Abstract
Human skin has long been known as a protective organ, acting as a mechanical barrier towards the external environment. More recent is the acquisition that in addition to this fundamental role, the complex architecture of the skin hosts a variety of immune and non-immune cells playing preeminent roles in immunological processes aimed at blocking infections, tumor progression and migration, and elimination of xenobiotics. On the other hand, dysregulated or excessive immunological response into the skin leads to autoimmune reactions culminating in a variety of skin pathological manifestations. Among them is psoriasis, a multifactorial, immune-mediated disease with a strong genetic basis. Psoriasis affects 2–3% of the population; it is associated with cardiovascular comorbidities, and in up to 30% of the cases, with psoriatic arthritis. The pathogenesis of psoriasis is due to the complex interplay between the genetic background of the patient, environmental factors, and both innate and adaptive responses. Moreover, an autoimmune component and the comprehension of the mechanisms linking chronic skin inflammation with systemic and joint manifestations in psoriatic patients is still a major challenge. The understanding of these mechanisms may offer a valuable chance to find targetable molecules to treat the disease and prevent its progression to severe systemic conditions.
1. Introduction
Psoriasis is a chronic–relapsing inflammatory skin disease with a multifactorial etiology, with a strong genetic basis and an autoimmune component. It affects on average 3% of the population and it can appear at any age but is more frequent between the ages of 15 and 30. Although the exact etiology of psoriasis is unknown, according to a widely shared view it can be caused by non-specific triggering factors such as mild trauma, drugs, stress, and bacterial infections which can initiate the inflammatory processes that lead to the development of the disease [1]. Recent studies suggest that a damaging insult to keratinocytes in genetically predisposed individuals can trigger inflammatory pathways that activate the inflammasome NLRP3, resulting in the production of pro-inflammatory cytokines such as IL-1β and CXCL8/IL-8 that mediate the subsequent inflammatory cascade of events leading to the development of clinical manifestations of the pathology [2,3]. The immunological mechanism that drives the amplification of inflammation in the skin is centered on the interaction between dendritic cells (DCs) and T cells that generates a self-sustaining inflammatory cycle around the TNF-α/IL-23/IL-17 axis, underlying the development of psoriatic plaques [4]. Psoriasis is associated with systemic and joint manifestations; in fact, 30% of patients develop psoriatic arthritis (PsA) and comorbidities such as increased risk of cardiovascular diseases and metabolic syndrome [5,6,7,8,9]. For these reasons, it is defined as a systemic disease.
According to the current view, the encounter of one of many potential environmental factors may induce keratinocyte stress leading to the release of self-DNA, self-RNA, and antimicrobial peptide complexes into the extracellular compartment [10]. These complexes activate, via TLR9, TLR7 and TLR8 myeloids (mDCs) that produce TNF-α, IL-23, IL-12, and the plasmacytoid (pDCs) that produce IFN-α. DCs migrate into regional lymph nodes and, by presenting antigens, can activate T cells. T cells play a major role in the pathogenesis of cutaneous psoriasis. Cells with the Th17 phenotype are markedly amplified in psoriatic skin lesions, and IL-17 was also shown as an excellent target for the most cytokine blocking therapies [11,12]. CD8+ T cells mainly residing in the epidermis also play a role in the pathogenesis of the disease.
It is, however, unknown the link between T cell responses arising and amplifying in the skin and the development of extra cutaneous manifestations and comorbidities. Of particular interest is the link with psoriatic arthritis, which usually starts from the entheses and in most cases develops 8–10 years after the appearance of the cutaneous symptoms [13,14]. The development of psoriatic arthritis has been shown to be preceded by a long asymptomatic phase in which signs of synovio–entheseal inflammation are evident only by diagnostic imaging techniques [15].
The link between T cells and the asymptomatic phase may provide a new reading key to understanding psoriasis and associated comorbidities, opening new perspectives for therapeutic interventions. Here, we took advantage of the increasing knowledge accumulating in the field of cutaneous immunology to envisage possible physiological mechanisms of skin immunology for the establishment of psoriasis and its systemic manifestations.
We reviewed the most updated literature on T cell mechanisms linking cutaneous and systemic immunity and integrate them with literature data on the role of T cells in psoriatic disease. The scenario that emerges could substantially contribute to the understanding of this complex disease and its manifestations at distant sites.
2. T Cells and Immunology of the Skin
The skin is the largest organ of the body and a central barrier to maintain tissue homeostasis and prevent damage upon environmental triggers, as well as microbial assaults. It also represents an exclusive environment in which skin cells interact with immune cells to induce suitable immune responses. Also, the idea that skin cells interact with external microorganisms (bacteria, viruses, fungi, archea) in a delicate equilibrium that needs to be maintained to ensure skin health is gaining momentum. Alteration of entities in skin microbiota–host interactions would thus favor the establishment of potentially pathogenetic conditions [16].
Although skin immune responses are mediated by different immune cell types, including Langerhans cells, mast cells, macrophages, neutrophils, and B and T lymphocytes, the initial trigger is often given by stimulation and activation of skin cells, including keratinocytes, fibroblasts, endothelial cells, and adipocytes. These cellular elements work as sensors by interacting with microorganisms as well as poisonous and irritating molecules, thus alerting the immune effector cells [17,18]. Degenerated and degraded structural cells or matrixes forming molecules can also act as stimuli to initiate a pathological response, ultimately leading to impairment of skin integrity and function.
The type, intensity, and duration of the immune responses largely depend on the nature of the stimuli and type of recruited immune cells. To this aim, the transition of resident structural cells to chemokine-secreting and inflammation receptor-expressing elements is fundamental. Hence, keratinocytes, in addition to synthesizing the major structural and isolating proteins of the skin, i.e., keratin, are endowed with the ability to act as sensor cells, alerting immune cells by releasing alarm signals such as intracellular adenosine triphosphate (ATP). Inflammasome-mediated responses through activation of the ATP receptor P2X7 induces IL-23 secretion [3,19,20]. IL-23, together with IL-12 and IL-17, are among the central cytokines involved in the pathogenesis of psoriasis [21,22]. Aberrant polarization of CD4+ T helper lymphocytes induce secretion of TNF-α, IFN-γ, IL-17, and IL-22, activating keratinocytes, thus amplifying inflammation in psoriatic plaques [23].
