Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases
Abstract
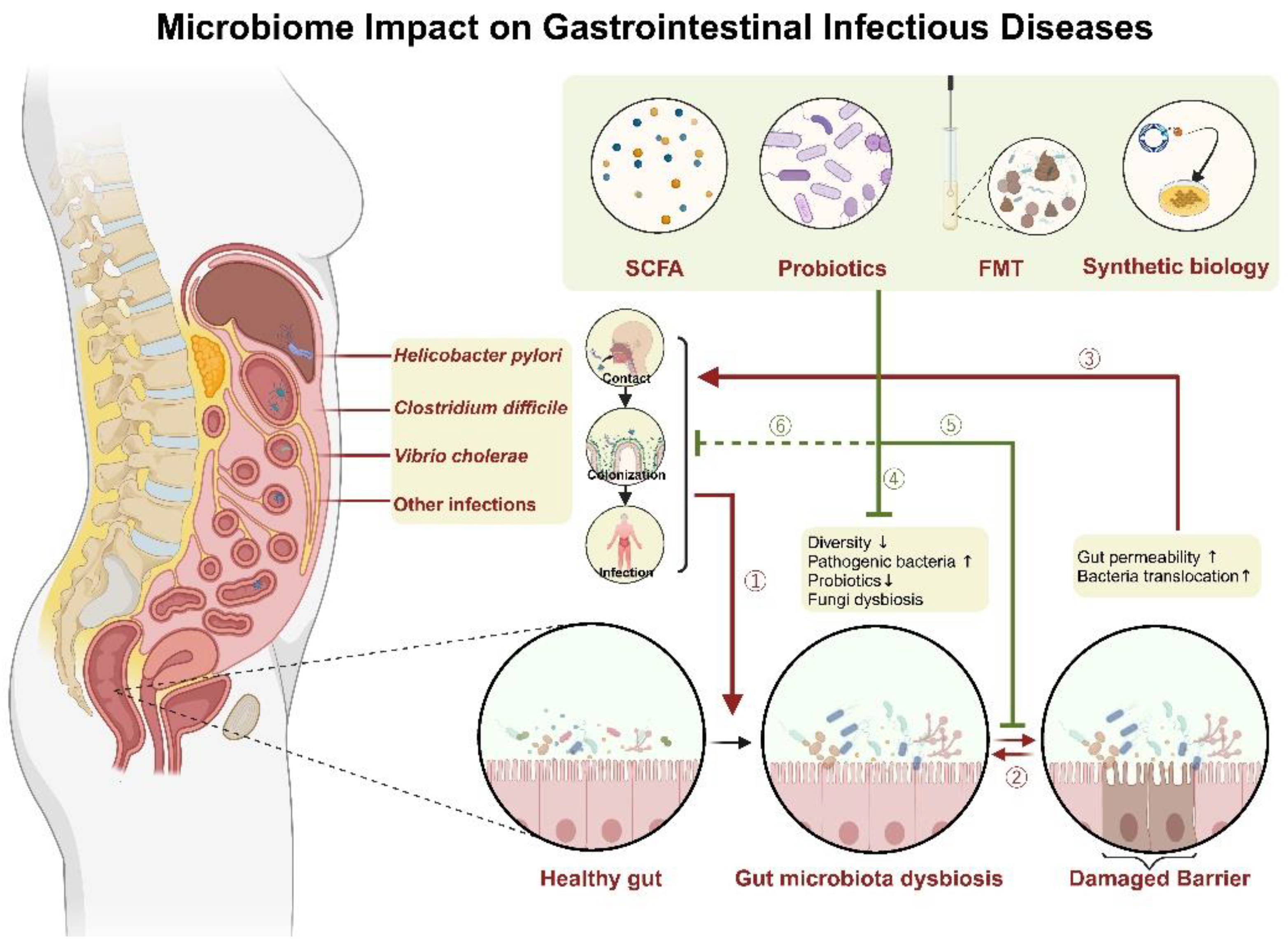
1. Introduction
2. Helicobacter pylori
2.1. Interaction of Gut Microbiome and Helicobacter pylori
2.2. Gut Microbiota Modulation for Therapy and Prevention
| Probiotic Genus | Specific Probiotics Used | Author | Year | Patient Status before Enrollment | Combination with Traditional Therapy * | Effect |
|---|---|---|---|---|---|---|
| Bacillus | B. clausii | Plomer [47] | 2020 | Untreated (in the previous 3 months) | Yes | Incidence and duration of diarrhea ↓ (p < 0.05) |
| Bifidobacterium | tetragenous | He [48] | 2022 | Untreated | Yes | Incidence of adverse events ↓ (p = 0.016) fluctuations of gastric microbiota ↓ |
| Clostridium | C. butyricum | Chen [23] | 2018 | Untreated | Yes | Relieves more symptoms |
| Lactobacillus (including Lacticaseibacillus, Lactiplantibacillus **) | Lactobacillus acidophilus and L. rhamnosus | Chen [49] | 2021 | Untreated | No | H. pylori bacterial load (measured using delta over baseline value, DOB) ↓ (p = 0.045) |
| L. crispatus/L. helveticus/L. plantarum | Wang [50] | 2022 | Untreated (in the previous 1 month) | No | Eradication rate ↑ (especially L. crispatus) (p = 0.0039) Gastrointestinal symptoms ↓ (p < 0.001) | |
| L. reuteri (non-viable) | Yang [51] | 2021 | Untreated | Yes | frequencies of abdominal distention (p = 0.01) and diarrhea ↓ (p = 0.022) GSRS score ↓ (p = 0.03) More beneficial microbiota profile | |
| Lacticaseibacillus paracasei and L. rhamnosus | Guillemard [29] | 2021 | Untreated | Yes | SCFA ↑ (p = 0.035) Faster recovery of gut microbiota | |
| L. reuteri | Dore [52] | 2022 | Untreated (in the previous 1 month) | Yes | Cannot restore gut microbiota | |
| Saccharomyces | S. boulardii | Qu [53] | 2022 | Treated and failed | No | Eradication rate ↑ during the first phase of treatment (p < 0.001) cost-effectiveness ratio ↑ |
| S. boulardii | Seddik [54] | 2019 | Untreated (in the previous 4 weeks) | Yes | Eradication rate ↑ (p = 0.02) Adverse events ↓ (p < 0.001) | |
| S. boulardii | Zhao [55] | 2021 | Untreated | Yes | Incidence of severe diarrhea ↓ (p = 0.04) Duration of diarrhea ↓ (p = 0.032) | |
| S. boulardii | Chang [56] | 2020 | Untreated (in the previous 1 month) | Yes | No effect on eradication rate and adverse reactions | |
| Mixed | L. plantarum and Pediococcus acidilactici | McNicholl [57] | 2018 | Treated | No | No difference in eradication rate, side effects, and compliance |
| Bacillus subtilis and Enterococcus faecium | Tang [39] | 2021 | Untreated | Yes | More beneficial microbiota profile | |
| L. acidophilus, Lactiplantibacillus. plantarum, Bifidobacterium lactis, S. boulardii | Viazis [58] | 2022 | Untreated | Yes | Eradication rate ↑ (p = 0.028) Side effects ↓ (p < 0.00001) |
3. Clostridium difficile
3.1. Interaction of Gut Microbiome and Clostridium difficile
3.2. Gut Microbiota Modulation for Therapy and Prevention
4. Cholera
4.1. Interaction of Gut Microbiome and Cholera
4.2. Microbiota in Disease Diagnosis and Prediction
4.3. Gut Microbiota Modulation for Therapy and Prevention
5. Enteric Viruses
5.1. Interaction of Gut Microbiome and Enteric Viruses
5.2. Gut Microbiota Modulation for Therapy and Prevention
6. Salmonella enterica Serovar Typhimurium
6.1. Interaction of Gut Microbiome and Salmonella enterica Serovar Typhimurium
6.2. Gut Microbiota Modulation for Therapy and Prevention
7. Other Infections
7.1. Pseudomonas aeruginosa
7.2. Staphylococcus aureus
7.3. Candida albicans
7.4. Giardia duodenalis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal. Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef]
- Uchiyama, R.; Chassaing, B.; Zhang, B.; Gewirtz, A.T. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J. Infect. Dis. 2014, 210, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.T.; Nice, T.J.; McCune, B.T.; Yokoyama, C.C.; Kambal, A.; Wheadon, M.; Diamond, M.S.; Ivanova, Y.; Artyomov, M.; Virgin, H.W. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 2015, 347, 266–269. [Google Scholar] [CrossRef]
- Shi, Z.; Zou, J.; Zhang, Z.; Zhao, X.; Noriega, J.; Zhang, B.; Zhao, C.; Ingle, H.; Bittinger, K.; Mattei, L.M.; et al. Segmented Filamentous Bacteria Prevent and Cure Rotavirus Infection. Cell 2019, 179, 644–658.e613. [Google Scholar] [CrossRef]
- Lei, S.; Samuel, H.; Twitchell, E.; Bui, T.; Ramesh, A.; Wen, K.; Weiss, M.; Li, G.; Yang, X.; Jiang, X.; et al. Enterobacter cloacae inhibits human norovirus infectivity in gnotobiotic pigs. Sci. Rep. 2016, 6, 25017. [Google Scholar] [CrossRef] [PubMed]
- Tsay, F.W.; Hsu, P.I.H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 65. [Google Scholar] [CrossRef]
- Fiorani, M.; Tohumcu, E.; Del Vecchio, L.E.; Porcari, S.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The Influence of Helicobacter pylori on Human Gastric and Gut Microbiota. Antibiotics 2023, 12, 765. [Google Scholar] [CrossRef]
- Dooyema, S.D.R.; Noto, J.M.; Wroblewski, L.E.; Piazuelo, M.B.; Krishna, U.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Peek, R.M. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes 2022, 14, 2105102. [Google Scholar] [CrossRef]
- von Rosenvinge, E.C.; Song, Y.; White, J.R.; Maddox, C.; Blanchard, T.; Fricke, W.F. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J. 2013, 7, 1354–1366. [Google Scholar] [CrossRef]
- Li, M.; Shao, D.; Zhou, J.; Gu, J.; Qin, J.; Li, X.; Hao, C.; Wei, W. Microbial Diversity and Composition in Six Different Gastrointestinal Sites among Participants Undergoing Upper Gastrointestinal Endoscopy in Henan, China. Microbiol. Spectr. 2022, 10, e0064521. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Zimmermann, K.; Nauck, M.; Völker, U.; Völzke, H.; Biffar, R.; et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci. Rep. 2019, 9, 20100. [Google Scholar] [CrossRef]
- Dash, N.R.; Khoder, G.; Nada, A.M.; Al Bataineh, M.T. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS ONE 2019, 14, e0218274. [Google Scholar] [CrossRef]
