Abstract
Schizophrenia is one of the most serious psychiatric disorders and is characterized by reductions in both brain volume and spine density in the frontal cortex. RhoA belongs to the RAS homolog (Rho) family and plays critical roles in neuronal development and structural plasticity via Rho-kinase. RhoA activity is regulated by GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs). Several variants in GAPs and GEFs associated with RhoA have been reported to be significantly associated with schizophrenia. Moreover, several mouse models carrying schizophrenia-associated gene variants involved in RhoA/Rho-kinase signaling have been developed. In this review, we summarize clinical evidence showing that variants in genes regulating RhoA activity are associated with schizophrenia. In the last half of the review, we discuss preclinical evidence indicating that RhoA/Rho-kinase is a potential therapeutic target of schizophrenia. In particular, Rho-kinase inhibitors exhibit anti-psychotic-like effects not only in Arhgap10 S490P/NHEJ mice, but also in pharmacologic models of schizophrenia (methamphetamine- and MK-801-treated mice). Accordingly, we propose that Rho-kinase inhibitors may have antipsychotic effects and reduce cognitive deficits in schizophrenia despite the presence or absence of genetic variants in small GTPase signaling pathways.
Keywords:
copy number variants; CNVs; single-nucleotide polymorphisms; SNPs; fasudil; Kalirin; dopamine; glutamate; RTN4R; KCTD13 1. Introduction
Schizophrenia is one of the most serious psychiatric disorders and affects approximately 1% of the population [1]. It typically emerges in late adolescence and early adulthood and involves positive symptoms (such as hallucinations, delusions, and formal thought disorder), negative symptoms (such as lack of volition, reduced speech output, and flattening of affect), and cognitive dysfunction (manifested, for instance, by deteriorations in working memory, executive function, and learning) [1,2,3].
Neuropathological and neurophysiological changes observed in patients with schizophrenia include enlargement of the lateral ventricles and a 2% decrease in gray matter volume [4]. Brain volume reduction involves the frontal lobe in particular, including the frontal cortex, which exhibits a reduced density of pyramidal neuron spines that are components of the postsynaptic site of most excitatory synapses [4,5,6,7,8,9]. Moreover, patients with schizophrenia show decreased prefrontal cortex (PFC) blood flow during the performance of cognitive tasks [10].
One of the major therapeutic targets of schizophrenia is the dopamine D2 receptor, and its antagonists, such as haloperidol, reduce positive symptoms but have a minimal effect on negative symptoms or cognitive deficits [1,11]. These drugs also have major side effects, including sedation, hyperprolactinemia, and the extrapyramidal symptoms of parkinsonism [1]. Second-generation antipsychotics such as risperidone and olanzapine have lower rates of such severe side effects, but their clinical efficacy and tolerability are not significantly improved [12]. Furthermore, 20–30% of patients show resistance to antipsychotic treatment [13]. Clozapine is the sole drug indicated for treatment-resistant schizophrenia and improves symptoms in only about 30–60% of patients [14,15,16,17]. Therefore, there is an urgent need for the development of effective schizophrenia treatments that are both effective and safe. To achieve this goal, it is necessary to understand the pathoetiology of the disease and to establish novel pathophysiologic animal models to expand upon existing classical pharmacologic animal models.
The etiology of schizophrenia involves both genetic vulnerabilities and environmental risk factors such as pregnancy and birth complications, childhood trauma, substance abuse, and psychosocial stress in adolescence [1,18]. In genome-wide association studies (GWASs) of schizophrenia, more than 200 genetic loci associated with neuronal function, including synaptic organization, differentiation, and transmission, have been shown to be associated with schizophrenia [19]. In addition to common variants, a small number of rare copy number variants (CNVs) and gene-disrupting variants, including the so-called rare-coding variants and protein-truncating variants, have been identified in schizophrenia with large effect sizes (odds ratios (ORs) of 2–60 fold and 3–50 fold, respectively) [1]. Several CNVs, such as 1q21.1, 2p16.3 (NRXN1), 3q29, 15q11.2, 15q13.3, and 22q11.2 have been consistently reported to be associated with schizophrenia [20,21]. Furthermore, a gene set analysis replicated previous findings (e.g., those implicating synapses and calcium signaling) and identified novel biological pathways such as those involved in the oxidative stress response, genomic integrity, and kinase and small GTPase signaling [22].