Cutaneous DCs and keratinocytes are able to perceive tissue damage derived from different sources, through receptors recognizing molecular patterns deriving from pathogens or host-derived molecules exposed following tissue damage [24,25,26,27]. Skin DCs consist of various populations located in the epidermis or dermis and respond to antigens and allergens with the production of chemokines, inflammatory cytokines, and biocidal molecules such as nitric oxide (NO). Langerhans cells, dermal DCs (dDCs), pDCs, macrophages, and the recently identified CD103+ DCs represent a fundamental part of the complex puzzle of skin defense, strategically positioned for antigen presentation to circulating but also resident lymphocytes [18,23,28].
CD103+ DCs of the type 1-conventional dendritic cell (cDC1) subtype are specialized in cross-presentation of cell-associated foreign antigens and upon activation will migrate to the skin-draining lymph nodes to activate naïve T cells, preparing them to differentiate into effectors [29]. The activated T cells can then migrate to the epidermis to control infection by producing signals that recruit additional immune effector cells. In addition, DCs of the type 2-conventional dendritic cell (cDC2) subgroups play a central role in the induction and maintenance of T cell tolerance of the skin towards both self-antigens and antigens derived from commensal bacteria [30,31].
Healthy skin contains a number of T cells that are more than double than the ones found in peripheral blood [32,33]. Most of them are memory T cells that have previously encountered antigens and can rapidly reactivate. CD8+ T cells are mainly found in the epidermis, whereas CD4+ T lymphocytes are found mainly in the dermis, where they have the role of amplifying the immune response. Among T cells, γδT cells are known to enhance wound healing by secretion of the insulin-like growth factor 1 (IGF-1) [34,35]. Depending on the Vδ and Vγ gene expressed, these cells contain different subpopulations, showing heterogeneous tissue colonization [36]. Vδ1 γδ T cells are mainly located in the dermis, while Vδ2 T cells are more abundant in peripheral blood and dermis [37]. Inappropriate activation of dermal γδT cells plays a role in psoriasis pathogenesis, since they are endowed with the ability to produce IL-17 [38].
Memory T cells in the blood have been initially classified into central memory that express CCR7 and CD62L high endothelial venule (HEV) homing molecules and constitutively recirculate between the blood and secondary lymphoid organs as well as effector memory T cells found only in the blood and lacking HEV homing molecules [39].
New light has been recently shed on the role of tissue-resident memory T lymphocytes (TRM) residing in the skin to grant a prompt response in case of re-infection of the tissue by microorganisms. Both helper CD4+ and cytotoxic CD8+ TRM have been found in the skin [40,41,42,43,44,45] and also act as responders in autoimmunity as well as in psoriasis and other skin diseases [46]. Resident memory T lymphocytes of the skin may allocate to this organ for years as they stably express CD69 and the integrin alpha E (CD103), which in turn binds E-cadherin expressed by keratinocytes. Moreover, they express skin homing molecules such as cutaneous leukocyte antigen (CLA) and the chemokine receptors CCR4 and CCR10. This is paralleled by down-regulation of membrane molecules involved in cell recirculation between tissues [47]. In the human epidermis there are indeed many CD4+ and CD8+ TRM [48]. Skin TRM are found at increased level in the upper hair follicles and require IL-7 and IL-15 signaling for their survival [49,50]. In the generation and persistence of TRM, a central role is played by transforming growth factor-β (TGF-β) which is required for the CD8+ TRM to be retained in the epidermis and mediates its effects through the CD103 induction on T cells [51].
In the epidermis, TGF-β produced by keratinocytes favors the differentiation of skin-recruited CD8+ T cells into TRM cells after antigen-specific activation and expansion in the lymph node and it also induces their persistence in the epidermis. Intriguingly, TRM activated by both antigen-specific and bystander activation can persist as TRM, however, under limiting conditions, antigen-specific TRM cells were more efficiently retained than bystander TRM cells. This mechanism may represent a selective pressure that favors the persistence and accumulation of antigen-specific TRM cells at the skin barrier and may, on the other hand, set the basis for postulating that a fraction of cells with effectors/terminally differentiated phenotype derived from non-antigen-specific cells may be found in the circulation under chronic inflammatory conditions (Figure 1).
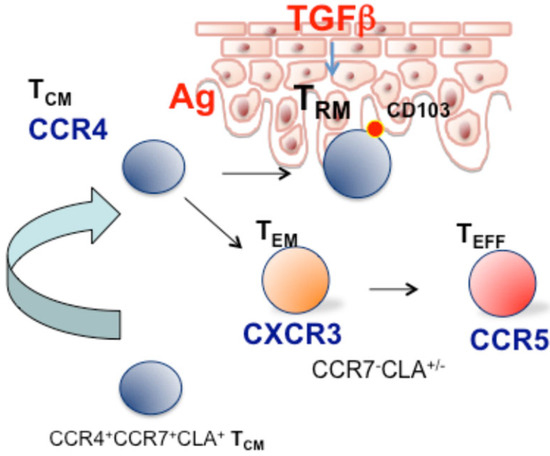
Figure 1.
Role of circulating CCR4+ CCR7+ TCM/TMM cells in skin patrolling. After antigens encounter or are under appropriate inflammatory conditions, (i.e., TGF-β), CCR4+ CCR7+ T cells recruited peripheral blood acquire a long-lived TRM phenotype. In the long term, antigen-specific TRM may accumulate, whereas TRM activated by bystander activation may lose the resident phenotype and be found in the circulation with CXCR3+, CCR5+ effector/EMRA phenotype.
