Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection
Abstract
:1. Introduction
2. Results
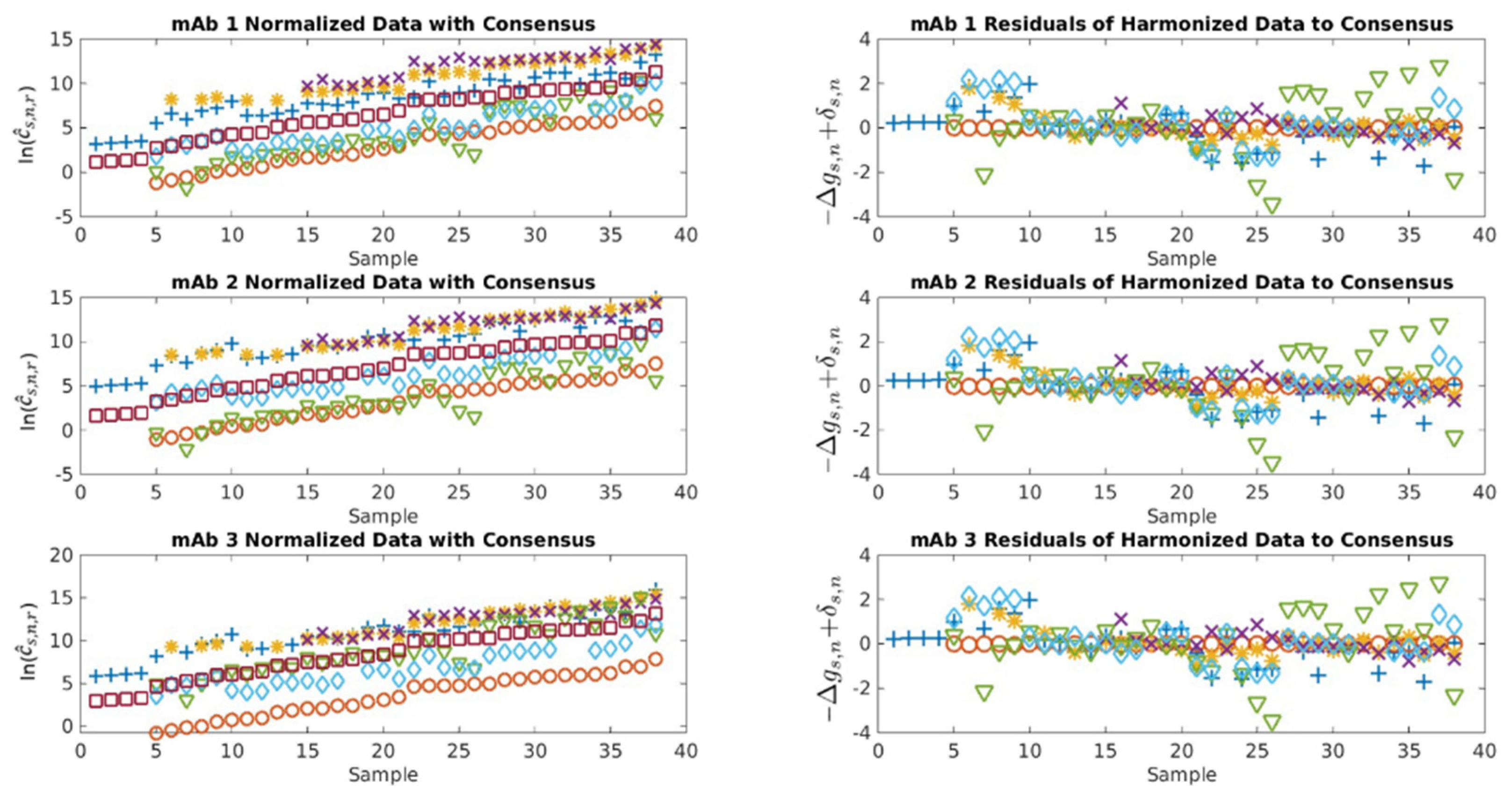
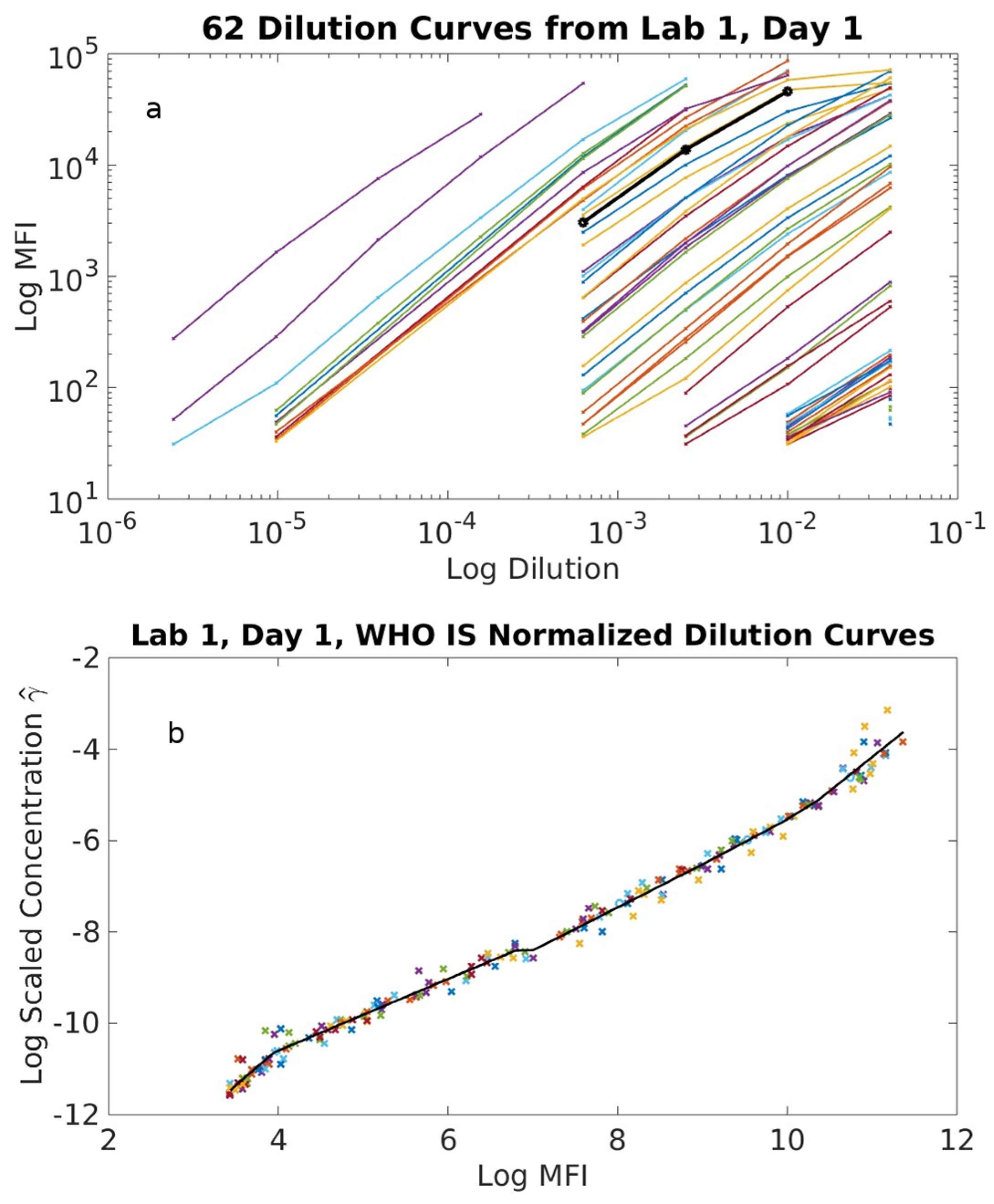
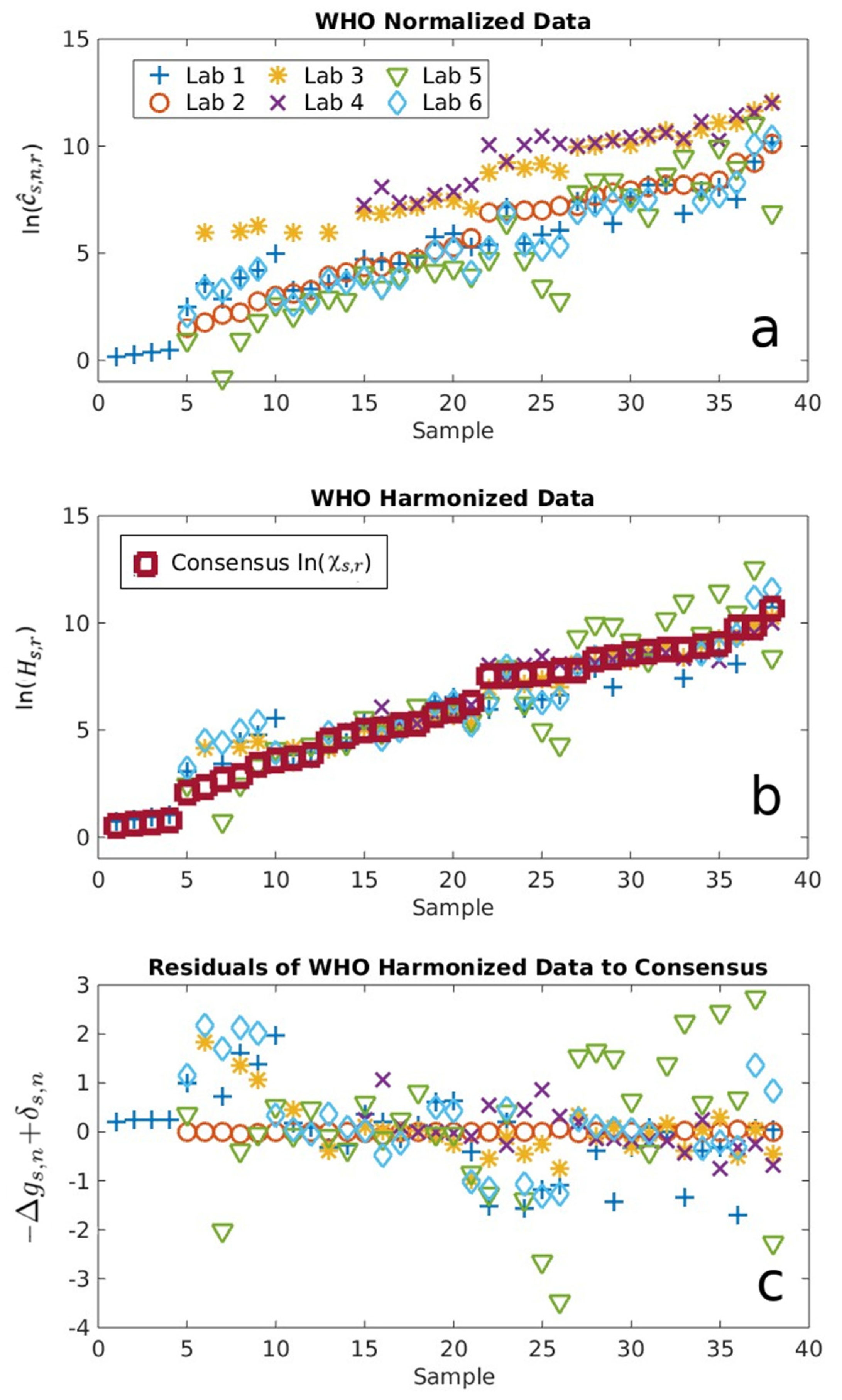
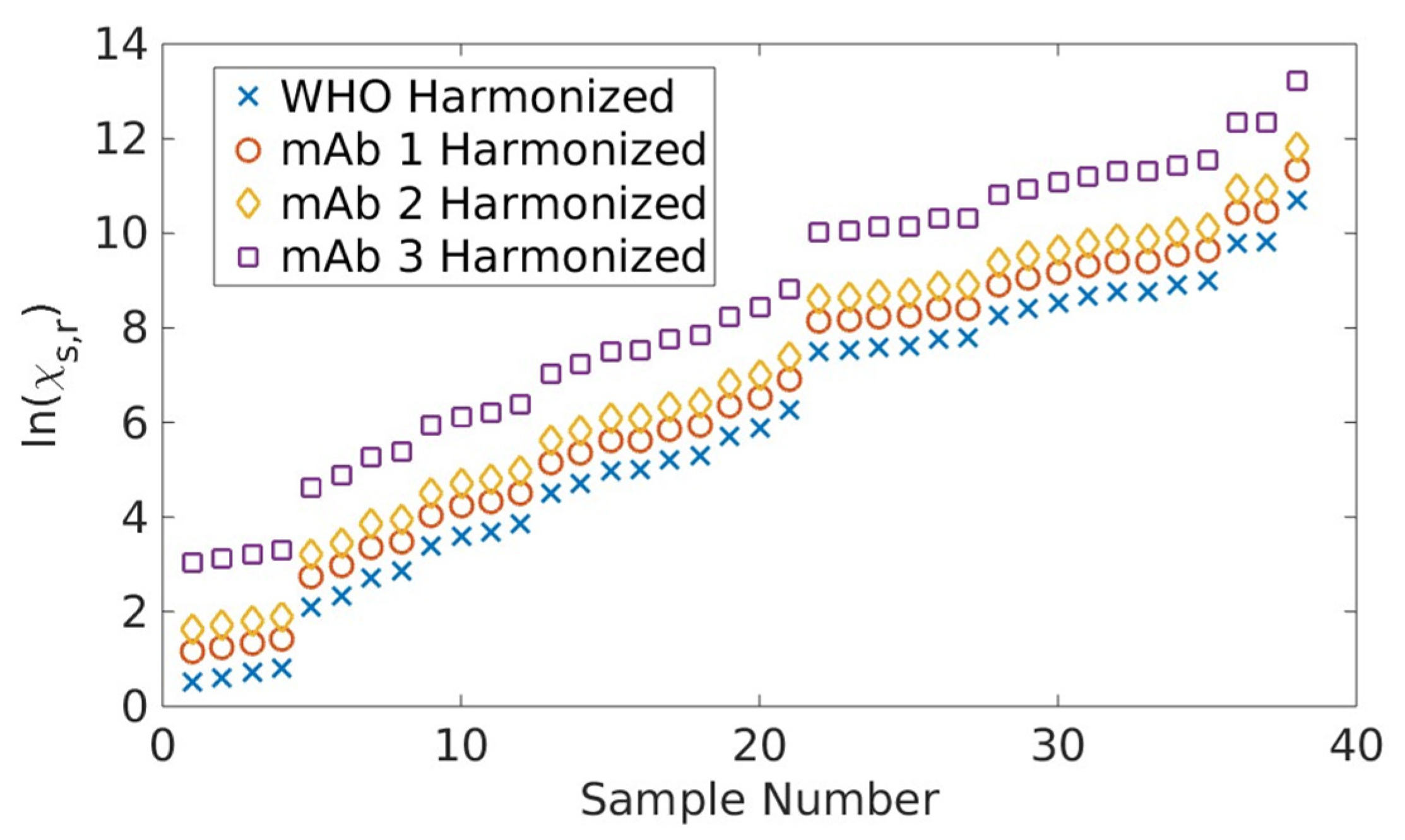
2.1. Serological Binding Assays
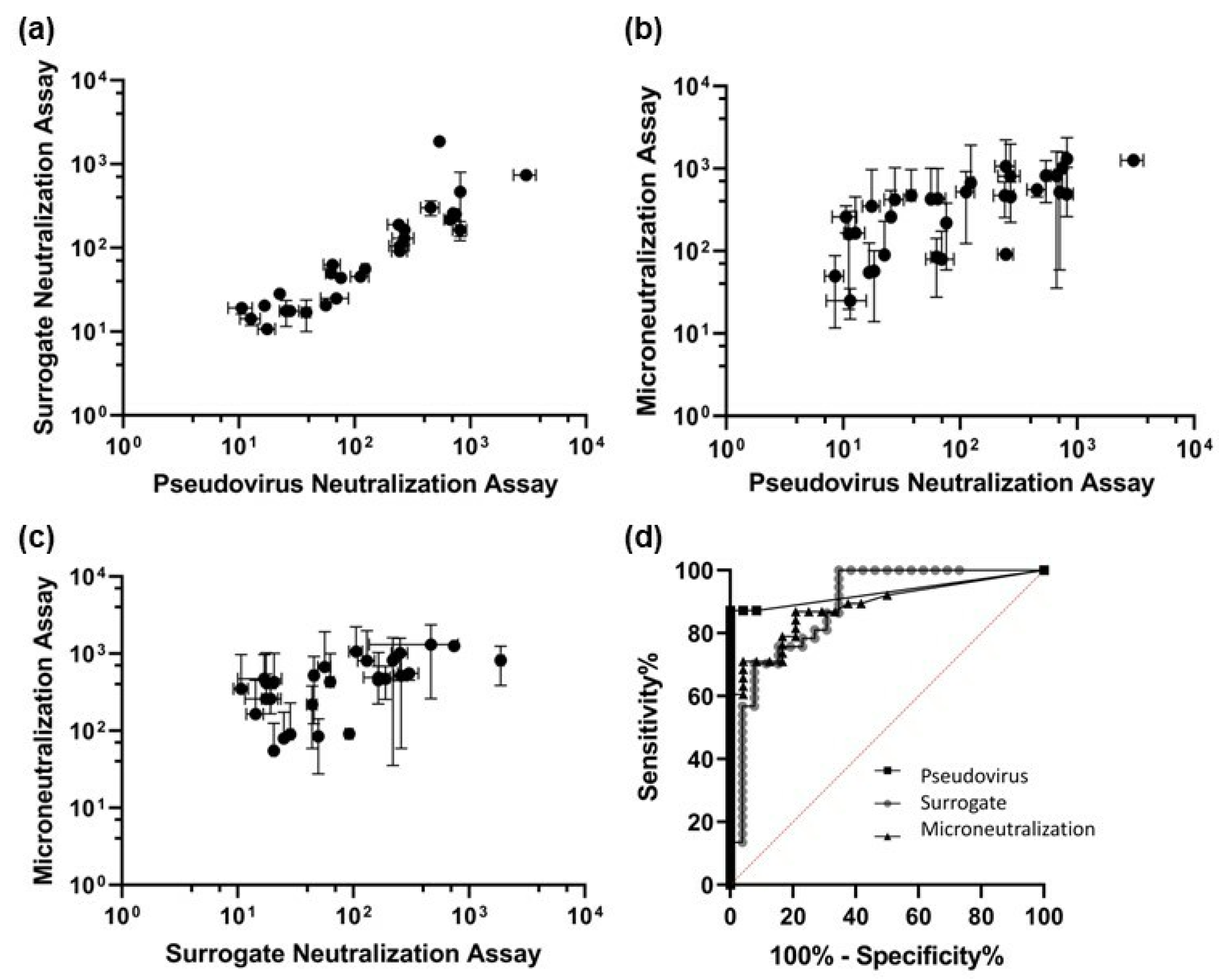
2.2. Neutralization Assays
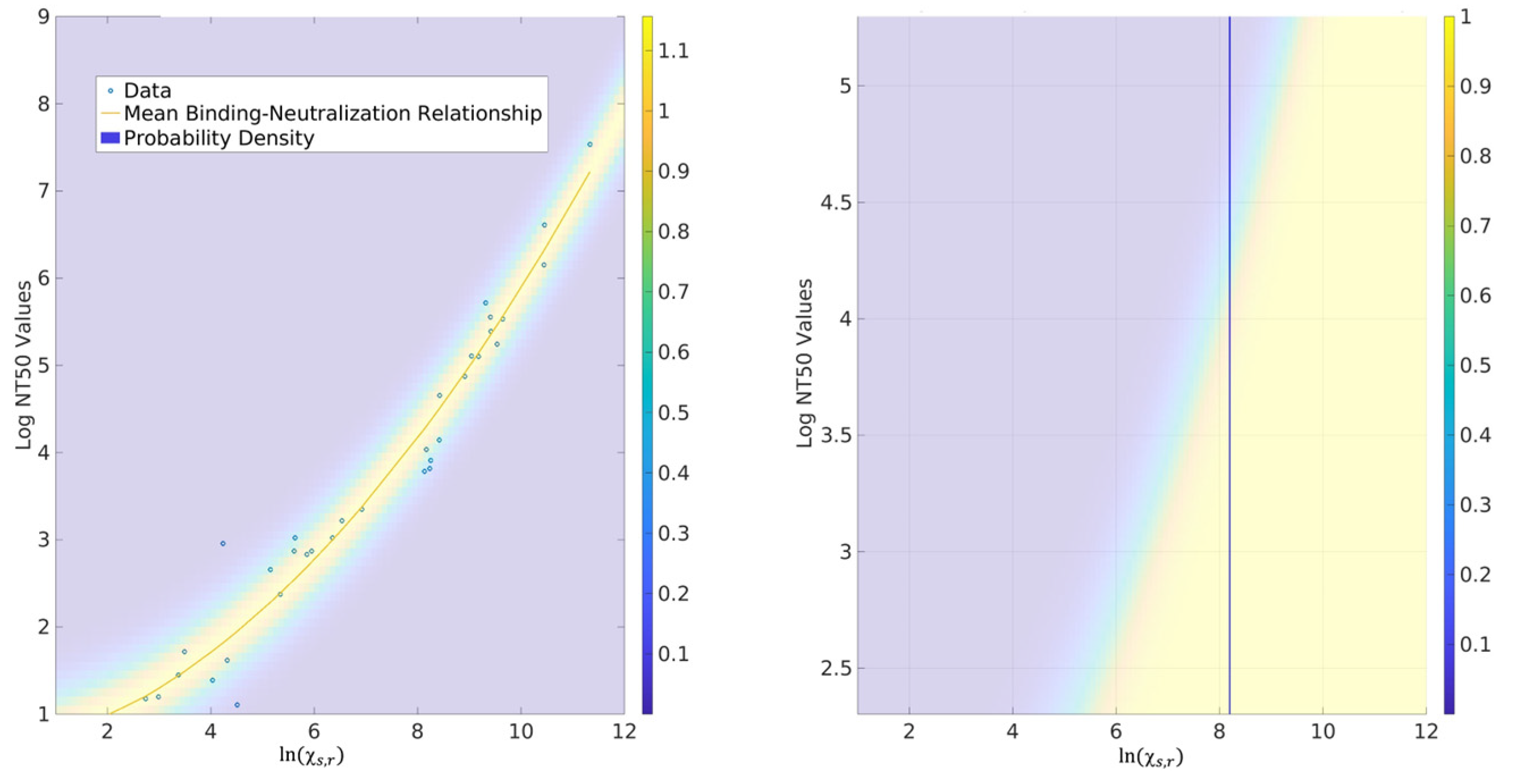
2.3. Probability for CoPs
3. Discussion
3.1. Serological Binding Assays
3.2. Neutralization Assays
