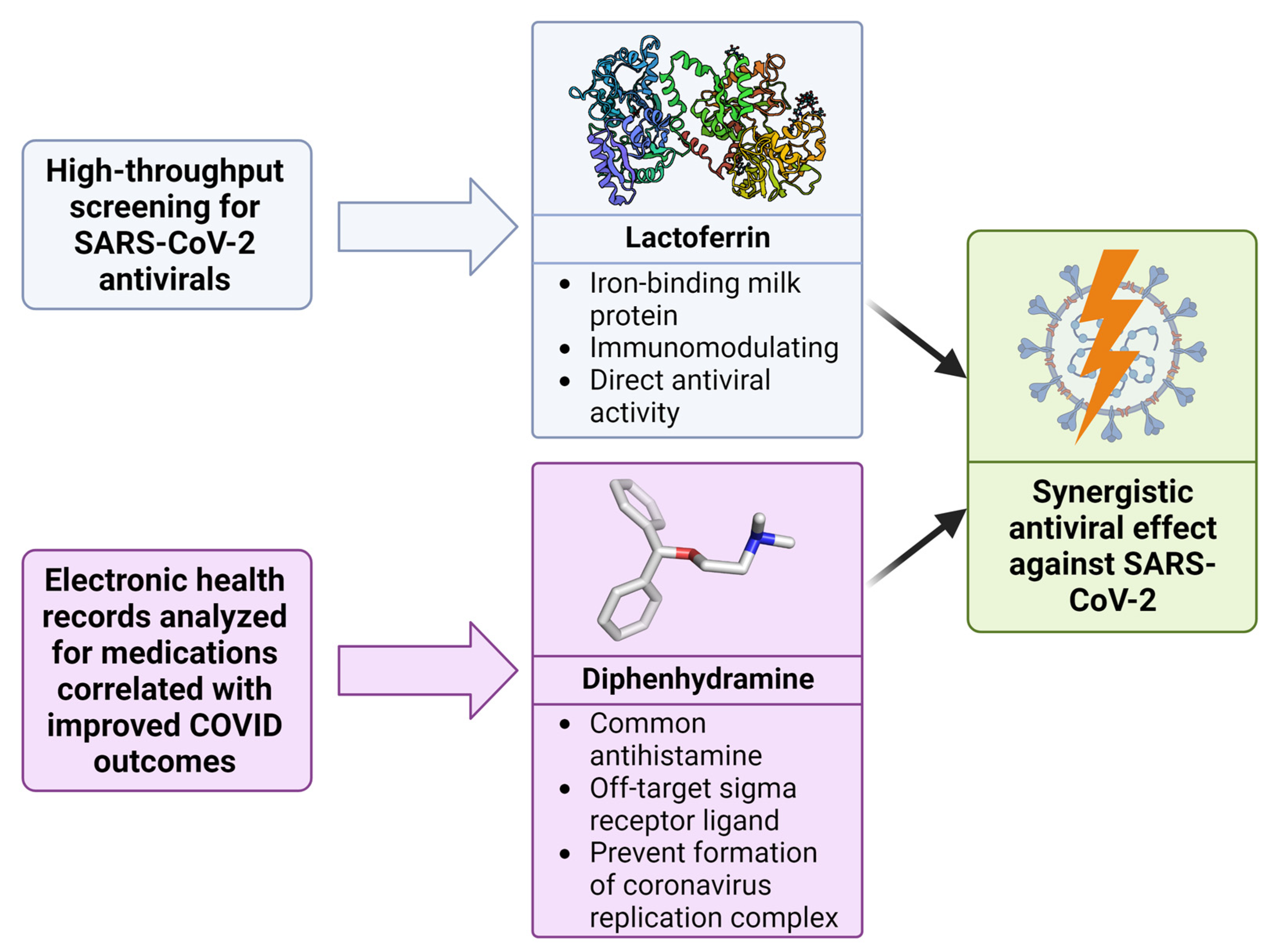
3.3. Repurposed Drugs That Inhibit the Coronavirus Life Cycle
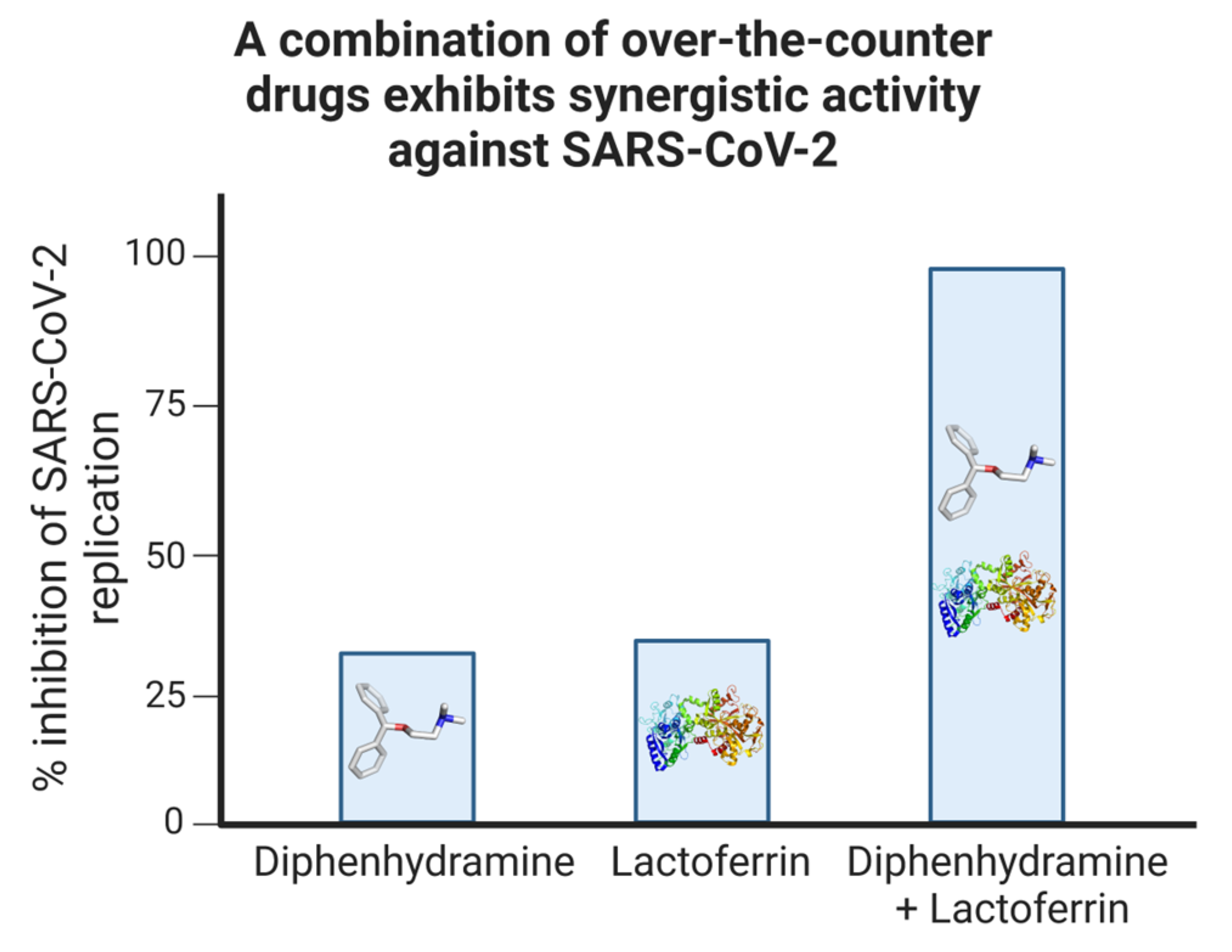
3.4. Antiviral Drug Combinations for COVID
4. Materials and Methods
4.1. Virus Culturing and Growth
4.2. Sigma Ligands and Other Chemicals Used in This Study
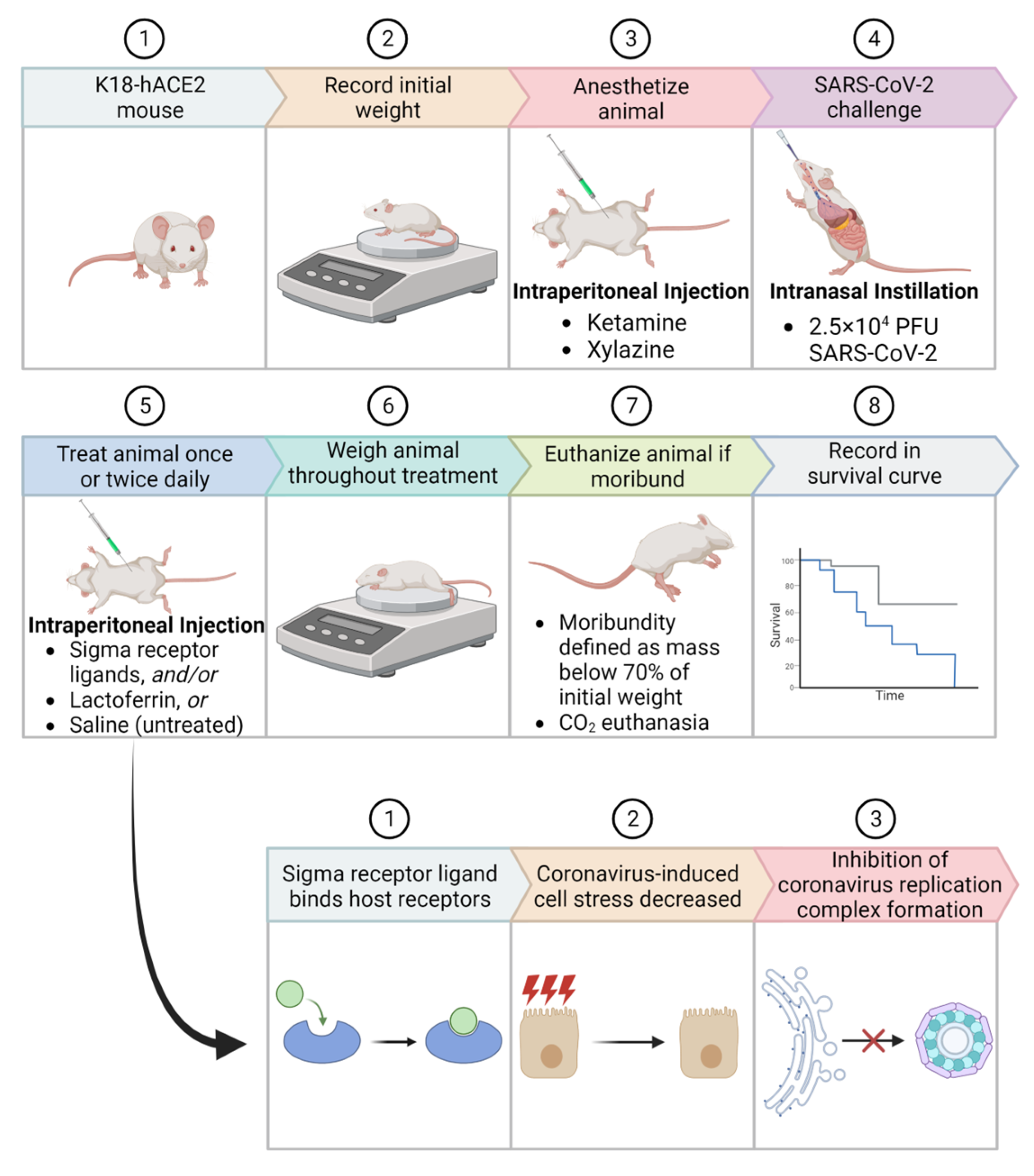
4.3. Animal Challenge Experiments
4.4. Modeling Interactions between SARS-CoV-2 Spike Protein and Ligands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, K.; Wang, C.; Sun, T. The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson’s Disease (PD) and Major Depressive Disorder (MDD). Front. Pharmacol. 2019, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. The Sigma-1 Receptor in Cellular Stress Signaling. Front. Neurosci. 2019, 13, 733. [Google Scholar] [CrossRef]
- Merlos, M.; Romero, L.; Zamanillo, D.; Plata-Salamán, C.; Vela, J.M. Sigma-1 Receptor and Pain. Handb. Exp. Pharmacol. 2017, 244, 131–161. [Google Scholar] [CrossRef]
- Sambo, D.O.; Lebowitz, J.J.; Khoshbouei, H. The Sigma-1 Receptor as a Regulator of Dopamine Neurotransmission: A Potential Therapeutic Target for Methamphetamine Addiction. Pharmacol. Ther. 2018, 186, 152–167. [Google Scholar] [CrossRef]