In addition to the prominent role of TRM, an indication of a skin-to-blood recirculating subset with TCM phenotype and expressing CCR4 was provided by Luster and co-workers showing, in a Kaede transgenic mouse model, that CD4 memory T cells can egress from the skin in a CCR7-dependent manner. Specifically, the authors characterized a recirculating memory CD4 T cell subset with phenotype CCR7+CD69-CD103-/CCR4+ that can enter the circulation and maintain the capability to migrate into normal skin [52]. Circulating CD8 T cells also have the ability to enter the skin in the absence of infection and were identified for their lack of CD69 expression. In support of the physiological role of this mechanism, it has been shown that loss of recirculating memory T cells impairs host defense to skin infection [53]. As an additional characterization, Clark and coworkers in 2015 have defined two distinct populations of CCR7+ recirculating T cells: the central memory T cells (TCM) expressing L-selectin and CCR7+ T cells negative for L-selectin, which were classified as migratory memory T cells (TMM) [48]. This circulating skin-tropic TMM subset produced cytokines at an intermediate level between TCM and effector memory T cells.
TMM cells were present in the dermis and absent from the epidermis. A significant fraction of these cells also expressed CD69, suggesting that they may be in a transitional state towards a more differentiated phenotype. About two thirds of TMM in both healthy skin and inflamed tissues, however, lack CD69. These cells, unlike TCM and naïve T cells, lack L-selectin and probably cannot migrate through high endothelial venues to enter the lymph nodes from the blood, but rather migrate directly back to the skin where they could either give rise to TRM or recirculate back to the blood. Possibly, both TRM and recirculating T cells could contribute to the pathogenesis of inflammatory and autoimmune diseases, and understanding all the steps of this process can be critical for the identification of therapies targeting pathogenic T cells involved in specific phases of disease manifestations.
A T cell-intrinsic skin-homing program is established during priming in the context of skin infection and maintained even without antigen re-exposure. According to a recent definition, CCR4 is a skin-homing receptor expressed by memory T cells which have been primed in lymph nodes draining the skin compartment [54,55,56]. Cells expressing CCR4 can enter the skin under normal non-inflammatory conditions, by interacting with low levels of the homing molecules E-selectin and CCL17, constitutively expressed in resting endothelium, that bind CLA and CCR4 on T cells [57,58].
Actually, an earlier study by Campbell and colleagues had already suggested the role of the chemokine receptor CCR4 as a skin-homing molecule mediating the migration of T cells from the blood to the dermis. The study shows that in peripheral blood a considerable number of memory T cells express the chemokine receptor CCR4 and respond to the CCL17 chemokine [59]. The CCL17 chemokine is constitutively expressed by skin epidermal cells and released under inflammatory conditions. In addition, CCR4 also binds to CCL22, which also plays a role in other tissues [60].
Binding of CCR4 to CCL17 causes the arrest of memory T cells under physiological flow integrin-dependent adhesion of skin memory T cells to the cell-adhesion molecule ICAM-1. CCR4/CCL17 axis therefore emerges as a central element for the interaction between circulating memory T cells and the skin vasculature, thus directing memory T cells to their original target tissues [59,61]. The chemokine receptor CCR4 would favor the trafficking of cutaneous memory type 2 Th cells as well as of other T cell phenotypes to the skin [62].
In the CD8 compartment, CCR4+ T cells were predominantly found in the CD27+CD28+CD45RA− memory subset and expressed the CCR7+CCR5− phenotype. CCR4+CD8+ T cells did not express perforin and granzymes A and B that are a feature of differentiated effectors. This evidence has suggested that they were more immature than CCR6+CD8 early effector memory T cells that express GraA and perforin and accordingly with more recent study contribute to place these cells in an early stage of the memory T cell differentiation pathway [63].
According to this view, analysis of CCR4 and skin-tropic molecules in the different stages of memory T cell differentiation in CD8+, CD4+CD45RA−CCR7+ (TCM), and CD45RA−CCR7− (TEM) cells indicate that the skin-homing CCR4 marker is expressed mainly in cells with a CCR7+ phenotype, both L-selectin+ and L-selectin−, thus including TCM and TMM subsets described by Clark and colleagues [48] CCR4+TCM/MM cells also express high level of CLA, whereas TEM expresses CXCR3 and CCR5 and lower levels of CCR4 and CLA [64]. This indeed supports the concept that progressive stages of memory T cell differentiation have different chemokine receptor profiles, and that skin-tropic features are progressively lost as the differentiation pathway progresses towards the effector memory/effector phenotype.
In another study, the question of whether CCR4+ TCM cells could represent a subset with high plasticity that, upon antigen encounter, can progressively shift their phenotype toward CXCR3+ TEM was addressed. Sorted CCR7+CD45RA−CXCR3− cells from peripheral blood mononuclear cells (PBMCs) stimulated with αCD3/αCD28 beads were analyzed at different time points. On day 3, after T cell receptor (TCR) stimulation, all CCR4−-enriched TCM cells shifted their phenotype to double positive CCR4+CXCR3+ TCM, and at later time points a small fraction of cells that were single positive for CXCR3 and a fraction of cells with the TEM phenotype were detected [65].
Under physiological conditions and in early inflammation, CCR4 could therefore be required for lymphocyte trafficking to the skin, whereas under severe inflammatory conditions other chemokines could play a major role in the recruitment of effector memory/effector T cells. Consistent with this view, different groups evidenced that T cell-mediated skin inflammation is largely independent of CCR4 and rather requires CXCR3 [61].
CCR4 central/migratory memory T cells could be crucial in skin patrolling rather than in mediating the recruitment of differentiated effectors cells to the inflamed skin. To explain this phenomenon, a T cell phenotype hierarchy in skin inflammation that places early memory and antigen-specific T cells upstream of the inflammatory cascade and a massive recruitment of CXCR3 effector from the blood as a downstream event of advanced inflammation were postulated [66].
The skin compartment had previously been proposed as a “peripheral lymphoid organ”, important for antigen encounters and lymphocyte differentiation [67]. The evidence accumulated in this field strengthens at least the role of the skin as a preferential trafficking site for TCM/MM in an early stage of differentiation, where it is possible that antigen encounter occurs. These cells after an antigen encounter and exposure to inflammatory stimuli could progress to a non-circulating TRM phenotype [40,68] (model depicted in Figure 1). In immunopathological skin conditions such as psoriasis, the expanded subset of TCM cells expressing CCR4 and CXCR3 could play a role in disease recurrence or redistribution to distant sites such as joint synovial tissues and enthesis.