3.3. Correlates of Protection (CoPs)
4. Materials and Methods
4.1. Interlaboratory Study Design
4.2. Centralized Serology Data Analysis
4.3. Neutralization Assays
4.4. Probability Models for CoPs
4.5. Disclaimer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Assays Used in the Study
Appendix B
References
- Krammer, F.; Simon, V. Serology assays to manage COVID-19. Science 2020, 368, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Gundlapalli, A.V.; Salerno, R.M.; Brooks, J.T.; Averhoff, F.; Petersen, L.; McDonald, L.; Lademarco, M.F. SARS-CoV-2 Serologic Assay Needs for the Next Phase of the US COVID-19 Pandemic Response. Open Forum Infect. Dis. 2020, 8, ofaa555. [Google Scholar] [CrossRef] [PubMed]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Donis, R.O.; Koup, R.A.; Fong, Y.; Plotkin, S.A.; Follmann, D. A COVID-19 Milestone Attained—A Correlate of Protection for Vaccines. N. Engl. J. Med. 2022, 387, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Corbett, K.S.; Nason, M.C.; Flach, B.; Gagne, M.; O’Connell, S.; Johnston, T.S.; Shah, S.N.; Edara, V.V.; Floyd, K.; Lai, L.; et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021, 373, eabj0299. [Google Scholar] [CrossRef]
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021, 27, 1147–1148. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Ozçürümez, M.K.; Ambrosch, A.; Frey, O.; Haselmann, V.; Holdenrieder, S.; Kiehntopf, M.; Neumaier, M.; Walter, M.; Wenzel, F.; Wölfel, R.; et al. SARS-CoV-2 antibody testing-questions to be asked. J. Allergy Clin. Immunol. 2020, 146, 35–43. [Google Scholar] [CrossRef] [PubMed]
- FDA, US Food and Drug Administration. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices: In Vitro Diagnostics EUAs. 2020. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas (accessed on 27 October 2023).
- Karger, A.B.; Brien, J.D.; Christen, J.M.; Dhakal, S.; Kemp, T.J.; Klein, S.L.; Pinto, L.A.; Premkumar, L.; Roback, J.D.; Binder, R.A.; et al. The Serological Sciences Network (SeroNet) for COVID-19: Depth and Breadth of Serology Assays and Plans for Assay Harmonization. mSphere 2022, 7, e0019322. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzo, G.; Bentley, E.M.; Hassall, M.; Routley, S.; Richardson, S.; Beranasconi, V.; Kristiansen, P.; Harvala, H.; Roberts, D.; Semple, M.G.; et al. Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody; WHO/BS/2020.2403; World Health Organization: Hertfordshire, UK, 2020; Available online: https://www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed on 27 October 2023).