- Fung, T.S.; Huang, M.; Liu, D.X. Coronavirus-Induced ER Stress Response and Its Involvement in Regulation of Coronavirus–Host Interactions. Virus Res. 2014, 194, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Bluhm, A.P.; Li, D.; Khan, J.Q.; Rohamare, M.; Rajamanickam, K.; Bhanumathy, K.K.; Lew, J.; Falzarano, D.; Vizeacoumar, F.J.; et al. Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells. Pathogens 2021, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, L.R.; Norris, M.H.; Vashisht, R.; Bluhm, A.P.; Li, D.; Liao, Y.-S.J.; Brown, A.; Butte, A.J.; Ostrov, D.A. Identification of Antiviral Antihistamines for COVID-19 Repurposing. Biochem. Biophys. Res. Commun. 2021, 538, 173–179. [Google Scholar] [CrossRef]
- Mirabelli, C.; Wotring, J.W.; Zhang, C.J.; McCarty, S.M.; Fursmidt, R.; Pretto, C.D.; Qiao, Y.; Zhang, Y.; Frum, T.; Kadambi, N.S.; et al. Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105815118. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.-Y.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B.; et al. COVID-19 Treatments and Pathogenesis Including Anosmia in K18-hACE2 Mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Strohmeier, S.; Amanat, F.; Gillespie, V.L.; Krammer, F.; García-Sastre, A.; Coughlan, L.; Schotsaert, M.; Uccellini, M.B. Comparison of Transgenic and Adenovirus hACE2 Mouse Models for SARS-CoV-2 Infection. Emerg. Microbes Infect. 2020, 9, 2433–2445. [Google Scholar] [CrossRef]