3. T Cells in Psoriasis Pathogenesis
Critical components of the T cell response involved in the initiation and amplification phases of cutaneous psoriasis have been described in the last two decades. It should be mentioned that in the psoriatic skin, CD103+ tissue resident memory T cells of CD8 lineage were found by Eidsmo and coworkers, describing the role of CD8 TRM cells with a Tc17 phenotype in specific disease memory in sites of recurrent psoriasis [46,69,70].
As regards to the role of T cells in the early formation of the psoriatic plaque, Bowcock and Krueger proposed a role for the perivascular aggregation of T cells and mature DCs in the dermis which, in this case, could work as an ectopic lymphoid aggregate or, according to a more recent definition, as a tertiary lymphoid structure [71].
Subsequent studies indicated the interaction between the chemokine, CCL19, and its ligand CCR7, which is typical of T cell activation in lymphoid structures as a key event for the recruitment of these cells also in psoriatic skin [72]. In the dermis of psoriatic plaques, the presence of a lymphoid aggregates has been reported by Mitsui and colleagues, evidencing also the expression of CCL19 and CCR7 [73]. This first suggested a role for the CCR7/CCL19 axis in disease pathogenesis. At the same time, a study by Bosè and colleagues showed that anti-TNF-α therapy in psoriasis patients led to the inhibition of the CCL19/CCR7 axis in psoriatic skin lesions and that this phenomenon was correlated with disease regression [74]. This importantly enlightened CCR7/CCL19 axis in skin lymphoid aggregates and showed that one of the mechanisms at the basis of the long-term effect of TNF-α-blocking treatment is actually the destruction of these structures. To this end, it is important to mention that among the multiple functions of TNF-α/β they also contribute to the maintenance of the secondary lymphoid organ architecture and promote angiogenesis through the induction of VEGF [75,76,77].
Along this line, data obtained by Canete and colleagues highlighted the presence of lymphoid aggregates in the synovial tissues of patients with psoriatic arthritis and showed that these structures were significantly reduced by anti-TNF-α therapy [78].
Deep phenotyping of the circulating T cell compartment performed in our laboratory showed enhancement in the percentage of memory T cell subsets expressing putative pathogenic double-positive IL-17 and IFN-γ non-classic Th1, described by Annunziato and colleagues [79], and CD8 cells, as compared to healthy subjects. Therefore, it was envisaged that in the dermal lymphoid structures of psoriatic plaques something essential for the pathogenesis of the systemic disease manifestations could happen [80,81,82,83]. In particular in these structure, TCM cells attracted from the blood through the CCR7/CCL19 chemokine axis could be activated either by an antigen encounter or by bystander activation and give rise to both TRM or TCM/TMM that egress the skin with an inflammatory phenotype, but at different stages of memory differentiation.
Then, a series of studies were developed to investigate the hypothesis that skin-to-blood recirculation of T cells could take part in the development of psoriatic arthritis and comorbidities [84].
4. T Cells in the Pathogenesis of Psoriatic Arthritis
Psoriatic arthritis develops in up to 30% of patients with cutaneous psoriasis and in the majority of cases it follows the development of the cutaneous manifestations for 8 to 10 years [85].
Enthesitis is indeed a feature of psoriatic arthritis and it is increasingly evident that, in the joints of patients with psoriatic arthritis, inflammation can start from the entheses, the attachment sites of ligament to bone, during a subclinical phase of the disease [86,87]. In the subclinical phase of entheseal, inflammation psoriatic arthritis (PsA) is silent and is only evidenced by diagnostic imaging techniques such as ultrasonography, magnetic resonance imaging (MRI), and computed tomography that have empowered the concept of an evolution from cutaneous to synovio-entheseal inflammation [13,14,15]. This evidence also opened the possibility to investigate the events linked to the development of psoriatic arthritis in patients with psoriasis.
For a better understanding of the link between cutaneous psoriasis and psoriatic arthritis, it is important to underline that psoriasis has an autoimmune component and T cells reactive to autoantigens cathelicidin LL-37, melanocytic ADAMTSL5, lipid antigen PLA2G4D, and keratin 17 have been identified in patients’ peripheral blood [88,89,90]. The possibility that activated T cells reacting or cross-reacting with a self-component may enter the bloodstream and reach distant organs should therefore be considered as a causal link with joint manifestations and systemic inflammation.
In patients with psoriatic arthritis, Curran and colleagues analyzed the clonotype of T cells in the inflamed joint, peripheral blood, and skin samples by sequencing theTCRβ chain genes [91]. This pivotal work identified three populations of T cells in joint tissues: one highly represented population of polyclonal CD4 T cells that did not persist in the tissue after treatment with methotrexate, a second population of moderately expanded inflammation-related clones either of CD4 or CD8 lineage, and finally, a small population of highly expanded clones which were only of the CD8 lineage. These CD8 T clones showed a marked expansion in both peripheral blood and synovial fluid and persisted during methotrexate treatment.
These expanded CD8 T cell clones were proposed as potential drivers and could link psoriasis and PsA [92,93,94].
As regards the major polyclonal CD4 T cell population that has been described both in blood and in inflamed tissue, it could potentially represent a recirculating population of the Th17/Th1 subset originating in psoriatic plaques that traffic to the joints [91]. On this basis, the previously mentioned hierarchy in the T cell-mediated cascade of events characterizing psoriatic disease, comprising an autoimmune clonally expanded CD8+ T cell subset followed by Th17 and Th1 CD4+ T cells expanded either by cross-stimulation or by bystander activation and a major downstream recruitment of CXCR3+ T cells with different specificities induced by the increased expression of the chemokine CXCL10 has been hypothesized [95]. Accordingly, our group and a parallel study of scRNAseq showed accumulation of T cells with CXCR3 and an effector phenotype in the synovial fluid, paired by a massive increase in the CXCL10 chemokine which binds CXCR3 [81,96].
Given the amount of evidence collected in the field of cutaneous immunity in physiological conditions, the concept could be extended to skin immunopathology, by hypothesizing that in human diseases of barrier tissues, such as in psoriasis, a similar dynamic balance between tissue resident memory T cells and the pool of recirculating T cells could be involved in the pathogenesis of the disease [75].
