Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging
Abstract
1. Introduction
2. Results
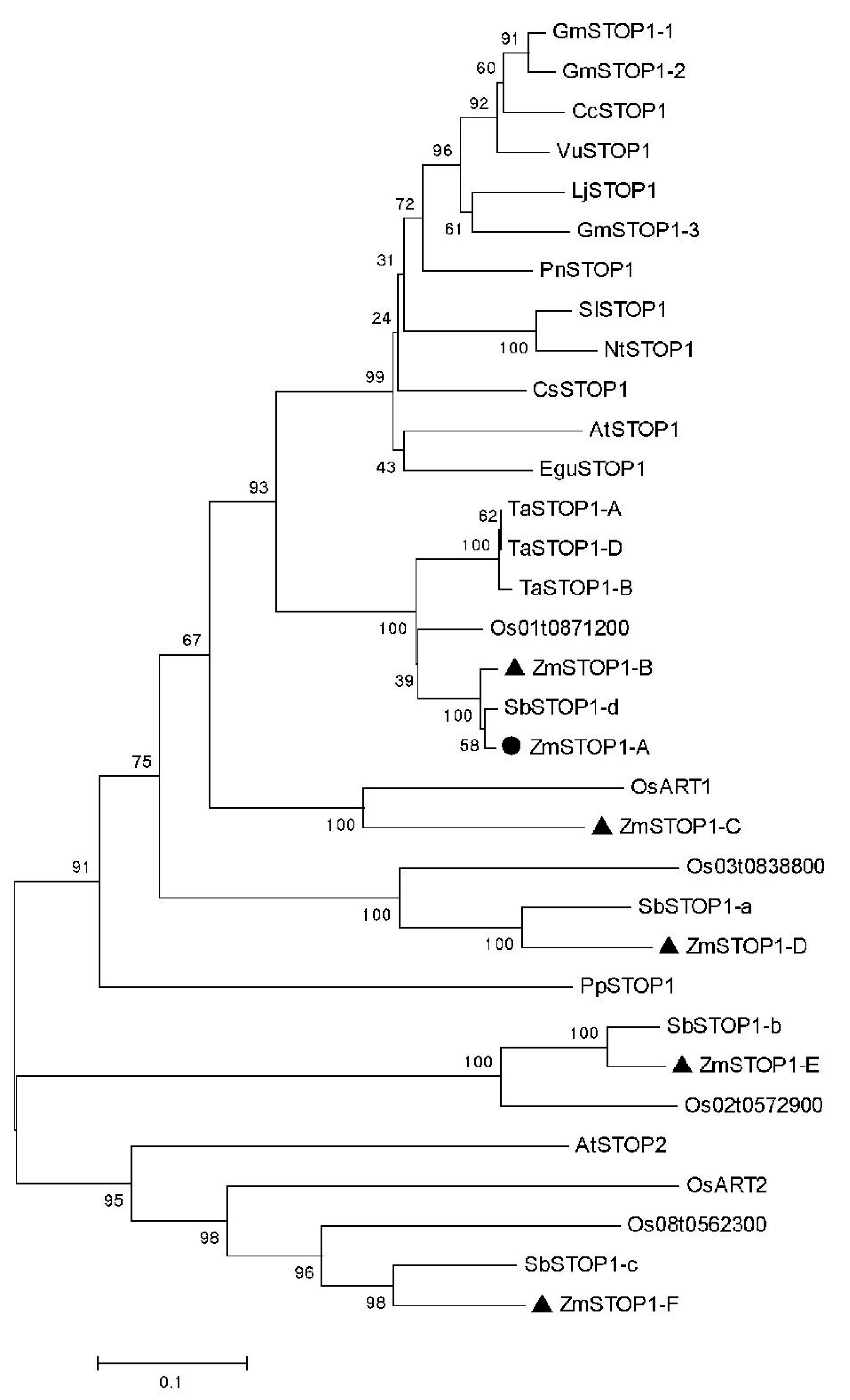
2.1. Identification and Phylogenetic Analysis of ZmSTOP1-A
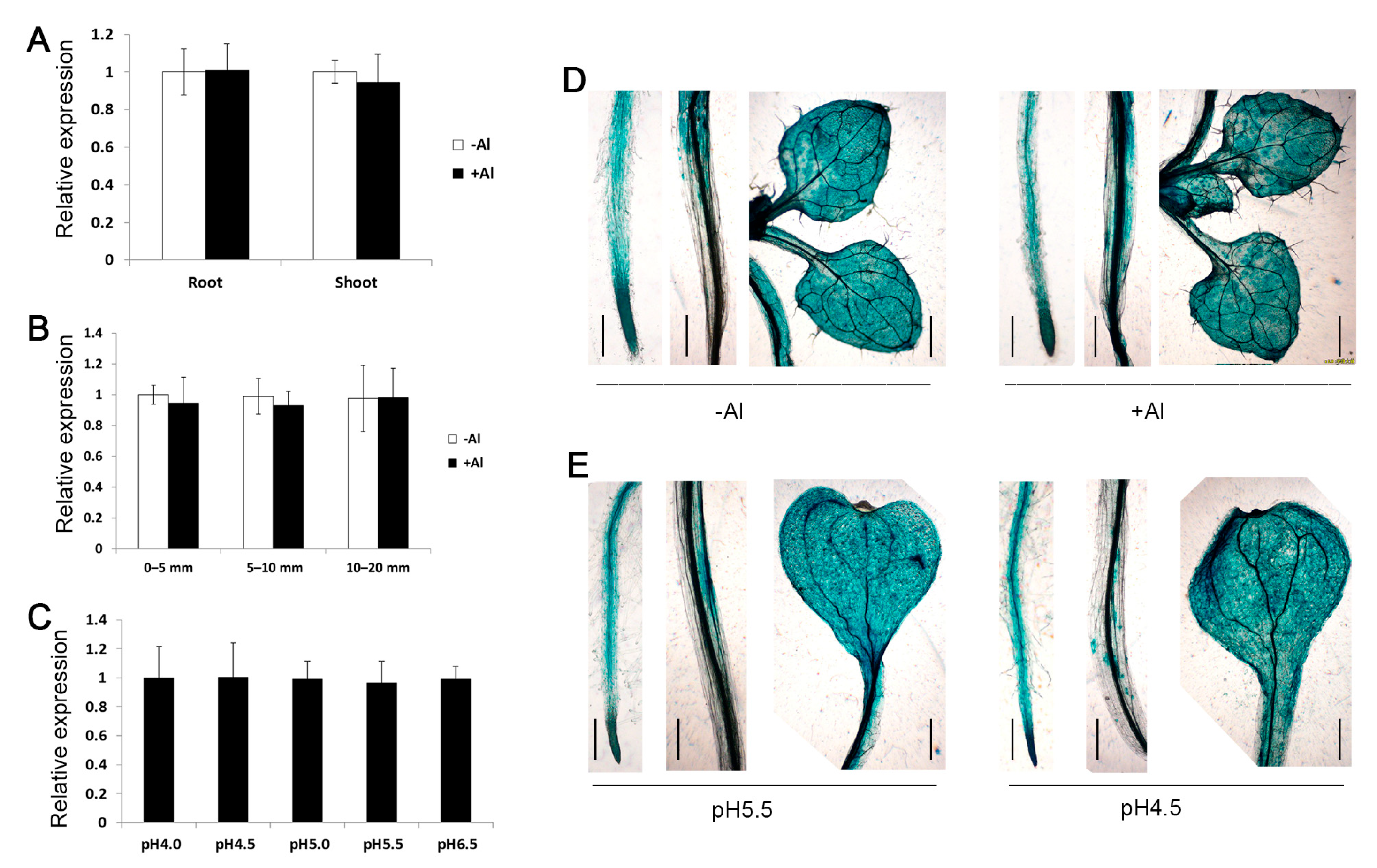
2.2. Expression Pattern of ZmSTOP1-A
2.3. Subcellular Localization and Transcriptional Activation of ZmSTOP1-A
2.4. Ectopic Expression of ZmSTOP1-A Enhanced Al and Low-pH Tolerance in Transgenic Arabidopsis
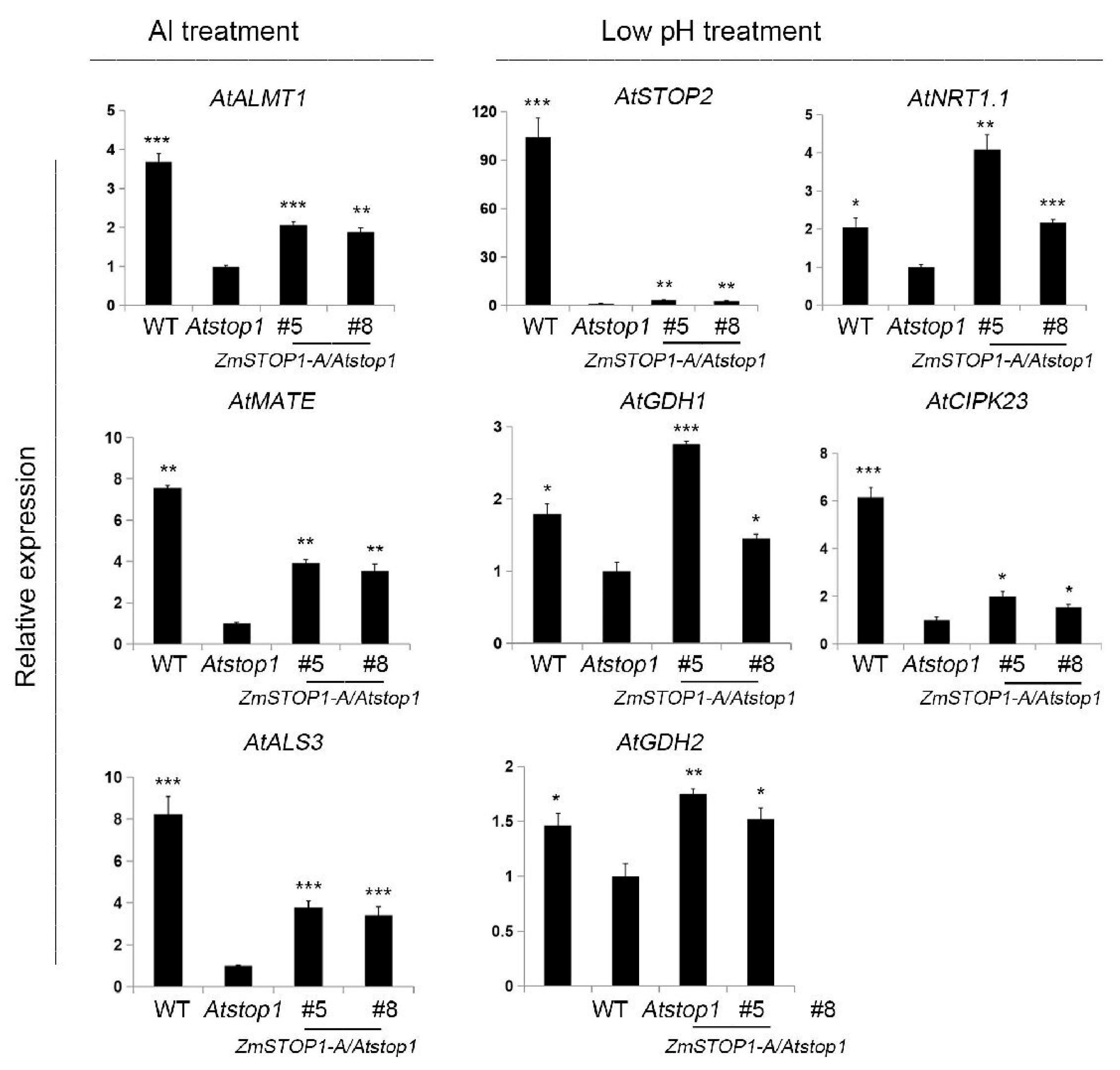
2.5. ZmSTOP1-A Partially Rescued Al Tolerance of Atstop1 Attributed to the Secretion of Organic Acids
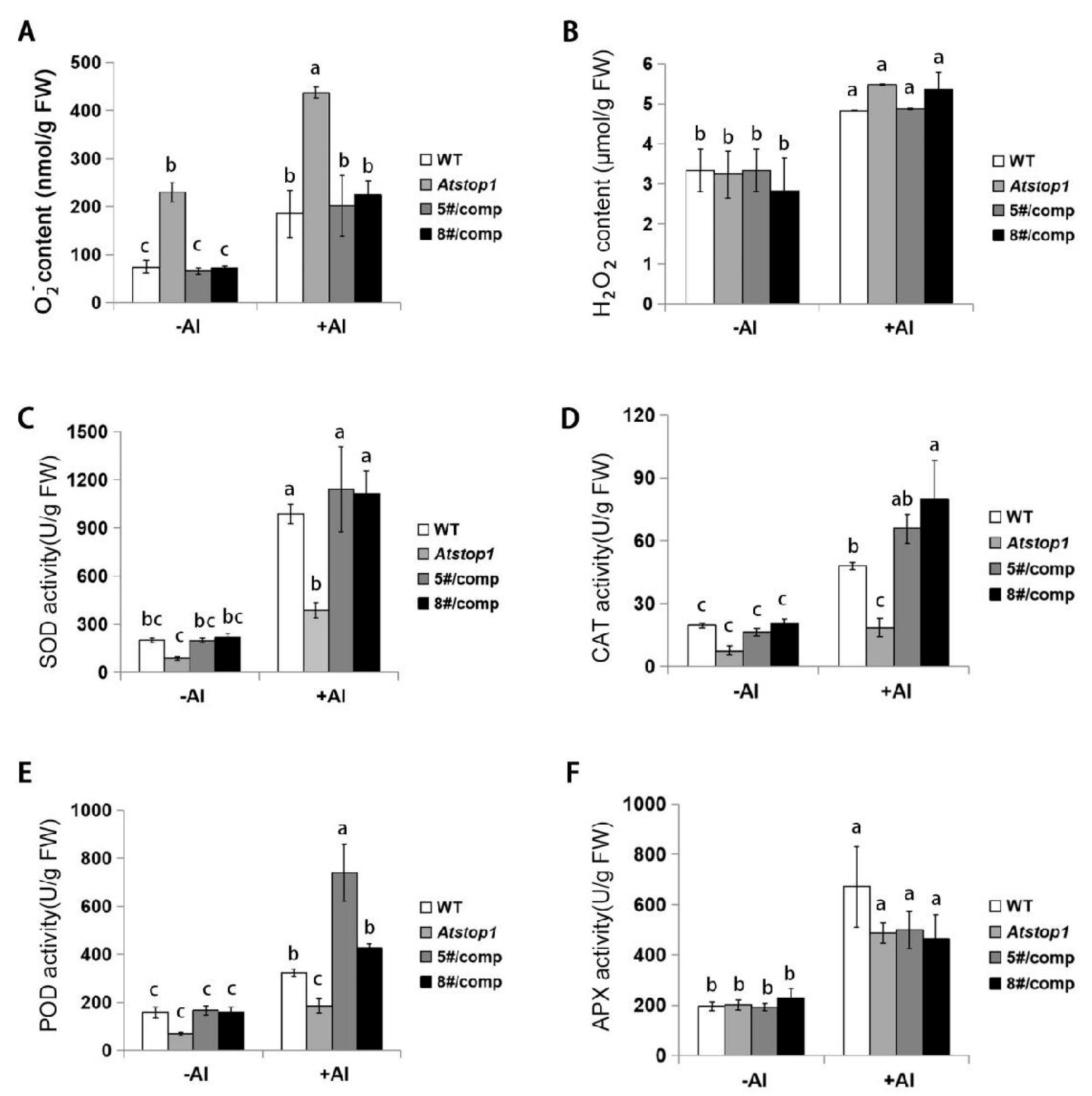
2.6. ZmSTOP1-A Responds to Al-Induced Oxidative Stress in the Atstop1 Mutant
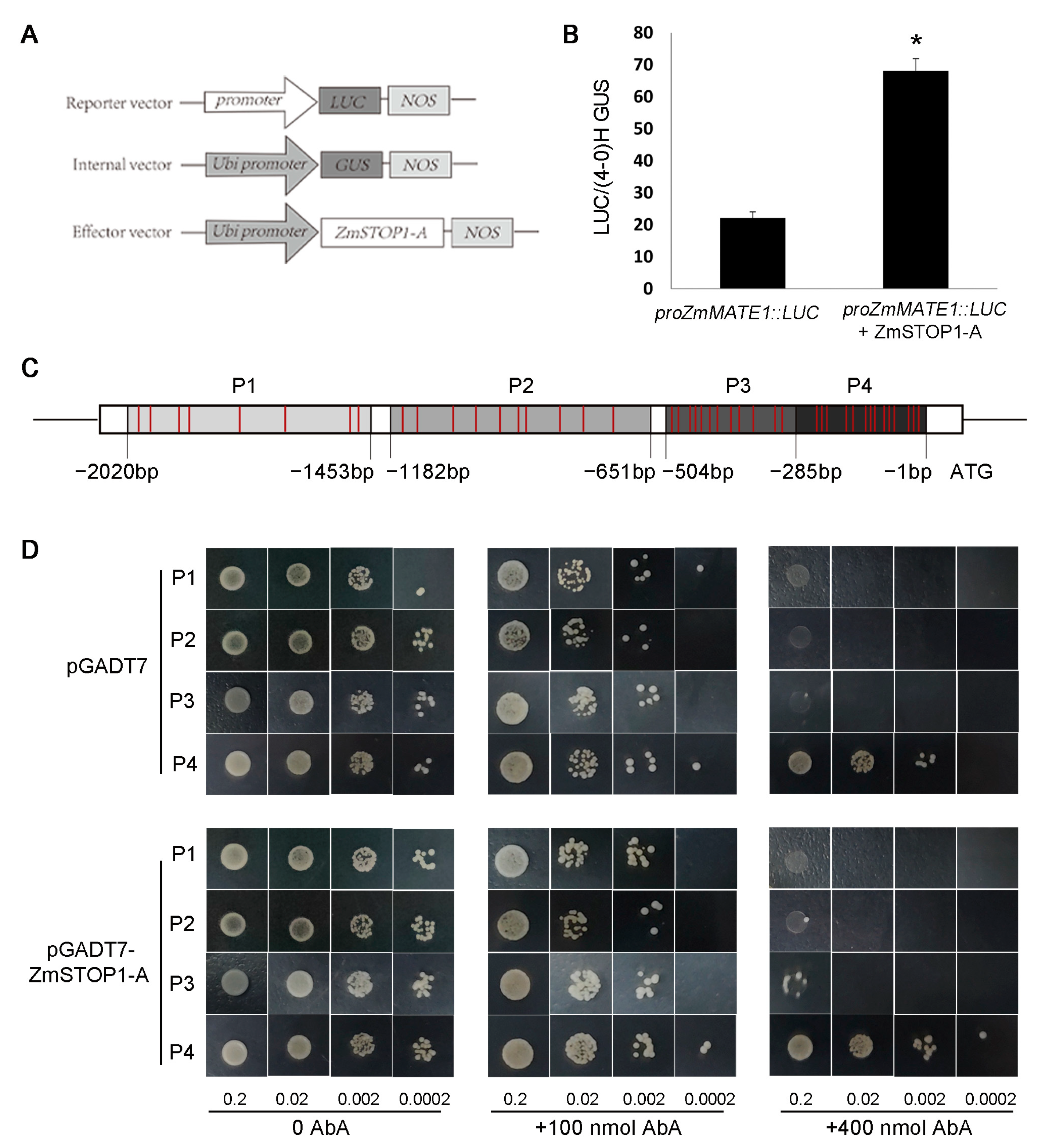
2.7. ZmSTOP1-A Directly Regulated ZmMATE1 Expression in Maize
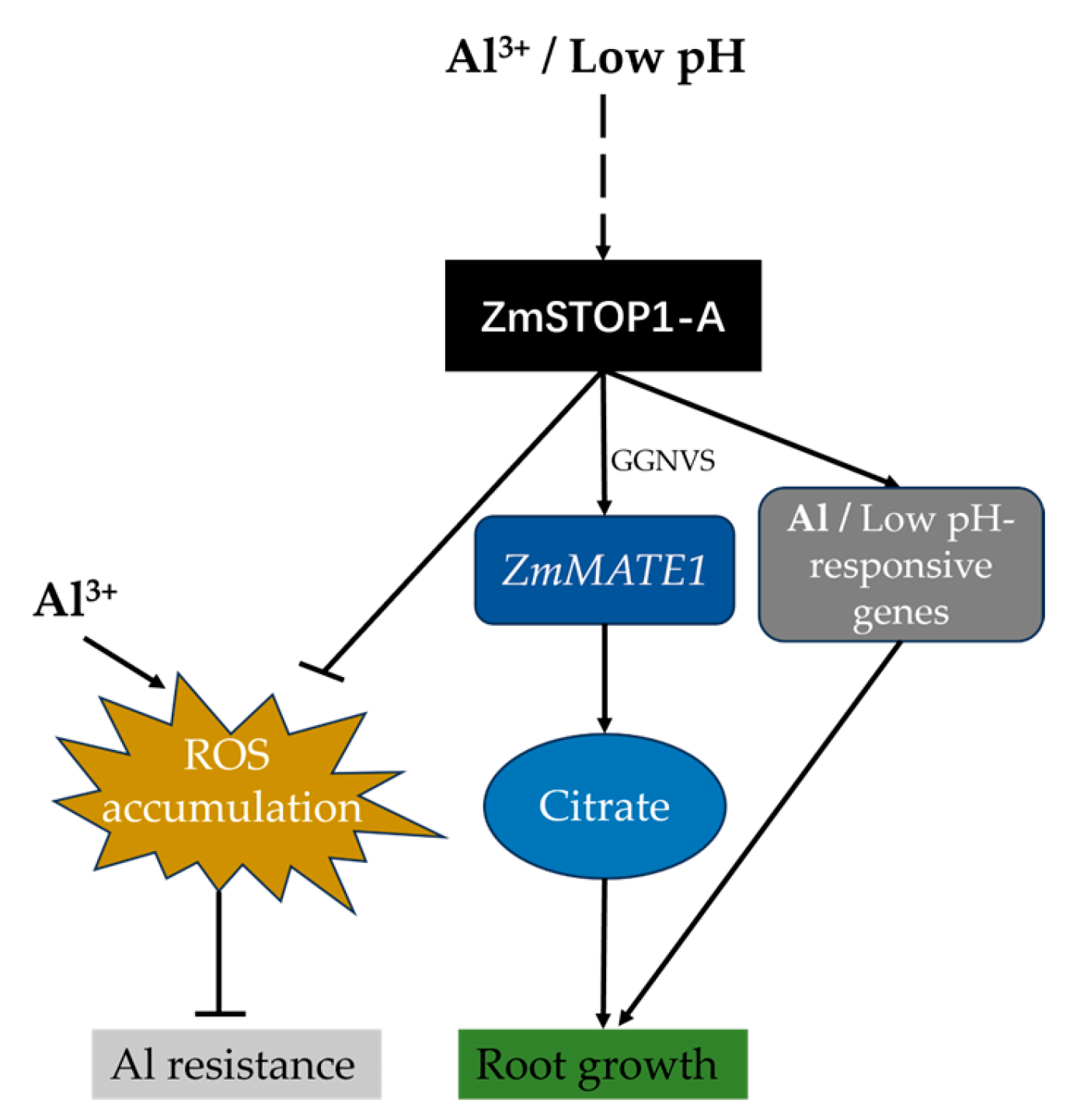
3. Discussion
3.1. ZmSTOP1-A Is Involved in Both Al Stress and Low-pH Stress Responses
3.2. ZmSTOP1-A Enhanced Al Tolerance in Arabidopsis Mainly through External Extrusion Mechanism
3.3. ZmSTOP1-A Eliminated the Al-Induced Oxidative Stress in Atstop1
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Phylogenetic Tree Construction
4.3. RNA Extraction and Quantitative Real-Time RT-PCR
4.4. Subcellular Localization
4.5. GUS Staining Assay
4.6. Determination of Organic Acids Secretion and Al Content