- Suárez-Jaramillo, A.; Baldeón, M.E.; Prado, B.; Fornasini, M.; Cohen, H.; Flores, N.; Salvador, I.; Cargua, O.; Realpe, J.; Cárdenas, P.A. Duodenal microbiome in patients with or without Helicobacter pylori infection. Helicobacter 2020, 25, e12753. [Google Scholar] [CrossRef]
- Liu, C.; Ng, S.K.; Ding, Y.; Lin, Y.; Liu, W.; Wong, S.H.; Sung, J.J.; Yu, J. Meta-analysis of mucosal microbiota reveals universal microbial signatures and dysbiosis in gastric carcinogenesis. Oncogene 2022, 41, 3599–3610. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Wan, C.; Wang, Z. The relationship of gastric microbiota and Helicobacter pylori infection in pediatrics population. Helicobacter 2020, 25, e12676. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Waskito, L.A.; El-Serag, H.B.; Ajami, N.J.; Nusi, I.A.; Syam, A.F.; Matsumoto, T.; Rezkitha, Y.A.A.; Doohan, D.; Fauzia, K.A.; et al. Gastric microbiota and Helicobacter pylori in Indonesian population. Helicobacter 2020, 25, e12695. [Google Scholar] [CrossRef]
- Chen, C.C.; Liou, J.M.; Lee, Y.C.; Hong, T.C.; El-Omar, E.M.; Wu, M.S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1909459. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Zai, H.; Fukui, Y.; Kato, Y.; Kumade, E.; Watanabe, T.; Furusyo, N.; Nakajima, H.; Arai, K.; Ishii, Y.; et al. Impact of Helicobacter pylori infection on fluid duodenal microbial community structure and microbial metabolic pathways. BMC Microbiol. 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhang, Y.; Gerhard, M.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; Liu, W.D.; et al. Association between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. Front. Cell. Infect. Microbiol. 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Ward, A.; Vasquez-Achaya, F.; Silva-Caso, W.; Aguilar-Luis, M.A.; Mazulis, F.; Urteaga, N.; Del Valle-Mendoza, J. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res. Notes 2018, 11, 468. [Google Scholar] [CrossRef]
- Ralser, A.; Dietl, A.; Jarosch, S.; Engelsberger, V.; Wanisch, A.; Janssen, K.P.; Middelhoff, M.; Vieth, M.; Quante, M.; Haller, D.; et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 2023, 72, 1258–1270. [Google Scholar] [CrossRef]
- Noto, J.M.; Zackular, J.P.; Varga, M.G.; Delgado, A.; Romero-Gallo, J.; Scholz, M.B.; Piazuelo, M.B.; Skaar, E.P.; Peek, R.M., Jr. Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic Histologic Phenotype. mBio 2019, 10, e00955-19. [Google Scholar] [CrossRef]
- Jaswal, K.; Todd, O.A.; Behnsen, J. Neglected gut microbiome: Interactions of the non-bacterial gut microbiota with enteric pathogens. Gut Microbes 2023, 15, 2226916. [Google Scholar] [CrossRef]
- Guillemard, E.; Poirel, M.; Schäfer, F.; Quinquis, L.; Rossoni, C.; Keicher, C.; Wagner, F.; Szajewska, H.; Barbut, F.; Derrien, M.; et al. A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy. Nutrients 2021, 13, 3171. [Google Scholar] [CrossRef] [PubMed]
- Massarrat, S.; Saniee, P.; Siavoshi, F.; Mokhtari, R.; Mansour-Ghanaei, F.; Khalili-Samani, S. The Effect of Helicobacter pylori Infection, Aging, and Consumption of Proton Pump Inhibitor on Fungal Colonization in the Stomach of Dyspeptic Patients. Front. Microbiol. 2016, 7, 801. [Google Scholar] [CrossRef]
- Sangster, W.; Hegarty, J.P.; Schieffer, K.M.; Wright, J.R.; Hackman, J.; Toole, D.R.; Lamendella, R.; Stewart, D.B., Sr. Bacterial and Fungal Microbiota Changes Distinguish C. difficile Infection from Other Forms of Diarrhea: Results of a Prospective Inpatient Study. Front. Microbiol. 2016, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef]
- Stewart, D.B., Sr.; Wright, J.R.; Fowler, M.; McLimans, C.J.; Tokarev, V.; Amaniera, I.; Baker, O.; Wong, H.T.; Brabec, J.; Drucker, R.; et al. Integrated Meta-omics Reveals a Fungus-Associated Bacteriome and Distinct Functional Pathways in Clostridioides difficile Infection. mSphere 2019, 4, e00454-19. [Google Scholar] [CrossRef] [PubMed]
- Lamendella, R.; Wright, J.R.; Hackman, J.; McLimans, C.; Toole, D.R.; Bernard Rubio, W.; Drucker, R.; Wong, H.T.; Sabey, K.; Hegarty, J.P.; et al. Antibiotic Treatments for Clostridium difficile Infection Are Associated with Distinct Bacterial and Fungal Community Structures. mSphere 2018, 3, e00572-17. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Ke, S.; Kelly, C.P.; Pollock, N.R.; Villafuerte Gálvez, J.A.; Daugherty, K.; Xu, H.; Yao, J.; Chen, Y.; et al. Analysis of Intestinal Mycobiota of Patients with Clostridioides difficile Infection among a Prospective Inpatient Cohort. Microbiol. Spectr. 2022, 10, e0136222. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Ke, S.; Villafuerte Gálvez, J.A.; Pollock, N.R.; Barrett, C.; Sprague, R.; Daugherty, K.; Xu, H.; Lin, Q.; et al. Fecal Mycobiota Combined with Host Immune Factors Distinguish Clostridioides difficile Infection from Asymptomatic Carriage. Gastroenterology 2021, 160, 2328–2339.e2326. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, X.S.; Guo, G.Y.; Zhou, M.G.; Yu, B. Effect of Helicobacter pylori Eradication on Human Gastric Microbiota: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 899248. [Google Scholar] [CrossRef]
- Serrano, C.A.; Pierre, R.; Van Der Pol, W.J.; Morrow, C.D.; Smith, P.D.; Harris, P.R. Eradication of Helicobacter pylori in Children Restores the Structure of the Gastric Bacterial Community to That of Noninfected Children. Gastroenterology 2019, 157, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Tang, L.; Huang, C.; Tian, C.; Chen, L.; He, Z.; Yang, G.; Zuo, L.; Zhao, G.; Liu, E.; et al. The Effect of Probiotics Supplementation on Gut Microbiota after Helicobacter pylori Eradication: A Multicenter Randomized Controlled Trial. Infect. Dis. Ther. 2021, 10, 317–333. [Google Scholar] [CrossRef]
- Martín-Núñez, G.M.; Cornejo-Pareja, I.; Clemente-Postigo, M.; Tinahones, F.J.; Moreno-Indias, I. Helicobacter pylori Eradication Therapy Affect the Gut Microbiota and Ghrelin Levels. Front. Med. 2021, 8, 712908. [Google Scholar] [CrossRef]
- Boyanova, L.; Gergova, G.; Markovska, R.; Yordanov, D.; Mitov, I. Bacteriocin-like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains. Lett. Appl. Microbiol. 2017, 65, 469–474. [Google Scholar] [CrossRef]