- Banos, A.; Lacasa, J. Spatio-Temporal Exploration of SARS Epidemic. Cybergeo Eur. J. Geogr. 2007. Systems, Modelling, Geostatistics, document 408. [Google Scholar] [CrossRef]
- CSR MERS Outbreaks. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed on 19 February 2023).
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Li, R.; Ling, J.; Grace, D.; Nguyen-Viet, H.; Lindahl, J.F. Live and Wet Markets: Food Access versus the Risk of Disease Emergence. Trends Microbiol. 2021, 29, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, H.; He, D. Differences in Case-Fatality-Rate of Emerging SARS-CoV-2 Variants. Public Health Pract. 2023, 5, 100350. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 21 March 2023).
- Vaughan, A. Omicron Emerges. New Sci. 2021, 252, 7. [Google Scholar] [CrossRef]
- Charness, M.E.; Gupta, K.; Stack, G.; Strymish, J.; Adams, E.; Lindy, D.C.; Mohri, H.; Ho, D.D. Rebound of SARS-CoV-2 Infection after Nirmatrelvir–Ritonavir Treatment. N. Engl. J. Med. 2022, 387, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, D.; Liu, C.; Donckers, K.; Stoycheva, A.; Boland, S.; Stevens, S.K.; De Vita, C.; Vanmechelen, B.; Maes, P.; Trüeb, B.; et al. The Substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance to Nirmatrelvir; Microbiology: Saint Cloud, MN, USA, 2022. [Google Scholar]