4.7. Transient Expression Assay in Maize Protoplasts
4.8. Transcriptional Activity Detection and Yeast One-Hybrid Assay
4.9. Determination of ROS Content and Antioxidant Enzyme Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uexküll, H.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pi Eros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Toxicity factors in acidic forest soils: Attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur. J. Soil Sci. 2003, 54, 323–333. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pi Eros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; et al. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol. 2006, 47, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.V.; Liu, J.; Guimarães, C.T.; Lana, U.G.P.; Alves, V.M.C.; Wang, Y.; Schaffert, R.E.; Hoekenga, O.A.; Piñeros, M.A.; Shaff, J.E.; et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef]
- Ezaki, B.; Suzuki, M.; Motoda, H.; Kawamura, M.; Nakashima, S.; Matsumoto, H. Mechanism of gene expression of Arabidopsis glutathione S-transferase, AtGST1, and AtGST11 in response to aluminum stress. Plant Physiol. 2004, 134, 1672–1682. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; How, J.; Xu, H.; Chen, L.; Li, K. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 2017, 95, 157–168. [Google Scholar] [CrossRef]
- Du, H.; Huang, Y.; Qu, M.; Li, Y.; Hu, X.; Yang, W.; Li, H.; He, W.; Ding, J.; Liu, C. A Maize ZmAT6 gene confers aluminum tolerance via reactive oxygen species scavenging. Front. Plant Sci. 2020, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, R.; Xie, Y.; Jiang, C.; Yu, Y. Recent advances in understanding mechanisms of plant tolerance and response to aluminum toxicity. Sustainability 2021, 13, 1782. [Google Scholar] [CrossRef]
- Sawaki, Y.; Iuchi, S.; Kobayashi, Y.; Kobayashi, Y.; Ikka, T.; Sakurai, N.; Fujita, M.; Shinozaki, K.; Shibata, D.; Kobayashi, M.; et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009, 150, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lou, H.Q.; Gong, Y.L.; Liu, M.Y.; Cao, M.J.; Liu, Y.; Yang, J.L.; Zheng, S.J. Characterization of an inducible C2 H2 -type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytol. 2015, 208, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, Y.; Cai, S.; Kuang, L.; Shen, Q.; Wu, D.; Zhang, G. The zinc finger transcription factor ATF1 regulates aluminum tolerance in barley. J. Exp. Bot. 2020, 71, 6512–6523. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Yamaji, N.; Feng Ma, J. Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiol. 2011, 156, 925–931. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ito, H.; Kobayashi, Y.; Ikka, T.; Morita, A.; Kobayashi, M.; Imaizumi, R.; Aoki, T.; Komatsu, K.; Sakata, Y.; et al. Characterization of AtSTOP1 orthologous genes in tobacco and other plant species. Plant Physiol. 