- Fakharian, F.; Sadeghi, A.; Pouresmaeili, F.; Soleimani, N.; Yadegar, A. Immunomodulatory effects of live and pasteurized Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori-triggered inflammation in gastric epithelial cells in vitro. Mol. Biol. Rep. 2023, 50, 6795–6805. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Marín-Vega, A.M.; Ilabaca, A.; Albarracín, L.; Marcial, G.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. Characterization of the immunomodulatory and anti-Helicobacter pylori properties of the human gastric isolate Lactobacillus rhamnosus UCO-25A. Biofouling 2019, 35, 922–937. [Google Scholar] [CrossRef]
- Do, A.D.; Su, C.H.; Hsu, Y.M. Antagonistic Activities of Lactobacillus rhamnosus JB3 Against Helicobacter pylori Infection Through Lipid Raft Formation. Front. Immunol. 2021, 12, 796177. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Lin, T.L.; Huang, M.Z.; Li, S.W.; Wu, H.Y.; Chiu, Y.F.; Yang, C.Y.; Chiu, C.H.; Lai, H.C. Gut Commensal Parabacteroides goldsteinii MTS01 Alters Gut Microbiota Composition and Reduces Cholesterol to Mitigate Helicobacter pylori-Induced Pathogenesis. Front. Immunol. 2022, 13, 916848. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sepúlveda, C.; Sánchez-Alonzo, K.; Olivares-Muñoz, J.; Gutiérrez-Zamorano, C.; Smith, C.T.; Carvajal, R.I.; Sáez-Carrillo, K.; González, C.; García-Cancino, A. Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved. Foods 2022, 11, 1668. [Google Scholar] [CrossRef]
- Plomer, M.; Iii Perez, M.; Greifenberg, D.M. Effect of Bacillus clausii Capsules in Reducing Adverse Effects Associated with Helicobacter pylori Eradication Therapy: A Randomized, Double-Blind, Controlled Trial. Infect. Dis. Ther. 2020, 9, 867–878. [Google Scholar] [CrossRef]
- He, C.; Xie, Y.; Zhu, Y.; Zhuang, K.; Huo, L.; Yu, Y.; Guo, Q.; Shu, X.; Xiong, Z.; Zhang, Z.; et al. Probiotics modulate gastrointestinal microbiota after Helicobacter pylori eradication: A multicenter randomized double-blind placebo-controlled trial. Front. Immunol. 2022, 13, 1033063. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chen, C.C.; Huang, Y.C.; Tseng, C.C.; Hsu, J.T.; Lin, Y.F.; Fang, Y.J.; Wu, M.S.; Liou, J.M. The efficacy of Lactobacillus acidophilus and rhamnosus in the reduction of bacterial load of Helicobacter pylori and modification of gut microbiota-a double-blind, placebo-controlled, randomized trial. Helicobacter 2021, 26, e12857. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Yu, L.; Tian, F.; Lu, W.; Wang, G.; Chen, W.; Wang, J.; Zhai, Q. Evaluation of the Potential Protective Effects of Lactobacillus Strains against Helicobacter pylori Infection: A Randomized, Double-Blinded, Placebo-Controlled Trial. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 6432750. [Google Scholar] [CrossRef]
- Yang, C.; Liang, L.; Lv, P.; Liu, L.; Wang, S.; Wang, Z.; Chen, Y. Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: A randomized double-blind placebo-controlled trial. Helicobacter 2021, 26, e12856. [Google Scholar] [CrossRef]
- Dore, M.P.; Sau, R.; Niolu, C.; Abbondio, M.; Tanca, A.; Bibbò, S.; Loria, M.; Pes, G.M.; Uzzau, S. Metagenomic Changes of Gut Microbiota following Treatment of Helicobacter pylori Infection with a Simplified Low-Dose Quadruple Therapy with Bismuth or Lactobacillus reuteri. Nutrients 2022, 14, 2789. [Google Scholar] [CrossRef]
- Qu, P.; Liu, X.; Xia, X.; Xie, X.; Luo, J.; Cheng, S.; Chi, J.; Liu, P.; Li, H.; Zhao, W.; et al. Saccharomyces boulardii Allows Partial Patients to Avoid Reusing Bismuth Quadruple for Helicobacter pylori Rescue Therapy: A Single-Center Randomized Controlled Study. Front. Cell. Infect. Microbiol. 2022, 12, 903002. [Google Scholar] [CrossRef] [PubMed]
- Seddik, H.; Boutallaka, H.; Elkoti, I.; Nejjari, F.; Berraida, R.; Berrag, S.; Loubaris, K.; Sentissi, S.; Benkirane, A. Saccharomyces boulardii CNCM I-745 plus sequential therapy for Helicobacter pylori infections: A randomized, open-label trial. Eur. J. Clin. Pharmacol. 2019, 75, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Aruna; Xiao, J.; Song, J.; Huang, T.; Li, S.; Kou, J.; Huang, L.; Ji, D.; et al. Saccharomyces boulardii Combined with Quadruple Therapy for Helicobacter pylori Eradication Decreased the Duration and Severity of Diarrhea: A Multi-Center Prospective Randomized Controlled Trial. Front. Med. 2021, 8, 776955. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Park, Y.M.; Oh, C.H.; Oh, S.J.; Cho, J.H.; Kim, J.W.; Jang, J.Y. Effects of probiotics or broccoli supplementation on Helicobacter pylori eradication with standard clarithromycin-based triple therapy. Korean J. Intern. Med. 2020, 35, 574–581. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Molina-Infante, J.; Lucendo, A.J.; Calleja, J.L.; Pérez-Aisa, Á.; Modolell, I.; Aldeguer, X.; Calafat, M.; Comino, L.; Ramas, M.; et al. Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: A randomized, double-blind, placebo-controlled trial. Helicobacter 2018, 23, e12529. [Google Scholar] [CrossRef]
- Viazis, N.; Argyriou, K.; Kotzampassi, K.; Christodoulou, D.K.; Apostolopoulos, P.; Georgopoulos, S.D.; Liatsos, C.; Giouleme, O.; Koustenis, K.; Veretanos, C.; et al. A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients 2022, 14, 632. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Guery, B.; Galperine, T.; Barbut, F. Clostridioides difficile: Diagnosis and treatments. BMJ 2019, 366, l4609. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Young, V.B. Clostridium difficile and the microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef]
- Piccioni, A.; Rosa, F.; Manca, F.; Pignataro, G.; Zanza, C.; Savioli, G.; Covino, M.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; et al. Gut Microbiota and Clostridium difficile: What We Know and the New Frontiers. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef]
- Mani, J.; Levy, S.; Angelova, A.; Hazrati, S.; Fassnacht, R.; Subramanian, P.; Richards, T.; Niederhuber, J.E.; Maxwell, G.L.; Hourigan, S.K. Epidemiological and microbiome associations of Clostridioides difficile carriage in infancy and early childhood. Gut Microbes 2023, 15, 2203969. [Google Scholar] [CrossRef]