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Toward harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D.; Carta, M. Improvements and limits of anti SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization. Diagnosis 2021, 9, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Charlton, C.; Plitt, S.; Thompson, L.A.; Braun, S.; Day, J.; Osiowy, C.; Tipples, G.; Kanji, J.N. Comparison of SARS-CoV-2 spike antibody quantitative titer reporting using the World Health Organization International Standard Units by four commercial assays. J. Clin. Virol. 2022, 156, 105292. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.M.; Atkinson, E.; Rigsby, P.; Elsley, W.; Bernasconi, V.; Kristiansen, P.; Harvala, H.; Turtle, L.C.; Dobson, S.; Wendel, S.; et al. Establishment of the 2nd WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin and Reference Panel for Antibodies to SARS-CoV-2 Variants of Concern; WHO/BS/2022.2427; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/m/item/who-bs-2022.2427 (accessed on 27 October 2023).
- Bentley, E.M.; Rigsby, P.; Elsley, W.; Bernasconi, V.; Kristiansen, P.A.; Wendel, S.; Fachini, R.; Devine, J.; Shongwe, N.; Rose, N.J.; et al. Expansion of WHO Reference Panel for Antibodies to SARS-CoV-2 Variants of Concern; WHO/BS/2023.2450; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/m/item/WHO-BS-2023-2450 (accessed on 27 October 2023).
- Dimech, W. The Standardization and Control of Serology and Nucleic Acid Testing for Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e0003521. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; El-Ella, D.M.A.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mona, O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Chen, R.E.; Winkler, E.S.; Case, J.B.; Aziati, I.D.; Bricker, T.L.; Joshi, A.; Darling, T.L.; Ying, B.; Errico, J.B.; Shrihari, S.; et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature 2021, 596, 103–108. [Google Scholar] [CrossRef]
- Patrone, P.N.; Wang, L.; Lin-Gibson, S.; Kearsley, A.J. Unertainty Quantification of Antibody Measurements: Physical Principles and Implications for Standardization. 2023; in review. [Google Scholar]
- Scheff, S.W. Nonparametric Statistics. In Fundamental Statistical Principles for the Neurobiologist; Academic Press: Cambridge, MA, USA, 2016; pp. 157–182. [Google Scholar]
- Li, X.; Pang, L.; Yin, Y.; Zhang, Y.; Xu, S.; Xu, D.; Shen, T. Patient Clinical Factors at Admission Affect the Levels of Neutralization Antibodies Six Months after Recovering from COVID-19. Viruses 2022, 14, 80. [Google Scholar] [CrossRef]
- Amanat, F.; White, K.M.; Miorin, L.; Strohmeier, S.; McMahon, M.; Meade, P.; Liu, W.; Albrecht, R.A.; Simon, V.; Martinez-Sobrido, L.; et al. An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening. Curr. Protoc. Microbil. 2020, 58, e108. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.T.; Li, Z.; Samson, R.; Payman, S.-T.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P., 2nd; Girardin, R.C.; Rathod, B.; et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef] [PubMed]
- Haslwanter, D.; Dieterle, M.E.; Wec, A.Z.; O’brien, C.M.; Sakharkar, M.; Florez, C.; Tong, K.; Garrett Rappazzo, C.; Lasso, G.; Vergnolle, O.; et al. A Combination of Receptor-Binding Domain and N-Terminal Domain Neutralizing Antibodies Limits the Generation of SARS-CoV-2 Spike Neutralization-Escape Mutants. mBio 2021, 12, e0247321. [Google Scholar] [CrossRef] [PubMed]
- Septisetyani, E.P.; Prasetyaningrum, P.W.; Anam, K.; Santoso, A. SARS-CoV-2 Antibody Neutralization Assay Platforms Based on Epitopes Sources: Live Virus, Pseudovirus, and Recombinant S Glycoprotein RBD. Immune Netw. 2021, 21, e39. [Google Scholar] [CrossRef]