Consistent with the role enlightened for CCR4 in skin-homing T cells and in the cells egressing the skin with a TCM phenotype, CCR4+ CD4 T cells in the circulation correlated positively with the severity of the cutaneous disease [82,97]. CCR4+ memory T cells could therefore represent a recirculating population responsible for systemic manifestations associated with severe psoriasis.
By contrast, analysis of the possible link between skin psoriasis and systemic inflammation indicates mainly a correlation with the percentage of CD8 T cells in a terminally differentiated phenotype (TEM and TEFF). This has been observed in different studies, evidencing a correlation between CCR4+ CCR5+ CD8 TEFF with serum level of C-reactive protein (CRP) and with (psoriasis area and severity index) PASI score in patients with psoriatic disease. Accordingly, in patients with psoriasis and PsA, a correlation between CD8 TEMRA-expressing CD69 early activation markers and the level of serum CRP was observed. Regarding CD4 T cells, a positive correlation with serum CRP was observed only with the percentage of CCR6+ terminally differentiated effector memory cells re-expressing CD45RA (EMRA) in patients with psoriasis. This suggests an involvement of terminally differentiated CD8 T cells, and to a minor extent of Th17 TEMRA, in the systemic inflammatory state [82,97]. In patients with PsA, analysis of blood and synovial fluid memory CD4+ and CD8+ T cells indicates a shift from the CCR4+ phenotype to CCR4+CXCR3+ and CXCR3+CCR4-, respectively [81].
Particularly intriguing is the link between psoriasis and PsA.
In light of the role that emerged for CCR4 in the recirculation of memory T cells forming the skin to the peripheral blood, we addressed the possibility that CCR4 cells are clonally linked to the self-reactive clones found in the blood and joint tissues of PsA patients.
To understand the mechanistic and clonal link between skin-primed T cells and clone expansion in the peripheral blood of psoriatic disease patients or in the synovial fluid of PsA individuals, an imiquimod-induced psoriasis-like inflammation model in K5-mOVA.tg mice expressing ovalbumin on the membrane of keratinocytes adoptively transferred with ovalbumin-specific OT-I.tg naïve CD8+ T cells was used to add the antigen-specific T cell component to the model of psoriasis-like inflammation [98].
In this mouse model, it was shown that prolonged skin inflammation induced by imiquimod favors the activation of OT-I CD8+ T cells specific to the cutaneous self-antigen [65]. These cells had a CCR4+ phenotype and were found in peripheral blood and in an expanded pool of memory T cells in the spleen. Therefore, the mechanistic evidence is that psoriasis-like skin inflammation induced by imiquimod-activated T cells specific to cutaneous self-antigens recirculate from skin to blood to spleen.
To understand if an analogous mechanism could play a role in spreading tissue damage and inflammation to the joints in human PsA, we analyzed scRNA-seq data paired with TCR αβ chain sequencing in paired samples of PsA patients’ peripheral blood and synovial fluid CD8 T cells [96]. Clonotype analysis showed a clonal expansions in the CCR4+ TCM subset in the peripheral blood of PsA patients as compared to their CCR4 negative counterpart; moreover, there was a clonal link between these expanded CCR4+ clones in the blood and clones found in the expanded CXCR3+ effectors in the synovial fluid of PsA patients [65]. This finding reinforces the importance of antigen-specific cells (likely self-antigen-specific T cells) entering the systemic circulation in generating inflammation and damage to the joints of PsA patients, with a possible shift in their phenotype towards more differentiated and cytotoxic effectors (Figure 2).
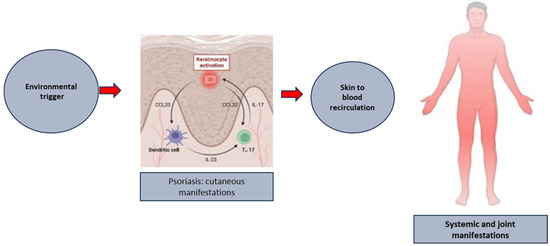
Figure 2.
The putative link between the formation of established psoriatic plaques and the development of systemic and joint manifestation of the disease.
Together, this evidence sheds light on the possibility to design alternative therapeutic strategies, aimed at both preventing the exit of activate/antigen-specific T cells from the skin and/or their re-localization to a distant site. This could be achieved either by intervening in the early phase of T cell activation, thus preventing amplification or by inhibiting re-localization. This latter approach may represent a challenge, as one should be able to specifically inhibit specific recirculating/potentially autoreactive subsets without altering the physiological immune responses.
5. Conclusions
In this review, we collected results from studies providing evidence that T cells primed in the skin-draining lymph nodes and expanded during the formation of psoriatic plaques may form a tissue resident memory T cell population specific to cutaneous self-antigens or cross-reactive microbial antigens or may enter the circulation with a CCR7+ TCM phenotype and spread cross-reactive and activated T cells through peripheral blood to a distant site. The clonotypic link between T cells in peripheral blood expressing skin-homing chemokine receptor CCR4 and the effector cells expanded in PsA synovial fluid supports the concept that T cell egressing from the skin and trafficking to the joints can play a role in the evolution from cutaneous to synovio-entheseal inflammation.