- Rea, M.C.; O’Sullivan, O.; Shanahan, F.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Hill, C. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J. Clin. Microbiol. 2012, 50, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.; Levenez, F.; Fouqueray, C.; Doré, J.; Collignon, A.; Lepage, P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J. Clin. Microbiol. 2011, 49, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kearney, S.; Li, N.; Bogart, E.; Bullock, K.; Gerber, G.K.; Bry, L.; Clish, C.B.; Alm, E.; Korzenik, J.R. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment. Pharmacol. Ther. 2016, 43, 1142–1153. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.R.; Kraft, C.S.; Dhere, T.; Henn, M.R.; Lombardo, M.J.; Vulic, M.; Ohsumi, T.; Winkler, J.; et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents Recurrent Clostridium difficile Infection. J. Infect. Dis. 2016, 214, 173–181. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E., Jr.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef]
- Manges, A.R.; Labbe, A.; Loo, V.G.; Atherton, J.K.; Behr, M.A.; Masson, L.; Tellis, P.A.; Brousseau, R. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J. Infect. Dis. 2010, 202, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.M.; Rogers, M.A.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 2014, 5, e01021-14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, Y.; Seo, M.R.; Bae, M.H.; Kim, B.; Rho, M.; Pai, H. Quantitative characterization of Clostridioides difficile population in the gut microbiome of patients with C. difficile infection and their association with clinical factors. Sci. Rep. 2020, 10, 17608. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Pollock, N.R.; Wang, X.W.; Chen, X.; Daugherty, K.; Lin, Q.; Xu, H.; Garey, K.W.; Gonzales-Luna, A.J.; Kelly, C.P.; et al. Integrating gut microbiome and host immune markers to understand the pathogenesis of Clostridioides difficile infection. Gut Microbes 2021, 13, 1935186. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Macfarlane, G.T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 2002, 51, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Jenior, M.L.; Keenan, O.; Hart, J.L.; Specker, J.; Abbas, A.; Rangel, P.C.; Di, C.; Green, J.; Bustin, K.A.; et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Simmonds, A.; Annavajhala, M.K.; Nunez, M.P.; Macesic, N.; Park, H.; Uhlemann, A.C. Intestinal Dysbiosis and Risk of Posttransplant Clostridioides difficile Infection in a Longitudinal Cohort of Liver Transplant Recipients. mSphere 2022, 7, e0036122. [Google Scholar] [CrossRef]
- Panpetch, W.; Somboonna, N.; Palasuk, M.; Hiengrach, P.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS ONE 2019, 14, e0210798. [Google Scholar] [CrossRef]
- van Leeuwen, P.T.; van der Peet, J.M.; Bikker, F.J.; Hoogenkamp, M.A.; Oliveira Paiva, A.M.; Kostidis, S.; Mayboroda, O.A.; Smits, W.K.; Krom, B.P. Interspecies Interactions between Clostridium difficile and Candida albicans. mSphere 2016, 1, e00187-16. [Google Scholar] [CrossRef]
- Markey, L.; Shaban, L.; Green, E.R.; Lemon, K.P.; Mecsas, J.; Kumamoto, C.A. Pre-colonization with the commensal fungus Candida albicans reduces murine susceptibility to Clostridium difficile infection. Gut Microbes 2018, 9, 497–509. [Google Scholar] [CrossRef]
- Orenstein, R.; Dubberke, E.R.; Khanna, S.; Lee, C.H.; Yoho, D.; Johnson, S.; Hecht, G.; DuPont, H.L.; Gerding, D.N.; Blount, K.F.; et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: Results from an open-label phase 2 clinical trial. BMC Infect. Dis. 2022, 22, 245. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.L.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef]
- Khanna, S.; Assi, M.; Lee, C.; Yoho, D.; Louie, T.; Knapple, W.; Aguilar, H.; Garcia-Diaz, J.; Wang, G.P.; Berry, S.M.; et al. Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection. Drugs 2022, 82, 1527–1538. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e1323. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Shanahan, F. Fecal microbiota-based treatment for recurrent Clostridioides difficile infection. Cell. 2023, 186, 1087. [Google Scholar] [CrossRef]
- Lodise, T.; Guo, A.; Yang, M.; Cook, E.E.; Song, W.; Yang, D.; Wang, Q.; Zhao, A.; Bochan, M. Budget Impact Analysis of REBYOTA™ (Fecal Microbiota, Live-jslm [FMBL]) for Preventing Recurrent Clostridioides difficile Infection in the US. Adv. Ther. 2023, 40, 2801–2819. [Google Scholar] [CrossRef]
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; Gravenor, M.B.; et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013, 382, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, Cd006095. [Google Scholar] [CrossRef] [PubMed]
- Alberda, C.; Marcushamer, S.; Hewer, T.; Journault, N.; Kutsogiannis, D. Feasibility of a Lactobacillus casei Drink in the Intensive Care Unit for Prevention of Antibiotic Associated Diarrhea and Clostridium difficile. Nutrients 2018, 10, 539. [Google Scholar] [CrossRef]
- Louie, T.; Golan, Y.; Khanna, S.; Bobilev, D.; Erpelding, N.; Fratazzi, C.; Carini, M.; Menon, R.; Ruisi, M.; Norman, J.M.; et al. VE303, a Defined Bacterial Consortium, for Prevention of Recurrent Clostridioides difficile Infection: A Randomized Clinical Trial. JAMA 2023, 329, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Pensinger, D.A.; Fisher, A.T.; Dobrila, H.A.; Van Treuren, W.; Gardner, J.O.; Higginbottom, S.K.; Carter, M.M.; Schumann, B.; Bertozzi, C.R.; Anikst, V.; et al. Butyrate Differentiates Permissiveness to Clostridioides difficile Infection and Influences Growth of Diverse C. difficile Isolates. Infect. Immun. 2023, 91, e0057022. [Google Scholar] [CrossRef]
- May, T.; Mackie, R.I.; Fahey, G.C., Jr.; Cremin, J.C.; Garleb, K.A. Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand. J. Gastroenterol. 1994, 29, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Li, F.; Xiang, L.; Lv, P.; Chen, Y. Treatment with butyrate alleviates dextran sulfate sodium and Clostridium difficile-induced colitis by preventing activity of Th17 cells via regulation of SIRT1/mTOR in mice. J. Nutr. Biochem. 2023, 111, 109155. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Nagao-Kitamoto, H.; Kitamoto, S.; Kim, C.H.; Kamada, N. The Butyrate-Producing Bacterium Clostridium butyricum Suppresses Clostridioides difficile Infection via Neutrophil- and Antimicrobial Cytokine-Dependent but GPR43/109a-Independent Mechanisms. J. Immunol. 2021, 206, 1576–1585. [Google Scholar] [CrossRef]