- Zhou, Y.; Gammeltoft, K.A.; Ryberg, L.A.; Pham, L.V.; Tjørnelund, H.D.; Binderup, A.; Duarte Hernandez, C.R.; Fernandez-Antunez, C.; Offersgaard, A.; Fahnøe, U.; et al. Nirmatrelvir-Resistant SARS-CoV-2 Variants with High Fitness in an Infectious Cell Culture System. Sci. Adv. 2022, 8, eadd7197. [Google Scholar] [CrossRef]
- Hu, Y.; Lewandowski, E.M.; Tan, H.; Zhang, X.; Morgan, R.T.; Zhang, X.; Jacobs, L.M.C.; Butler, S.G.; Gongora, M.V.; Choy, J.; et al. Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir; Pharmacology and Toxicology: Salt Lake City, UT, USA, 2022. [Google Scholar]
- Matthew, A.N.; Leidner, F.; Lockbaum, G.J.; Henes, M.; Zephyr, J.; Hou, S.; Rao, D.N.; Timm, J.; Rusere, L.N.; Ragland, D.A.; et al. Drug Design Strategies to Avoid Resistance in Direct-Acting Antivirals and Beyond. Chem. Rev. 2021, 121, 3238–3270. [Google Scholar] [CrossRef]
- Sanderson, T.; Hisner, R.; Donovan-Banfield, I.; Hartman, H.; Løchen, A.; Peacock, T.P.; Ruis, C. A Molnupiravir-Associated Mutational Signature in Global SARS-CoV-2 Genomes. Nature 2023. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Merck’s COVID Pill Loses Its Lustre: What That Means for the Pandemic. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (accessed on 26 September 2023).