Author Contributions
E.R. and D.F. wrote the manuscript and prepared the figures. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the National Recovery and Resilience Plan (NRRP), Mission 04 Component 2 Investment 1.5—NextGenerationEU, call for tender n. 3277 dated 30/12/2021; Award Number: 0001052 dated 23/06/2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and classification of psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef]
- Ferrara, F.; Pambianchi, E.; Pecorelli, A.; Woodby, B.; Messano, N.; Therrien, J.P.; Lila, M.A.; Valacchi, G. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2020, 152, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Casciano, F.; Secchiero, P.; Reali, E. Purinergic Signaling and Inflammasome Activation in Psoriasis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9449. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Brembilla, N.C. Unmet Needs in the Field of Psoriasis: Pathogenesis and Treatment. Clin. Rev. Allergy Immunol. 2017, 55, 295–311. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Gladman, D.D.; Chandran, V. Cardiovascular comorbidities in psoriasis and psoriatic arthritis: Pathogenesis, consequences for patient management, and future research agenda: A report from the GRAPPA 2009 annual meeting. J. Rheumatol. 2011, 38, 567–571. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Kirby, B.; Fitzgerald, O.; van de Kerkhof, P.C. New developments in our understanding of psoriatic arthritis and their impact on the diagnosis and clinical management of the disease. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 264–270. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Menter, A. Burden of disease: Psoriasis and psoriatic arthritis. Am. J. Clin. Dermatol. 2013, 14, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewe, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewe, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef]
- Scher, J.U.; Ogdie, A.; Merola, J.F.; Ritchlin, C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 2019, 15, 153–166. [Google Scholar] [CrossRef]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Merola, J.F. A clinical perspective on risk factors and signs of subclinical and early psoriatic arthritis among patients with psoriasis. J. Dermatol. Treat. 2022, 33, 1907–1915. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Richmond, J.M.; Harris, J.E. Immunology and skin in health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Perez, J.A.; Killeen, M.E.; Yang, Y.; Carey, C.D.; Falo, L.D., Jr.; Mathers, A.R. Extracellular ATP and IL-23 Form a Local Inflammatory Circuit Leading to the Development of a Neutrophil-Dependent Psoriasiform Dermatitis. J. Investig. Dermatol. 2018, 138, 2595–2605. [Google Scholar] [CrossRef]
- Fenini, G.; Karakaya, T.; Hennig, P. The NLRP1 Inflammasome in Human Skin and Beyond. Int. J. Mol. Sci. 2020, 21, 4788. [Google Scholar] [CrossRef]
- Seyedmirzaei, H.; Rezaei, N. Cytokine alterations in psoriasis: An updated review. Expert Rev. Clin. Immunol. 2021, 17, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.L.; Tsai, T.F. Non-immune functions of inflammatory cytokines targeted by anti-psoriatic biologics: A review. Inflamm. Res. 2022, 71, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Fuentes-Duculan, J.; Eungdamrong, N.J.; Abello, M.V.; Novitskaya, I.; Pierson, K.C.; Gonzalez, J.; Krueger, J.G.; Lowes, M.A. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Investig. Dermatol. 2009, 129, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Fuentes-Duculan, J.; Eungdamrong, N.J.; Johnson-Huang, L.M.; Nograles, K.E.; White, T.R.; Pierson, K.C.; Lentini, T.; Suarez-Farinas, M.; Lowes, M.A.; et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J. Allergy Clin. Immunol. 2010, 125, 1261–1268.e1269. [Google Scholar] [CrossRef]
- Zaba, L.C.; Fuentes-Duculan, J.; Steinman, R.M.; Krueger, J.G.; Lowes, M.A. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Investig. 2007, 117, 2517–2525. [Google Scholar] [CrossRef]
- Zaba, L.C.; Krueger, J.G.; Lowes, M.A. Resident and “inflammatory” dendritic cells in human skin. J. Investig. Dermatol. 2009, 129, 302–308. [Google Scholar] [CrossRef]
- Wohn, C.; Ober-Blobaum, J.L.; Haak, S.; Pantelyushin, S.; Cheong, C.; Zahner, S.P.; Onderwater, S.; Kant, M.; Weighardt, H.; Holzmann, B.; et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10723–10728. [Google Scholar] [CrossRef]
- Bedoui, S.; Whitney, P.G.; Waithman, J.; Eidsmo, L.; Wakim, L.; Caminschi, I.; Allan, R.S.; Wojtasiak, M.; Shortman, K.; Carbone, F.R.; et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009, 10, 488–495. [Google Scholar] [CrossRef]
- Gratchev, A.; Schledzewski, K.; Guillot, P.; Goerdt, S. Alternatively activated antigen-presenting cells: Molecular repertoire, immune regulation, and healing. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 272–279. [Google Scholar] [CrossRef]
- Weckel, A.; Dhariwala, M.O.; Ly, K.; Tran, V.M.; Ojewumi, O.T.; Riggs, J.B.; Gonzalez, J.R.; Dwyer, L.R.; Okoro, J.N.; Leech, J.M.; et al. Long-term tolerance to skin commensals is established neonatally through a specialized dendritic cell subgroup. Immunity 2023, 56, 1239–1254.e1237. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A. Skin-resident T cells: The ups and downs of on site immunity. J. Investig. Dermatol. 2010, 130, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Chong, B.F.; Mirchandani, N.; Yamanaka, K.; Murphy, G.F.; Dowgiert, R.K.; Kupper, T.S. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Investig. Dermatol. 2006, 126, 1059–1070. [Google Scholar] [CrossRef]
- Hu, W.; Shang, R.; Yang, J.; Chen, C.; Liu, Z.; Liang, G.; He, W.; Luo, G. Skin γδ T Cells and Their Function in Wound Healing. Front. Immunol. 2022, 13, 875076. [Google Scholar] [CrossRef] [PubMed]