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; de Sales, E.S.É.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.e757. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, L.; Li, F.; Deng, W.; Lv, P.; Chen, Y. Butyrate Protects against Clostridium difficile Infection by Regulating Bile Acid Metabolism. Microbiol. Spectr. 2023, 11, e0447922. [Google Scholar] [CrossRef]
- Koh, E.; Hwang, I.Y.; Lee, H.L.; De Sotto, R.; Lee, J.W.J.; Lee, Y.S.; March, J.C.; Chang, M.W. Engineering probiotics to inhibit Clostridioides difficile infection by dynamic regulation of intestinal metabolism. Nat. Commun. 2022, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Strokappe, N.M.; Hultberg, A.; Truusalu, K.; Smidt, I.; Mikelsaar, R.H.; Mikelsaar, M.; Verrips, T.; Hammarström, L.; Marcotte, H. Neutralization of Clostridium difficile Toxin B Mediated by Engineered Lactobacilli That Produce Single-Domain Antibodies. Infect. Immun. 2016, 84, 395–406. [Google Scholar] [CrossRef]
- Vedantam, G.; Kochanowsky, J.; Lindsey, J.; Mallozzi, M.; Roxas, J.L.; Adamson, C.; Anwar, F.; Clark, A.; Claus-Walker, R.; Mansoor, A.; et al. An Engineered Synthetic Biologic Protects Against Clostridium difficile Infection. Front. Microbiol. 2018, 9, 2080. [Google Scholar] [CrossRef]
- Kanungo, S.; Azman, A.S.; Ramamurthy, T.; Deen, J.; Dutta, S. Cholera. Lancet 2022, 399, 1429–1440. [Google Scholar] [CrossRef]
- Sack, D.A.; Debes, A.K.; Ateudjieu, J.; Bwire, G.; Ali, M.; Ngwa, M.C.; Mwaba, J.; Chilengi, R.; Orach, C.C.; Boru, W.; et al. Contrasting Epidemiology of Cholera in Bangladesh and Africa. J. Infect. Dis. 2021, 224, s701–s709. [Google Scholar] [CrossRef]
- Fast, D.; Petkau, K.; Ferguson, M.; Shin, M.; Galenza, A.; Kostiuk, B.; Pukatzki, S.; Foley, E. Vibrio cholerae-Symbiont Interactions Inhibit Intestinal Repair in Drosophila. Cell. Rep. 2020, 30, 1088–1100.e1085. [Google Scholar] [CrossRef] [PubMed]
- Flaugnatti, N.; Isaac, S.; Lemos Rocha, L.F.; Stutzmann, S.; Rendueles, O.; Stoudmann, C.; Vesel, N.; Garcia-Garcera, M.; Buffet, A.; Sana, T.G.; et al. Human commensal gut Proteobacteria withstand type VI secretion attacks through immunity protein-independent mechanisms. Nat. Commun. 2021, 12, 5751. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, X.; Chen, G.; Park, C.; Liu, Z. Crosstalks between Gut Microbiota and Vibrio cholerae. Front. Cell. Infect. Microbiol. 2020, 10, 582554. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Mitchell, J.D.; Cho, J.Y.; Liu, R.; Macbeth, J.C.; Hsiao, A. Interpersonal Gut Microbiome Variation Drives Susceptibility and Resistance to Cholera Infection. Cell 2020, 181, 1533–1546.e1513. [Google Scholar] [CrossRef]
- Hsiao, A.; Ahmed, A.M.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A., Jr.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef]
- Pauer, H.; Teixeira, F.L.; Robinson, A.V.; Parente, T.E.; De Melo, M.A.F.; Lobo, L.A.; Domingues, R.; Allen-Vercoe, E.; Ferreira, R.B.R.; Antunes, L.C.M. Bioactive small molecules produced by the human gut microbiome modulate Vibrio cholerae sessile and planktonic lifestyles. Gut Microbes 2021, 13, 1918993. [Google Scholar] [CrossRef]
- Chen, J.; Byun, H.; Liu, R.; Jung, I.J.; Pu, Q.; Zhu, C.Y.; Tanchoco, E.; Alavi, S.; Degnan, P.H.; Ma, A.T.; et al. A commensal-encoded genotoxin drives restriction of Vibrio cholerae colonization and host gut microbiome remodeling. Proc. Natl. Acad. Sci. USA 2022, 119, e2121180119. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Cubillos-Ruiz, A.; Cameron, D.E.; Collins, J.J. Probiotic strains detect and suppress cholera in mice. Sci. Transl. Med. 2018, 10, eaao2586. [Google Scholar] [CrossRef]
- Nair, G.B.; Ramamurthy, T.; Sur, D.; Kurakawa, T.; Takahashi, T.; Nomoto, K.; Takeda, Y. Vibrio cholerae/mimicus in fecal microbiota of healthy children in a cholera endemic urban slum setting in Kolkata, India. Microbiol. Immunol. 2012, 56, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Midani, F.S.; Weil, A.A.; Chowdhury, F.; Begum, Y.A.; Khan, A.I.; Debela, M.D.; Durand, H.K.; Reese, A.T.; Nimmagadda, S.N.; Silverman, J.D.; et al. Human Gut Microbiota Predicts Susceptibility to Vibrio cholerae Infection. J. Infect. Dis. 2018, 218, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Levade, I.; Saber, M.M.; Midani, F.S.; Chowdhury, F.; Khan, A.I.; Begum, Y.A.; Ryan, E.T.; David, L.A.; Calderwood, S.B.; Harris, J.B.; et al. Predicting Vibrio cholerae Infection and Disease Severity Using Metagenomics in a Prospective Cohort Study. J. Infect. Dis. 2021, 223, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xiao, Y.; Huang, X.; Chen, F.; Sun, M.; Bilotta, A.J.; Xu, L.; Lu, Y.; Yao, S.; Zhao, Q.; et al. Microbiota Metabolite Short-Chain Fatty Acids Facilitate Mucosal Adjuvant Activity of Cholera Toxin through GPR43. J. Immunol. 2019, 203, 282–292. [Google Scholar] [CrossRef]
- Rabbani, G.H.; Albert, M.J.; Rahman, H.; Chowdhury, A.K. Short-chain fatty acids inhibit fluid and electrolyte loss induced by cholera toxin in proximal colon of rabbit in vivo. Dig. Dis. Sci. 1999, 44, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Hoq, M.M.; Chowdhury, A.K.; Suau, A.; Magne, F.; Endtz, H.P.; Alam, M.; Rahman, M.; Pochart, P.; Desjeux, J.F.; et al. Short-chain fatty acids and commensal microbiota in the faeces of severely malnourished children with cholera rehydrated with three different carbohydrates. Eur. J. Clin. Nutr. 2010, 64, 1116–1124. [Google Scholar] [CrossRef]
- Jayaraman, P.; Holowko, M.B.; Yeoh, J.W.; Lim, S.; Poh, C.L. Repurposing a Two-Component System-Based Biosensor for the Killing of Vibrio cholerae. ACS Synth. Biol. 2017, 6, 1403–1415. [Google Scholar] [CrossRef]