2013, 162, 1937–1946. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Zhou, M.; Zhu, Q.Y.; Du, W.X.; Zhu, Y.X.; Liu, X.X.; Lin, X.Y.; Zheng, S.J.; Jin, C.W. STOP1 activates NRT1.1-mediated nitrate uptake to create a favorable rhizospheric pH for plant adaptation to acidity. Plant Cell 2021, 12, 3658–3674. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Benito, C.; Prieto, P.; de Andrade Menezes, R.; Rodrigues-Pousada, C.; Guedes-Pinto, H.; Martins-Lopes, P. Molecular characterization of TaSTOP1 homoeologues and their response to aluminum and proton (H+) toxicity in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Tsutsui, T.; Yokosho, K.; Yamaji, N.; Ma, J.F. Functional characterization of an aluminum (Al)-inducible transcription factor, ART2, revealed a different pathway for Al tolerance in rice. New Phytol. 2018, 220, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Barreiro, M.D.G. An overview into aluminum toxicity in maize. Bulg. J. Plant Physiol. 2002, 28, 96–112. [Google Scholar]
- Maron, L.G.; Piñeros, M.A.; Guimarães, C.T.; Magalhaes, J.V.; Pleiman, J.K.; Mao, C.; Shaff, J.; Belicuas, S.N.; Kochian, L.V. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. Cell Mol. Biol. 2010, 61, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ryan, P.R.; Liu, C.; Li, H.; Hu, W.; Yan, W.; Huang, Y.; He, W.; Luo, B.; Zhang, X.; et al. ZmMATE6 from maize encodes a citrate transporter that enhances aluminum tolerance in transgenic Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2021, 311, 111016. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, C.; Jin, X.; Du, C.; Yu, Y.; Luo, S.; He, W.; Zhang, S. Overexpression of the aldehyde dehydrogenase gene ZmALDH confers aluminum tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Hu, X.; Yang, W.; Hu, W.; Yan, W.; Li, Y.; He, W.; Cao, M.; Zhang, X.; Luo, B.; et al. ZmXTH, a xyloglucan endotransglucosylase/hydrolase gene of maize, conferred aluminum tolerance in Arabidopsis. J. Plant Physiol. 2021, 266, 153520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Li, C.; Zhang, B.; Zhang, C.; Zhang, X.; Ding, Z. Auxin Efflux Carrier ZmPGP1 Mediates Root Growth Inhibition under Aluminum Stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef]
- Sawaki, Y.; Kobayashi, Y.; Kihara-Doi, T.; Nishikubo, N.; Kawazu, T.; Kobayashi, M.; Kobayashi, Y.; Iuchi, S.; Koyama, H.; Sato, S. Identification of a STOP1-like protein in Eucalyptus that regulates transcription of Al tolerance genes. Plant Sci. Int. J. Exp. Plant Biol. 2014, 223, 8–15. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Y.; Chen, Q.; Peng, W.; Peng, J.; Tian, J.; Liang, C.; Liao, H. Functional Conservation and Divergence of Soybean GmSTOP1 Members in Proton and Aluminum Tolerance. Front. Plant Sci. 2018, 9, 570. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Salvador, N.; Del Pozo, J.C.; Benito, C.; Gallego, F.J. The rye transcription factor ScSTOP1 regulates the tolerance to aluminum by activating the ALMT1 transporter. Plant Sci. Int. J. Exp. Plant Biol. 2021, 310, 110951. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, J.; You, J.; Liang, Y.; Guan, K.; Yan, S.; Zhan, M.; Yang, Z. Identification of STOP1-Like Proteins Associated with Aluminum Tolerance in Sweet sorghum (L.). Front. Plant Sci. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ohyama, Y.; Kobayashi, Y.; Ito, H.; Iuchi, S.; Fujita, M.; Zhao, C.R.; Tanveer, T.; Ganesan, M.; Kobayashi, M.; et al. STOP2 activates transcription of several genes for Al- and low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol. Plant. 2014, 7, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Daspute, A.A.; Kobayashi, Y.; Panda, S.K.; Fakrudin, B.; Kobayashi, Y.; Tokizawa, M.; Iuchi, S.; Choudhary, A.K.; Yamamoto, Y.Y.; Koyama, H. Characterization of CcSTOP1; a C2H2-type transcription factor regulates Al tolerance gene in pigeonpea. Planta 2018, 247, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yokosho, K.; Kashino, M.; Zhao, F.; Yamaji, N.; Ma, J.F. Adaptation to acidic soil