- Barrette, R.W.; Urbonas, J.; Silbart, L.K. Quantifying Specific Antibody Concentrations by Enzyme-Linked Immunosorbent Assay Using Slope Correction. Clin. Vaccine Immunol. 2006, 13, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bhardwaj, R.; Mostowski, H.; Patrone, P.N.; Kearsley, A.J.; Watson, J.; Lim, L.; Pichaandi, J.; Ornatsky, O.; Majonis, D.; et al. Establishing CD 19 B-cell reference control materials for comparable and quantitative cytometric expression analysis. PLoS ONE 2021, 16, e0248118. [Google Scholar]
- Giovanni, Y.D.V.; Formari, C.; Goldlust, I.; Mills, G.; Koh, S.B.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. An automated fitting procedure and software for dose-response curves with multiphasic features. Sci. Rep. 2015, 5, 14701. [Google Scholar]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Radvak, P.; Kwon, H.J.; Kosikova, M.; Ortega-Rodriguez, U.; Xiang, R.; Phue, J.N.; Shen, R.F.; Rozzelle, J.; Kapoor, N.; Rabara, T.; et al. SARS-CoV-2 B.1.1.7 (alpha) and B.1.351 (beta) variants induce pathogenic patterns in K18-hACE2 transgenic mice distinct from early strains. Nat. Commun. 2021, 12, 6559. [Google Scholar] [CrossRef]
- Tian, L.; Elsheikh, R.B.; Patrone, P.N.; Kearsley, A.J.; Gaigalas, A.K.; Inwood, S.; Gibson, S.L.; Esposito, D.; Wang, L. Towards Quantitative and Standardized Serological and Neutralization Assays for COVID-19. Int. J. Mol. Sci. 2021, 22, 2723. [Google Scholar] [CrossRef]
- Izac, J.; Kwee, E.; Tian, L.; Gaigalas, A.; Elsheikh, E.; Elliott, J.T.; Wang, L. Development of a Cell-Based SARS-CoV-2 Pseudovirus Neutralization Assay Using Imaging and Flow Cytometry Analysis. Int. J. Mol. Sci. 2023, 24, 12332. [Google Scholar] [CrossRef]

| Participants | Serological Binding Assay(s) | Neutralization Assay(s) |
|---|---|---|
| NIST | SARS-CoV-2 spike IgG assay (quantitative, Wuhan-Hu-1) |
|
| FDA |
| Live virus-based microneutralization assay (D614G, Alpha, Beta, Delta) |
| FNLCR/NCI |
| |
| Abbott | ARCHITECT i2000SR Immunoassay (quantitative, Wuhan-Hu-1, spike/RBD IgG, chemiluminescent microparticle immunoassay, FDA- and EUA-granted) | |
| Roche | Elecsys anti-SARS-CoV-2 S assay on cobas e 801 immunoanalyzer (quantitative (US: semi-quantitative), Wuhan-Hu-1, S1 RBD total Ig, double-antigen sandwich, ligand-binding assay, FDA- and EUA-granted) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Patrone, P.N.; Kearsley, A.J.; Izac, J.R.; Gaigalas, A.K.; Prostko, J.C.; Kwon, H.J.; Tang, W.; Kosikova, M.; Xie, H.; et al. Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection. Int. J. Mol. Sci. 2023, 24, 15705. https://doi.org/10.3390/ijms242115705
Wang L, Patrone PN, Kearsley AJ, Izac JR, Gaigalas AK, Prostko JC, Kwon HJ, Tang W, Kosikova M, Xie H, et al. Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection. International Journal of Molecular Sciences. 2023; 24(21):15705. https://doi.org/10.3390/ijms242115705
Chicago/Turabian StyleWang, Lili, Paul N. Patrone, Anthony J. Kearsley, Jerilyn R. Izac, Adolfas K. Gaigalas, John C. Prostko, Hyung Joon Kwon, Weichun Tang, Martina Kosikova, Hang Xie, and et al. 2023. "Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection" International Journal of Molecular Sciences 24, no. 21: 15705. https://doi.org/10.3390/ijms242115705
APA StyleWang, L., Patrone, P. N., Kearsley, A. J., Izac, J. R., Gaigalas, A. K., Prostko, J. C., Kwon, H. J., Tang, W., Kosikova, M., Xie, H., Tian, L., Elsheikh, E. B., Kwee, E. J., Kemp, T., Jochum, S., Thornburg, N., McDonald, L. C., Gundlapalli, A. V., & Lin-Gibson, S. (2023). Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection. International Journal of Molecular Sciences, 24(21), 15705. https://doi.org/10.3390/ijms242115705