- Matsuda, F.; Chowdhury, M.I.; Saha, A.; Asahara, T.; Nomoto, K.; Tarique, A.A.; Ahmed, T.; Nishibuchi, M.; Cravioto, A.; Qadri, F. Evaluation of a probiotics, Bifidobacterium breve BBG-01, for enhancement of immunogenicity of an oral inactivated cholera vaccine and safety: A randomized, double-blind, placebo-controlled trial in Bangladeshi children under 5 years of age. Vaccine 2011, 29, 1855–1858. [Google Scholar] [CrossRef]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef]
- Hubbard, T.P.; Billings, G.; Dörr, T.; Sit, B.; Warr, A.R.; Kuehl, C.J.; Kim, M.; Delgado, F.; Mekalanos, J.J.; Lewnard, J.A.; et al. A live vaccine rapidly protects against cholera in an infant rabbit model. Sci. Transl. Med. 2018, 10, eaap8423. [Google Scholar] [CrossRef]
- Sit, B.; Zhang, T.; Fakoya, B.; Akter, A.; Biswas, R.; Ryan, E.T.; Waldor, M.K. Oral immunization with a probiotic cholera vaccine induces broad protective immunity against Vibrio cholerae colonization and disease in mice. PLoS Negl. Trop. Dis. 2019, 13, e0007417. [Google Scholar] [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Bhar, S.; Hackett, S.; Engelken, H.; Joseph, R.; Keyhani, N.O.; Jones, M.K. Attach Me If You Can: Murine Norovirus Binds to Commensal Bacteria and Fungi. Viruses 2020, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Wu, J.; Yu, L.; Li, X.; Xie, K.; Zhang, M.; Ren, L.; Ji, Y.; Liu, Y. Crosstalk between imbalanced gut microbiota caused by antibiotic exposure and rotavirus replication in the intestine. Heliyon 2023, 9, e12718. [Google Scholar] [CrossRef]
- Karst, S.M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 2016, 14, 197–204. [Google Scholar] [CrossRef]
- Gozalbo-Rovira, R.; Santiso-Bellón, C.; Buesa, J.; Rubio-Del-Campo, A.; Vila-Vicent, S.; Muñoz, C.; Yebra, M.J.; Monedero, V.; Rodríguez-Díaz, J. Microbiota Depletion Promotes Human Rotavirus Replication in an Adult Mouse Model. Biomedicines 2021, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, S.; Kwon, M.S.; Lee, J.; Yu, D.H.; Song, R.H.; Choi, H.J.; Park, J. Rotavirus-mediated alteration of gut microbiota and its correlation with physiological characteristics in neonatal calves. J. Microbiol. 2019, 57, 113–121. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.; García-Mantrana, I.; Vila-Vicent, S.; Gozalbo-Rovira, R.; Buesa, J.; Monedero, V.; Collado, M.C. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci. Rep. 2017, 7, 45559. [Google Scholar] [CrossRef]
- Kandasamy, S.; Vlasova, A.N.; Fischer, D.; Kumar, A.; Chattha, K.S.; Rauf, A.; Shao, L.; Langel, S.N.; Rajashekara, G.; Saif, L.J. Differential Effects of Escherichia coli Nissle and Lactobacillus rhamnosus Strain GG on Human Rotavirus Binding, Infection, and B Cell Immunity. J. Immunol. 2016, 196, 1780–1789. [Google Scholar] [CrossRef]
- Van Winkle, J.A.; Peterson, S.T.; Kennedy, E.A.; Wheadon, M.J.; Ingle, H.; Desai, C.; Rodgers, R.; Constant, D.A.; Wright, A.P.; Li, L.; et al. Homeostatic interferon-lambda response to bacterial microbiota stimulates preemptive antiviral defense within discrete pockets of intestinal epithelium. eLife 2022, 11, e74072. [Google Scholar] [CrossRef]
- Grau, K.R.; Zhu, S.; Peterson, S.T.; Helm, E.W.; Philip, D.; Phillips, M.; Hernandez, A.; Turula, H.; Frasse, P.; Graziano, V.R.; et al. The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat. Microbiol. 2020, 5, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vlasova, A.N.; Deblais, L.; Huang, H.C.; Wijeratne, A.; Kandasamy, S.; Fischer, D.D.; Langel, S.N.; Paim, F.C.; Alhamo, M.A.; et al. Impact of nutrition and rotavirus infection on the infant gut microbiota in a humanized pig model. BMC Gastroenterol. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.M.; Walk, S.T.; Taube, S.; Taniuchi, M.; Houpt, E.R.; Wobus, C.E.; Young, V.B. Disruption of the human gut microbiota following Norovirus infection. PLoS ONE 2012, 7, e48224. [Google Scholar] [CrossRef]
- Kernbauer, E.; Ding, Y.; Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014, 516, 94–98. [Google Scholar] [CrossRef]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef]
- Park, M.S.; Kwon, B.; Ku, S.; Ji, G.E. The Efficacy of Bifidobacterium longum BORI and Lactobacillus acidophilus AD031 Probiotic Treatment in Infants with Rotavirus Infection. Nutrients 2017, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Breiman, A.; le Pendu, J.; Uyttendaele, M. Anti-viral Effect of Bifidobacterium adolescentis against Noroviruses. Front. Microbiol. 2016, 7, 864. [Google Scholar] [CrossRef][Green Version]
- Di, J.B.; Gai, Z.T. Protective efficacy of probiotics on the treatment of acute rotavirus diarrhea in children: An updated meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9675–9683. [Google Scholar] [CrossRef]
- Peña-Gil, N.; Santiso-Bellón, C.; Gozalbo-Rovira, R.; Buesa, J.; Monedero, V.; Rodríguez-Díaz, J. The Role of Host Glycobiology and Gut Microbiota in Rotavirus and Norovirus Infection, an Update. Int. J. Mol. Sci. 2021, 22, 3473. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Bronowski, C.; Sindhu, K.N.C.; Babji, S.; Benny, B.; Carmona-Vicente, N.; Chasweka, N.; Chinyama, E.; Cunliffe, N.A.; Dube, Q.; et al. Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants. Nat. Commun. 2021, 12, 7288. [Google Scholar] [CrossRef]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chattha, K.S.; Vlasova, A.N.; Kandasamy, S.; Rajashekara, G.; Saif, L.J. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J. Immunol. 2013, 191, 2446–2456. [Google Scholar] [CrossRef]
- Wang, H.; Gao, K.; Wen, K.; Allen, I.C.; Li, G.; Zhang, W.; Kocher, J.; Yang, X.; Giri-Rachman, E.; Li, G.H.; et al. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol. 2016, 16, 109. [Google Scholar] [CrossRef]
- Haselbeck, A.H.; Panzner, U.; Im, J.; Baker, S.; Meyer, C.G.; Marks, F. Current perspectives on invasive nontyphoidal Salmonella disease. Curr. Opin. Infect. Dis. 2017, 30, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.W.L.; Tsolis, R.M.; Bäumler, A.J. Salmonella versus the Microbiome. Microbiol. Mol. Biol. Rev. 2021, 85, e00027-19. [Google Scholar] [CrossRef]