is achieved by increased numbers of cis-acting elements regulating ALMT1 expression in Holcus lanatus. Plant J. 2013, 76, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Retrotransposon-Mediated Aluminum Tolerance through Enhanced Expression of the Citrate Transporter OsFRDL4. Plant Physiol. 2016, 172, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for Aluminum stress tolerance. Sci. Rep. 2019, 9, 8681. [Google Scholar] [CrossRef]
- Xu, L.M.; Liu, W.; Cui, B.M.; Wang, N.; Ding, J.Z.; Liu, C.; Gao, S.B.; Zhang, S.Z. Aluminium tolerance assessment of 141 maize germplasms in a solution culture. Univers. J. Agric. Res. 2017, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ligaba, A.; Maron, L.; Shaff, J.; Kochian, L.; Pineros, M. Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux. Plant Cell Environ. 2012, 35, 1185–1200. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Xiao, Q.; Luo, L.; Zhang, C.; Mao, C.; Du, J.; Long, T.; Cao, Y.; Yi, Q.; et al. Transcription factor ZmPLATZ2 positively regulate the starch synthesis in maize. Plant Growth Regul. 2021, 93, 291–302. [Google Scholar] [CrossRef]
- Jefferson, R.A. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Lou, H.Q.; Fan, W.; Jin, J.F.; Xu, J.M.; Chen, W.W.; Yang, J.L.; Zheng, S.J. A NAC-type transcription factor confers aluminum resistance by regulating cell wall-associated receptor kinase 1 and cell wall pectin. Plant Cell Environ. 2020, 43, 463–478. [Google Scholar] [CrossRef]