- Toulon, A.; Breton, L.; Taylor, K.R.; Tenenhaus, M.; Bhavsar, D.; Lanigan, C.; Rudolph, R.; Jameson, J.; Havran, W.L. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009, 206, 743–750. [Google Scholar] [CrossRef]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dimitrion, P.; Cvetkovski, S.; Zhou, L.; Mi, Q.S. Epidermal resident γδ T cell development and function in skin. Cell. Mol. Life Sci. 2021, 78, 573–580. [Google Scholar] [CrossRef]
- Qi, C.; Wang, Y.; Li, P.; Zhao, J. Gamma Delta T Cells and Their Pathogenic Role in Psoriasis. Front. Immunol. 2021, 12, 627139. [Google Scholar] [CrossRef]
- Nolz, J.C. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell. Mol. Life Sci. CMLS 2015, 72, 2461–2473. [Google Scholar] [CrossRef]
- Buggert, M.; Price, D.A.; Mackay, L.K.; Betts, M.R. Human circulating and tissue-resident memory CD8(+) T cells. Nat. Immunol. 2023, 24, 1076–1086. [Google Scholar] [CrossRef]
- Beura, L.K.; Fares-Frederickson, N.J.; Steinert, E.M.; Scott, M.C.; Thompson, E.A.; Fraser, K.A.; Schenkel, J.M.; Vezys, V.; Masopust, D. CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019, 216, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Fraser, K.A.; Schenkel, J.M.; Moran, A.; Abt, M.C.; Beura, L.K.; Lucas, P.J.; Artis, D.; Wherry, E.J.; Hogquist, K.; et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012, 188, 4866–4875. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Pauken, K.E.; Vezys, V.; Masopust, D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014, 346, 98–101. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Wiken, M.; Blomqvist, L.; Nylen, S.; Talme, T.; Stahle, M.; Eidsmo, L. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014, 192, 3111–3120. [Google Scholar] [CrossRef]
- Klicznik, M.M.; Morawski, P.A.; Hollbacher, B.; Varkhande, S.R.; Motley, S.J.; Kuri-Cervantes, L.; Goodwin, E.; Rosenblum, M.D.; Long, S.A.; Brachtl, G.; et al. Human CD4(+)CD103(+) cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 2019, 4, eaav8995. [Google Scholar] [CrossRef]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279ra239. [Google Scholar] [CrossRef]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef]
- Gratz, I.K.; Truong, H.A.; Yang, S.H.; Maurano, M.M.; Lee, K.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 2013, 190, 4483–4487. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Yan, S.; Tomura, M.; Kanagawa, O.; Luster, A.D. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J. Immunol. 2013, 190, 970–976. [Google Scholar] [CrossRef]
- Hirai, T.; Zenke, Y.; Yang, Y.; Bartholin, L.; Beura, L.K.; Masopust, D.; Kaplan, D.H. Keratinocyte-Mediated Activation of the Cytokine TGF-β Maintains Skin Recirculating Memory CD8(+) T Cells. Immunity 2019, 50, 1249–1261.e1245. [Google Scholar] [CrossRef]
- Mueller, S.N. Grand Challenges in Immunological Memory. Front. Immunol. 2017, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Baekkevold, E.S.; Wurbel, M.A.; Kivisäkk, P.; Wain, C.M.; Power, C.A.; Haraldsen, G.; Campbell, J.J. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J. Exp. Med. 2005, 201, 1045–1051. [Google Scholar] [CrossRef]
- Tubo, N.J.; McLachlan, J.B.; Campbell, J.J. Chemokine receptor requirements for epidermal T-cell trafficking. Am. J. Pathol. 2011, 178, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Soler, D.; Humphreys, T.L.; Spinola, S.M.; Campbell, J.J. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood 2003, 101, 1677–1682. [Google Scholar] [CrossRef]
- Campbell, J.J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400, 776–780. [Google Scholar] [CrossRef]
- Reiss, Y.; Proudfoot, A.E.; Power, C.A.; Campbell, J.J.; Butcher, E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 2001, 194, 1541–1547. [Google Scholar] [CrossRef]
- Campbell, J.J.; O’Connell, D.J.; Wurbel, M.A. Cutting Edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 2007, 178, 3358–3362. [Google Scholar] [CrossRef]
- Vestergaard, C.; Deleuran, M.; Gesser, B.; Grønhøj Larsen, C. Expression of the T-helper 2-specific chemokine receptor CCR4 on CCR10-positive lymphocytes in atopic dermatitis skin but not in psoriasis skin. Br. J. Dermatol. 2003, 149, 457–463. [Google Scholar] [CrossRef]
- Kondo, T.; Takiguchi, M. Human memory CCR4+CD8+ T cell subset has the ability to produce multiple cytokines. Int. Immunol. 2009, 21, 523–532. [Google Scholar] [CrossRef]
- Casciano, F.; Diani, M.; Altomare, A.; Granucci, F.; Secchiero, P.; Banfi, G.; Reali, E. CCR4(+) Skin-Tropic Phenotype as a Feature of Central Memory CD8(+) T Cells in Healthy Subjects and Psoriasis Patients. Front. Immunol. 2020, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Montico, G.; Mingozzi, F.; Casciano, F.; Protti, G.; Gornati, L.; Marzola, E.; Banfi, G.; Guerrini, R.; Secchiero, P.; Volinia, S.; et al. CCR4(+) CD8(+) T cells clonally expand to differentiated effectors in murine psoriasis and in human psoriatic arthritis. Eur. J. Immunol. 2023, 53, e2149702. [Google Scholar] [CrossRef] [PubMed]
- Casciano, F.; Pigatto, P.D.; Secchiero, P.; Gambari, R.; Reali, E. T Cell Hierarchy in the Pathogenesis of Psoriasis and Associated Cardiovascular Comorbidities. Front. Immunol. 2018, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Skin as a peripheral lymphoid organ: Revisiting the concept of skin-associated lymphoid tissues. J. Investig. Dermatol. 2011, 131, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef]
- Gallais Serezal, I.; Classon, C.; Cheuk, S.; Barrientos-Somarribas, M.; Wadman, E.; Martini, E.; Chang, D.; Xu Landen, N.; Ehrstrom, M.; Nylen, S.; et al. Resident T cells in resolved psoriasis steer tissue responses that stratify clinical outcome. J. Investig. Dermatol. 2018, 138, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Wiken, M.; Cheuk, S.; Gallais Serezal, I.; Baharom, F.; Stahle, M.; Smed-Sorensen, A.; Eidsmo, L. Dynamic Changes in Resident and Infiltrating Epidermal Dendritic Cells in Active and Resolved Psoriasis. J. Investig. Dermatol. 2017, 137, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Bowcock, A.M.; Krueger, J.G. Getting under the skin: The immunogenetics of psoriasis. Nat. Rev. Immunol. 2005, 5, 699–711. [Google Scholar] [CrossRef]