- Vogt, S.L.; Finlay, B.B. Gut microbiota-mediated protection against diarrheal infections. J. Travel. Med. 2017, 24, S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kabe, Y.; Kanai, A.; Sugiura, Y.; Hida, S.; Taniguchi, S.; Takahashi, T.; Matsui, H.; Yasukawa, Z.; Itou, H.; et al. Short-chain fatty acids bind to apoptosis-associated speck-like protein to activate inflammasome complex to prevent Salmonella infection. PLoS Biol. 2020, 18, e3000813. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, W.; Qin, N.; Ren, X.; Xia, X. Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models. Foods 2022, 11, 3493. [Google Scholar] [CrossRef]
- Bronner, D.N.; Faber, F.; Olsan, E.E.; Byndloss, M.X.; Sayed, N.A.; Xu, G.; Yoo, W.; Kim, D.; Ryu, S.; Lebrilla, C.B.; et al. Genetic Ablation of Butyrate Utilization Attenuates Gastrointestinal Salmonella Disease. Cell Host Microbe 2018, 23, 266–273.e264. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Liu, H.; Teng, Y.; Qin, N.; Ren, X.; Xia, X. Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice. J. Adv. Res. 2023, 52, 89–102. [Google Scholar] [CrossRef]
- Lemme-Dumit, J.M.; Cazorla, S.I.; Perdigón, G.D.V.; Maldonado-Galdeano, C. Probiotic Bacteria and Their Cell Walls Induce Th1-Type Immunity Against Salmonella Typhimurium Challenge. Front. Immunol. 2021, 12, 660854. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection-prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Dalmasso, G.; Arantes, R.M.; Doye, A.; Lemichez, E.; Lagadec, P.; Imbert, V.; Peyron, J.F.; Rampal, P.; Nicoli, J.R.; et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS ONE 2010, 5, e8925. [Google Scholar] [CrossRef]
- Liu, R.H.; Sun, A.Q.; Liao, Y.; Tang, Z.X.; Zhang, S.H.; Shan, X.; Hu, J.T. Lactiplantibacillus plantarum Regulated Intestinal Microbial Community and Cytokines to Inhibit Salmonella typhimurium Infection. Probiotics Antimicrob. Proteins 2023, 15, 1355–1370. [Google Scholar] [CrossRef]
- Acurcio, L.B.; Wuyts, S.; de Cicco Sandes, S.H.; Sant’anna, F.M.; Pedroso, S.; Bastos, R.W.; Dos Reis, D.C.; Vieira, A.F.; Cassali, G.D.; Lebeer, S.; et al. Milk Fermented by Lactobacillus paracasei NCC 2461 (ST11) Modulates the Immune Response and Microbiota to Exert its Protective Effects Against Salmonella typhimurium Infection in Mice. Probiotics Antimicrob. Proteins 2020, 12, 1398–1408. [Google Scholar] [CrossRef]
- Mazkour, S.; Shekarforoush, S.S.; Basiri, S.; Nazifi, S.; Yektaseresht, A.; Honarmand, M. Effects of two probiotic spores of Bacillus species on hematological, biochemical, and inflammatory parameters in Salmonella Typhimurium infected rats. Sci. Rep. 2020, 10, 8035. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Patel, P.; Bernhardt, C.; Biswas, D. Linoleic Acids Overproducing Lactobacillus casei Limits Growth, Survival, and Virulence of Salmonella Typhimurium and Enterohaemorrhagic Escherichia coli. Front. Microbiol. 2018, 9, 2663. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.B.; Souto, B.S.; Santos, N.C.M.; Pereira, J.A.; Tagliati, C.A.; Novaes, R.D.; Corsetti, P.P.; de Almeida, L.A. Murine response to the opportunistic bacterium Pseudomonas aeruginosa infection in gut dysbiosis caused by 5-fluorouracil chemotherapy-induced mucositis. Life Sci. 2022, 307, 120890. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, M.M.; Gent, J.F.; Kong, Y.; Halpin, A.L.; Pineles, L.; Harris, A.D.; Johnson, J.K. Gastrointestinal Microbiota Disruption and Risk of Colonization with Carbapenem-resistant Pseudomonas aeruginosa in Intensive Care Unit Patients. Clin. Infect. Dis. 2019, 69, 604–613. [Google Scholar] [CrossRef]
- Phuengmaung, P.; Somparn, P.; Panpetch, W.; Singkham-In, U.; Wannigama, D.L.; Chatsuwan, T.; Leelahavanichkul, A. Coexistence of Pseudomonas aeruginosa with Candida albicans Enhances Biofilm Thickness Through Alginate-Related Extracellular Matrix but Is Attenuated by N-acetyl-l-cysteine. Front. Cell. Infect. Microbiol. 2020, 10, 594336. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Wieërs, G.; Verbelen, V.; Van Den Driessche, M.; Melnik, E.; Vanheule, G.; Marot, J.C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota with Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health 2020, 8, 578089. [Google Scholar] [CrossRef] [PubMed]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Janapatla, R.P.; Dudek, A.; Chen, C.L.; Chuang, C.H.; Chien, K.Y.; Feng, Y.; Yeh, Y.M.; Wang, Y.H.; Chang, H.J.; Lee, Y.C.; et al. Marine prebiotics mediate decolonization of Pseudomonas aeruginosa from gut by inhibiting secreted virulence factor interactions with mucins and enriching Bacteroides population. J. Biomed. Sci. 2023, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Ni, Q.; Wang, C.; Zhang, L.; Li, Z.; Jiang, C.; Mao, E.; Peng, Y. Effects of intestinal colonization by Clostridium difficile and Staphylococcus aureus on microbiota diversity in healthy individuals in China. BMC Infect. Dis. 2018, 18, 207. [Google Scholar] [CrossRef]
- Mousa, W.K.; Ghemrawi, R.; Abu-Izneid, T.; Ramadan, A.; Al-Marzooq, F. Discovery of Lactomodulin, a Unique Microbiome-Derived Peptide That Exhibits Dual Anti-Inflammatory and Antimicrobial Activity against Multidrug-Resistant Pathogens. Int. J. Mol. Sci. 2023, 24, 6901. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Guo, D.; Gu, L.; Li, N.; Li, J. Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant Staphylococcus aureus enterocolitis. BMC Infect. Dis. 2015, 15, 265. [Google Scholar] [CrossRef]
- Gutierrez, D.; Weinstock, A.; Antharam, V.C.; Gu, H.; Jasbi, P.; Shi, X.; Dirks, B.; Krajmalnik-Brown, R.; Maldonado, J.; Guinan, J.; et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2020, 96, fiz187. [Google Scholar] [CrossRef]
- Pan, C.H.; Lo, H.J.; Yan, J.Y.; Hsiao, Y.J.; Hsueh, J.W.; Lin, D.W.; Lin, T.H.; Wu, S.H.; Chen, Y.C. Candida albicans Colonizes and Disseminates to the Gastrointestinal Tract in the Presence of the Microbiota in a Severe Combined Immunodeficient Mouse Model. Front. Microbiol. 2020, 11, 619878. [Google Scholar] [CrossRef]