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.-W.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Mihelc, E.M.; Baker, S.C.; Lanman, J.K. Coronavirus Infection Induces Progressive Restructuring of the Endoplasmic Reticulum Involving the Formation and Degradation of Double Membrane Vesicles. Virology 2021, 556, 9–22. [Google Scholar] [CrossRef]
- Ricciardi, S.; Guarino, A.M.; Giaquinto, L.; Polishchuk, E.V.; Santoro, M.; Di Tullio, G.; Wilson, C.; Panariello, F.; Soares, V.C.; Dias, S.S.G.; et al. The Role of NSP6 in the Biogenesis of the SARS-CoV-2 Replication Organelle. Nature 2022, 606, 761–768. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Waltzek, T.B.; McGeehan, E.; Loeb, J.C.; Hamilton, S.B.; Luetke, M.C. Isolation and Genetic Characterization of Human Coronavirus NL63 in Primary Human Renal Proximal Tubular Epithelial Cells Obtained from a Commercial Supplier, and Confirmation of Its Replication in Two Different Types of Human Primary Kidney Cells. Virol. J. 2013, 10, 213. [Google Scholar] [CrossRef]
- Crespo, J.; Calleja, J.L.; Fernández, I.; Sacristan, B.; Ruiz-Antorán, B.; Ampuero, J.; Hernández-Conde, M.; García-Samaniego, J.; Gea, F.; Buti, M.; et al. Real-World Effectiveness and Safety of Oral Combination Antiviral Therapy for Hepatitis C Virus Genotype 4 Infection. Clin. Gastroenterol. Hepatol. 2017, 15, 945–949.e1. [Google Scholar] [CrossRef]
- Moreno, S.; Perno, C.; Mallon, P.; Behrens, G.; Corbeau, P.; Routy, J.-P.; Darcis, G. Two-Drug vs. Three-Drug Combinations for HIV-1: Do We Have Enough Data to Make the Switch? HIV Med. 2019, 20, 2–12. [Google Scholar] [CrossRef]
- Sicari, V.; Zabbo, C.P. Diphenhydramine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schafer, A.; Cheng, H.; Xiong, R.; Soloveva, V.; Retterer, C.; Mo, F.; Bavari, S.; Thatcher, G.; Rong, L. Repurposing Potential of 1st Generation H1-Specific Antihistamines as Anti-Filovirus Therapeutics. Antiviral Res. 2018, 157, 47–56. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Li Volti, G.; Leonardi, S. Lactoferrin: Cytokine Modulation and Application in Clinical Practice. J. Clin. Med. 2021, 10, 5482. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration U.S. FDA. GRN 000465 [Cow’s Milk-Derived Lactoferrin]. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=465 (accessed on 22 March 2023).
- van der Strate, B.W.A.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D.K.F. Antiviral Activities of Lactoferrin. Antiviral Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Okada, S.; Tanaka, K.; Sato, T.; Ueno, H.; Saito, S.; Okusaka, T.; Sato, K.; Yamamoto, S.; Kakizoe, T. Dose-Response Trial of Lactoferrin in Patients with Chronic Hepatitis C. Jpn. J. Cancer Res. 2002, 93, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of Orally Administered Bovine Lactoferrin on the Growth of Adenomatous Colorectal Polyps in a Randomized, Placebo-Controlled Clinical Trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Matino, E.; Tavella, E.; Rizzi, M.; Avanzi, G.C.; Azzolina, D.; Battaglia, A.; Becco, P.; Bellan, M.; Bertinieri, G.; Bertoletti, M.; et al. Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial. Nutrients 2023, 15, 1285. [Google Scholar] [CrossRef]
- Navarro, R.; Paredes, J.L.; Tucto, L.; Medina, C.; Angles-Yanqui, E.; Nario, J.C.; Ruiz-Cabrejos, J.; Quintana, J.L.; Turpo-Espinoza, K.; Mejia-Cordero, F.; et al. Bovine Lactoferrin for the Prevention of COVID-19 Infection in Health Care Personnel: A Double-Blinded Randomized Clinical Trial (LF-COVID). BioMetals 2023, 36, 463–472. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public. Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 08–15. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef]