- Alanko, J.; Uçar, M.C.; Canigova, N.; Stopp, J.; Schwarz, J.; Merrin, J.; Hannezo, E.; Sixt, M. CCR7 acts as both a sensor and a sink for CCL19 to coordinate collective leukocyte migration. Sci. Immunol. 2023, 8, eadc9584. [Google Scholar] [CrossRef]
- Mitsui, H.; Suarez-Farinas, M.; Belkin, D.A.; Levenkova, N.; Fuentes-Duculan, J.; Coats, I.; Fujita, H.; Krueger, J.G. Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J. Investig. Dermatol. 2012, 132, 1615–1626. [Google Scholar] [CrossRef]
- Bose, F.; Petti, L.; Diani, M.; Moscheni, C.; Molteni, S.; Altomare, A.; Rossi, R.L.; Talarico, D.; Fontana, R.; Russo, V.; et al. Inhibition of CCR7/CCL19 axis in lesional skin is a critical event for clinical remission induced by TNF blockade in patients with psoriasis. Am. J. Pathol. 2013, 183, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, M.; Qiao, J.; Fu, Y.X. Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy. Cell. Mol. Immunol. 2017, 14, 809–818. [Google Scholar] [CrossRef]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: An early target in anticancer therapeutic strategy. In Vivo 2007, 21, 147–161. [Google Scholar] [PubMed]
- Canete, J.D.; Santiago, B.; Cantaert, T.; Sanmarti, R.; Palacin, A.; Celis, R.; Graell, E.; Gil-Torregrosa, B.; Baeten, D.; Pablos, J.L. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann. Rheum. Dis. 2007, 66, 720–726. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Human Th1 dichotomy: Origin, phenotype and biologic activities. Immunology 2014, 144, 343–351. [Google Scholar] [CrossRef]
- Diani, M.; Altomare, G.; Reali, E. T Helper Cell Subsets in Clinical Manifestations of Psoriasis. J. Immunol. Res. 2016, 2016, 7692024. [Google Scholar] [CrossRef]
- Diani, M.; Casciano, F.; Marongiu, L.; Longhi, M.; Altomare, A.; Pigatto, P.D.; Secchiero, P.; Gambari, R.; Banfi, G.; Manfredi, A.A.; et al. Increased frequency of activated CD8(+) T cell effectors in patients with psoriatic arthritis. Sci. Rep. 2019, 9, 10870. [Google Scholar] [CrossRef] [PubMed]
- Diani, M.; Galasso, M.; Cozzi, C.; Sgambelluri, F.; Altomare, A.; Cigni, C.; Frigerio, E.; Drago, L.; Volinia, S.; Granucci, F.; et al. Blood to skin recirculation of CD4+ memory T cells associates with cutaneous and systemic manifestations of psoriatic disease. Clin. Immunol. 2017, 180, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Cimaz, R.; Capone, M.; Santarlasci, V.; Querci, V.; Simonini, G.; Nencini, F.; Liotta, F.; Romagnani, S.; Maggi, E.; et al. Brief report: Etanercept inhibits the tumor necrosis factor α-driven shift of Th17 lymphocytes toward a nonclassic Th1 phenotype in juvenile idiopathic arthritis. Arthritis Rheumatol. 2014, 66, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Bose’, F.; Capsoni, F.; Molteni, S.; Raeli, L.; Diani, M.; Altomare, A.; Garavaglia, M.; Garutti, C.; Frigerio, E.; Banfi, G.; et al. Differential expression of interleukin-2 by anti-CD3-stimulated peripheral blood mononuclear cells in patients with psoriatic arthritis and patients with cutaneous psoriasis. Clin. Exp. Dermatol. 2014, 39, 385–390. [Google Scholar] [CrossRef]
- Dhir, V.; Aggarwal, A. Psoriatic arthritis: A critical review. Clin. Rev. Allergy Immunol. 2013, 44, 141–148. [Google Scholar] [CrossRef]
- Sherlock, J.P.; Buckley, C.D.; Cua, D.J. The critical role of interleukin-23 in spondyloarthropathy. Mol. Immunol. 2014, 57, 38–43. [Google Scholar] [CrossRef]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef]
- Arakawa, A.; Siewert, K.; Stohr, J.; Besgen, P.; Kim, S.M.; Ruhl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef]
- Curran, S.A.; FitzGerald, O.M.; Costello, P.J.; Selby, J.M.; Kane, D.J.; Bresnihan, B.; Winchester, R. Nucleotide sequencing of psoriatic arthritis tissue before and during methotrexate administration reveals a complex inflammatory T cell infiltrate with very few clones exhibiting features that suggest they drive the inflammatory process by recognizing autoantigens. J. Immunol. 2004, 172, 1935–1944. [Google Scholar] [PubMed]
- Costello, P.; Bresnihan, B.; O’Farrelly, C.; FitzGerald, O. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J. Rheumatol. 1999, 26, 1117–1124. [Google Scholar]
- Menon, B.; Gullick, N.J.; Walter, G.J.; Rajasekhar, M.; Garrood, T.; Evans, H.G.; Taams, L.S.; Kirkham, B.W. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014, 66, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Bioley, G.; Rivals, J.P.; Dudler, J.; Speiser, D.; Romero, P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008, 58, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.J.; McElrath, M.J.; Andersen-Nissen, E.; Frahm, N.; Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8(+) T cells. Nat. Commun. 2019, 10, 4987. [Google Scholar] [CrossRef] [PubMed]
- Penkava, F.; Velasco-Herrera, M.D.C.; Young, M.D.; Yager, N.; Nwosu, L.N.; Pratt, A.G.; Lara, A.L.; Guzzo, C.; Maroof, A.; Mamanova, L.; et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 2020, 11, 4767. [Google Scholar] [CrossRef]
- Sgambelluri, F.; Diani, M.; Altomare, A.; Frigerio, E.; Drago, L.; Granucci, F.; Banfi, G.; Altomare, G.; Reali, E. A role for CCR5(+)CD4 T cells in cutaneous psoriasis and for CD103(+) CCR4(+) CD8 Teff cells in the associated systemic inflammation. J. Autoimmun. 2016, 70, 80–90. [Google Scholar] [CrossRef]
- Azukizawa, H.; Kosaka, H.; Sano, S.; Heath, W.R.; Takahashi, I.; Gao, X.H.; Sumikawa, Y.; Okabe, M.; Yoshikawa, K.; Itami, S. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 2003, 33, 1879–1888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).