- Zaongo, S.D.; Ouyang, J.; Isnard, S.; Zhou, X.; Harypursat, V.; Cui, H.; Routy, J.P.; Chen, Y. Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes 2023, 15, 2167171. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Datta, A.; Hernandez-Franco, J.F.; Park, S.; Olson, M.R.; HogenEsch, H.; Thangamani, S. Bile Acid Regulates Mononuclear Phagocytes and T Helper 17 Cells to Control Candida albicans in the Intestine. J. Fungi 2022, 8, 610. [Google Scholar] [CrossRef]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Panpetch, W.; Worasilchai, N.; Chindamporn, A.; Tumwasorn, S.; Jaroonwitchawan, T.; Wilantho, A.; Chatthanathon, P.; Somboonna, N.; Leelahavanichkul, A. Administration of Candida albicans to Dextran Sulfate Solution Treated Mice Causes Intestinal Dysbiosis, Emergence and Dissemination of Intestinal Pseudomonas aeruginosa and Lethal Sepsis. Shock 2020, 53, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zeise, K.D.; Woods, R.J.; Huffnagle, G.B. Interplay between Candida albicans and Lactic Acid Bacteria in the Gastrointestinal Tract: Impact on Colonization Resistance, Microbial Carriage, Opportunistic Infection, and Host Immunity. Clin. Microbiol. Rev. 2021, 34, e0032320. [Google Scholar] [CrossRef]
- Matsuo, K.; Haku, A.; Bi, B.; Takahashi, H.; Kamada, N.; Yaguchi, T.; Saijo, S.; Yoneyama, M.; Goto, Y. Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol. Immunol. 2019, 63, 155–163. [Google Scholar] [CrossRef]
- Authier, H.; Salon, M.; Rahabi, M.; Bertrand, B.; Blondeau, C.; Kuylle, S.; Holowacz, S.; Coste, A. Oral Administration of Lactobacillus helveticus LA401 and Lactobacillus gasseri LA806 Combination Attenuates Oesophageal and Gastrointestinal Candidiasis and Consequent Gut Inflammation in Mice. J. Fungi 2021, 7, 57. [Google Scholar] [CrossRef]
- Graf, K.; Last, A.; Gratz, R.; Allert, S.; Linde, S.; Westermann, M.; Gröger, M.; Mosig, A.S.; Gresnigt, M.S.; Hube, B. Keeping Candida commensal: How lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis. Model. Mech. 2019, 12, dmm039719. [Google Scholar] [CrossRef]
- Kondori, N.; Nowrouzian, F.; Ajdari, M.; Hesselmar, B.; Saalman, R.; Wold, A.E.; Adlerberth, I. Candida species as commensal gut colonizers: A study of 133 longitudinally followed Swedish infants. Med. Mycol. 2020, 58, 485–492. [Google Scholar] [CrossRef]
- Peroumal, D.; Sahu, S.R.; Kumari, P.; Utkalaja, B.G.; Acharya, N. Commensal Fungus Candida albicans Maintains a Long-Term Mutualistic Relationship with the Host To Modulate Gut Microbiota and Metabolism. Microbiol. Spectr. 2022, 10, e0246222. [Google Scholar] [CrossRef]
- Tso, G.H.W.; Reales-Calderon, J.A.; Tan, A.S.M.; Sem, X.; Le, G.T.T.; Tan, T.G.; Lai, G.C.; Srinivasan, K.G.; Yurieva, M.; Liao, W.; et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 2018, 362, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Belkessa, S.; Ait-Salem, E.; Laatamna, A.; Houali, K.; Sönksen, U.W.; Hakem, A.; Bouchene, Z.; Ghalmi, F.; Stensvold, C.R. Prevalence and Clinical Manifestations of Giardia intestinalis and Other Intestinal Parasites in Children and Adults in Algeria. Am. J. Trop. Med. Hyg. 2021, 104, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, Y.E.; Yıldız, M.R. Microbiota and parasite relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef]
- Barash, N.R.; Maloney, J.G.; Singer, S.M.; Dawson, S.C. Giardia Alters Commensal Microbial Diversity throughout the Murine Gut. Infect. Immun. 2017, 85, e00948-16. [Google Scholar] [CrossRef] [PubMed]
- Dashti, N.; Zarebavani, M. Probiotics in the management of Giardia duodenalis: An update on potential mechanisms and outcomes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1869–1878. [Google Scholar] [CrossRef]
| Author | Disease States vs. Control * | Microbiome Specimen | Fungi Change | |
|---|---|---|---|---|
| Helicobacter pylori untreated | Suárez-Jaramillo [17] | 37 infected vs. 38 uninfected | Proximal duodenum biopsy | No Agaricomycetes |
| Dash [16] | 12 infected vs. 48 uninfected | Fecal | Candida glabrata and other unclassified fungi ↑ ** | |
| Helicobacter pylori treated (triple therapy) | Guillemard [29] | 136 infected, triple therapy, before vs. after | Fecal | Fungi to bacteria Shannon ratio transient ↑ Candida transient ↑ |
| Clostridium difficile untreated | Sangster [31] | 12 CDI vs. 12 non-CDI diarrhea | Fecal | Penicillium ↑ |
| Zuo [32] | 34 CDI vs. 24 HC | Fecal | Diversity, evenness, and richness ↓ Ascomycota phylum ↑ Candida albicans ↑ | |
| Stewart [33] | 18 CDI vs. 31 non-CDI diarrhea | Fecal | Aspergillus and Penicillium ↑ Oscillospira, Comamonadaceae, Microbacteriaceae, and Cytophagaceae genus ↓ | |
| Lamendella [34] | 10 CDI vs. 10 non-CDI diarrhea | Fecal | Ascomycota phylum, Pleosporales order, and Dothideomycetes class ↑ Pichiaceae family ↓ | |
| Cao [35] | 58 CDI vs. 91 non-CDI (28 asymptomatic carriers, 32 HC, and 31 non-CDI diarrhea) | Fecal | Diversity and richness ↓ Ascomycota phylum, Pichia genus, and Suhomyces genus ↑ Basidiomycota phylum ↓ | |
| Cao [36] | 58 CDI vs. 28 asymptomatic carrier | Fecal | Diversity and richness ↓ Ascomycota phylum ↑ Basidiomycota phylum, Aspergillus, and Cladosporium genus ↓ | |
| 58 CDI vs. 32 HC | ||||
| Clostridium difficile treated | Zuo [32] | 9 CDI-FMT Responder vs. 7 CDI-FMT non-Responder | Fecal | Richness and diversity ↑ Saccharomyces, Aspergillus, and Penicillum genus ↑ Candida genus and Candida albicans ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Min, Y.; Pang, K.; Wu, D. Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases. Int. J. Mol. Sci. 2023, 24, 15654. https://doi.org/10.3390/ijms242115654
Han Z, Min Y, Pang K, Wu D. Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases. International Journal of Molecular Sciences. 2023; 24(21):15654. https://doi.org/10.3390/ijms242115654
Chicago/Turabian StyleHan, Ziying, Yiyang Min, Ke Pang, and Dong Wu. 2023. "Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases" International Journal of Molecular Sciences 24, no. 21: 15654. https://doi.org/10.3390/ijms242115654
APA StyleHan, Z., Min, Y., Pang, K., & Wu, D. (2023). Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases. International Journal of Molecular Sciences, 24(21), 15654. https://doi.org/10.3390/ijms242115654