- Erich, E.; Claus, S.; Adelheid, H. Determination of Superoxide Free Radical Ion and Hydrogen Peroxide as Products of Photosynthetic Oxygen Reduction. Z. Naturf. C 1975, 30, 53–57. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Hu, X.; Zang, L.; Liu, X.; Wei, Y.; Wang, X.; Jin, X.; Du, C.; Yu, Y.; He, W.; et al. Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging. Int. J. Mol. Sci. 2023, 24, 15669. https://doi.org/10.3390/ijms242115669
Liu C, Hu X, Zang L, Liu X, Wei Y, Wang X, Jin X, Du C, Yu Y, He W, et al. Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging. International Journal of Molecular Sciences. 2023; 24(21):15669. https://doi.org/10.3390/ijms242115669
Chicago/Turabian StyleLiu, Chan, Xiaoqi Hu, Lei Zang, Xiaofeng Liu, Yuhui Wei, Xue Wang, Xinwu Jin, Chengfeng Du, Yan Yu, Wenzhu He, and et al. 2023. "Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging" International Journal of Molecular Sciences 24, no. 21: 15669. https://doi.org/10.3390/ijms242115669
APA StyleLiu, C., Hu, X., Zang, L., Liu, X., Wei, Y., Wang, X., Jin, X., Du, C., Yu, Y., He, W., & Zhang, S. (2023). Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging. International Journal of Molecular Sciences, 24(21), 15669. https://doi.org/10.3390/ijms242115669