- Piacentini, R.; Centi, L.; Miotto, M.; Milanetti, E.; Di Rienzo, L.; Pitea, M.; Piazza, P.; Ruocco, G.; Boffi, A.; Parisi, G. Lactoferrin Inhibition of the Complex Formation between ACE2 Receptor and SARS CoV-2 Recognition Binding Domain. Int. J. Mol. Sci. 2022, 23, 5436. [Google Scholar] [CrossRef] [PubMed]
- Cappanera, S.; Palumbo, M.; Kwan, S.H.; Priante, G.; Martella, L.A.; Saraca, L.M.; Sicari, F.; Vernelli, C.; Di Giuli, C.; Andreani, P.; et al. When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. J. Clin. Med. 2021, 10, 297. [Google Scholar] [CrossRef]
- Grodzki, M.; Bluhm, A.P.; Schaefer, M.; Tagmount, A.; Russo, M.; Sobh, A.; Rafiee, R.; Vulpe, C.D.; Karst, S.M.; Norris, M.H. Genome-Scale CRISPR Screens Identify Host Factors That Promote Human Coronavirus Infection. Genome Med. 2022, 14, 10. [Google Scholar] [CrossRef]
- Cirino, T.J.; Eans, S.O.; Medina, J.M.; Wilson, L.L.; Mottinelli, M.; Intagliata, S.; McCurdy, C.R.; McLaughlin, J.P. Characterization of Sigma 1 Receptor Antagonist CM-304 and Its Analog, AZ-66: Novel Therapeutics Against Allodynia and Induced Pain. Front. Pharmacol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Shen, B.; Zavaleta, C.L.; Nielsen, C.H.; Mesangeau, C.; Vuppala, P.K.; Chan, C.; Avery, B.A.; Fishback, J.A.; Matsumoto, R.R.; et al. New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects. J. Med. Chem. 2012, 55, 8272–8282. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, M.J.; Robson, M.J.; Abdelazeem, A.H.; Mesangeau, C.; Jamalapuram, S.; Avery, B.A.; McCurdy, C.R.; Matsumoto, R.R. Synthesis and Pharmacological Characterization of a Novel Sigma Receptor Ligand with Improved Metabolic Stability and Antagonistic Effects Against Methamphetamine. AAPS J. 2012, 14, 43–51. [Google Scholar] [CrossRef]
- Intagliata, S.; Sharma, A.; King, T.I.; Mesangeau, C.; Seminerio, M.; Chin, F.T.; Wilson, L.L.; Matsumoto, R.R.; McLaughlin, J.P.; Avery, B.A.; et al. Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-Dihydroisoquinolin-2(1H)-Yl)Butyl)-3-Methyl-1H-Benzo[d]Imidazol-2(3H)-One (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo. AAPS J. 2020, 22, 94. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Park, J.-G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allué-Guardia, A.; Olmo-Fontánez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.; et al. Lethality of SARS-CoV-2 Infection in K18 Human Angiotensin-Converting Enzyme 2 Transgenic Mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A Web Server for Protein–Protein and Protein–DNA/RNA Docking Based on a Hybrid Strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-Ray Structures Reveal a Large Hinge-Bending Motion Important for Inhibitor Binding and Catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-Dimensional Structure of Diferric Bovine Lactoferrin at 2.8 Å Resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-Directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkowitz, R.L.; Bluhm, A.P.; Knox, G.W.; McCurdy, C.R.; Ostrov, D.A.; Norris, M.H. Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics. Int. J. Mol. Sci. 2023, 24, 15718. https://doi.org/10.3390/ijms242115718
Berkowitz RL, Bluhm AP, Knox GW, McCurdy CR, Ostrov DA, Norris MH. Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics. International Journal of Molecular Sciences. 2023; 24(21):15718. https://doi.org/10.3390/ijms242115718
Chicago/Turabian StyleBerkowitz, Reed L., Andrew P. Bluhm, Glenn W. Knox, Christopher R. McCurdy, David A. Ostrov, and Michael H. Norris. 2023. "Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics" International Journal of Molecular Sciences 24, no. 21: 15718. https://doi.org/10.3390/ijms242115718
APA StyleBerkowitz, R. L., Bluhm, A. P., Knox, G. W., McCurdy, C. R., Ostrov, D. A., & Norris, M. H. (2023). Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics. International Journal of Molecular Sciences, 24(21), 15718. https://doi.org/10.3390/ijms242115718