The Rho GTPase family plays a role in spine morphology by regulating actin dynamics [23]. It is associated with psychiatric diseases such as schizophrenia and depression, and also with neurodevelopmental disorders including autism spectrum disorders and intellectual disabilities [24,25,26,27,28,29,30,31]. Variants in genes upstream of the Rho GTPase family, such as KALRN and p250GAP, have been reported to be associated with schizophrenia [25,27]. However, few reviews have summarized schizophrenia-associated variants of genes regulating the Rho GTPase family. Here, we focus on RhoA, one of the Rho GTPases, and summarize its genetic association with schizophrenia and the effect of RhoA signaling modification in animal models of schizophrenia.
2. Rho Family Activity Is Regulated by GTPase-Activating Proteins (GAPs) and Guanine Nucleotide Exchange Factors (GEFs)
RhoA belongs to the RAS homolog (Rho) family, along with cell division control protein 42 (Cdc42) and RAS-related C3 botulinum toxin substrate 1 (Rac1) [32]. Rho family proteins contain a conserved GDP/GTP binding domain and switch their activity by cycling between GDP-bound (inactive) and GTP-bound (active) states [32,33]. This cycling is regulated by GAPs, GEFs, and guanine nucleotide dissociation inhibitors (GDIs) [32,34]. GAPs consist of more than 70 members, and conversion from a GTP-bound form to a GDP-bound form suppresses their activity [32,35]. In contrast, GEFs (>74 members) accelerate the exchange of tightly bound GDP for GTP, resulting in the activation of Rho family proteins [32,35]. GAPs and GEFs exhibit high selectivity for RhoA, Cdc42, and Rac1 [35,36]. GDIs, of which there are only three members in the human genome, form soluble complexes with GDP-bound Rho protein and control its cycling between the cytosol and membrane [32,34].
3. Rho Family Protein Effectors and Their Physiological Roles in the Brain
Rho family proteins are associated with over 70 potential effector proteins [37]. Rho-kinase, a serine/threonine kinase, is a representative downstream effector of RhoA [38]. In vascular smooth muscle, for example, Rho-kinase phosphorylates myosin phosphatase-targeting subunit 1 (MYPT1) at Thr696 and Thr853. This converts MYPT1 to an inactivated state, increases the phosphorylation of myosin light chain, and promotes actomyosin contractility [39,40,41]. P21-activated kinase (PAK) acts as a downstream effector for Cdc42 and Rac1 and affects actin dynamics by regulating the LIM kinase–cofilin pathway [42,43]. PAK also inhibits myosin light chain kinase, resulting in decreased myosin light chain phosphorylation and, thus, decreased actomyosin contractility [44].
Rho GTPases regulate cell morphology. For instance, RhoA promotes stress fiber formation and focal adhesions in cells [45]. Rho GTPases also modulate neuronal development. For instance, RhoA inhibits growth of dendrites and axons, while Rac1 and Cdc42 promote axonal elongation [42,46]. In addition, RhoA/Rho-kinase signaling promotes spine shrinkage and destabilization, while Rac1 and Cdc42/PAK signaling promotes spine stabilization and maintenance [23,47]. Accordingly, Rho GTPase signaling is involved in neuronal maturation through the regulation of actin dynamics.
4. Schizophrenia-Associated Genes Involved in Small GTPase RhoA Signaling
Recently, several variants of RhoA-associated GAPs, including ARHGAP10 [48], ARHGAP18 [49,50,51], and p250GAP [52], and GEFs such as KALRN [53,54,55,56] and ARHGEF11 [57], were reported to be significantly associated with the development of schizophrenia. In addition, variants in genes that activate RhoA via GEF, such as RTN4R [58,59,60], or in those that degrade RhoA, such as KCTD13 [61], were also identified in schizophrenia (Table 1, Figure 1).

Table 1.
Genetic variants of RhoA GAPs/GEFs in schizophrenia.
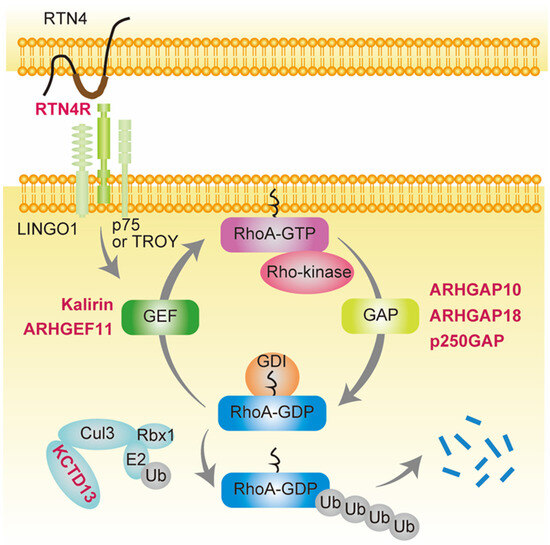
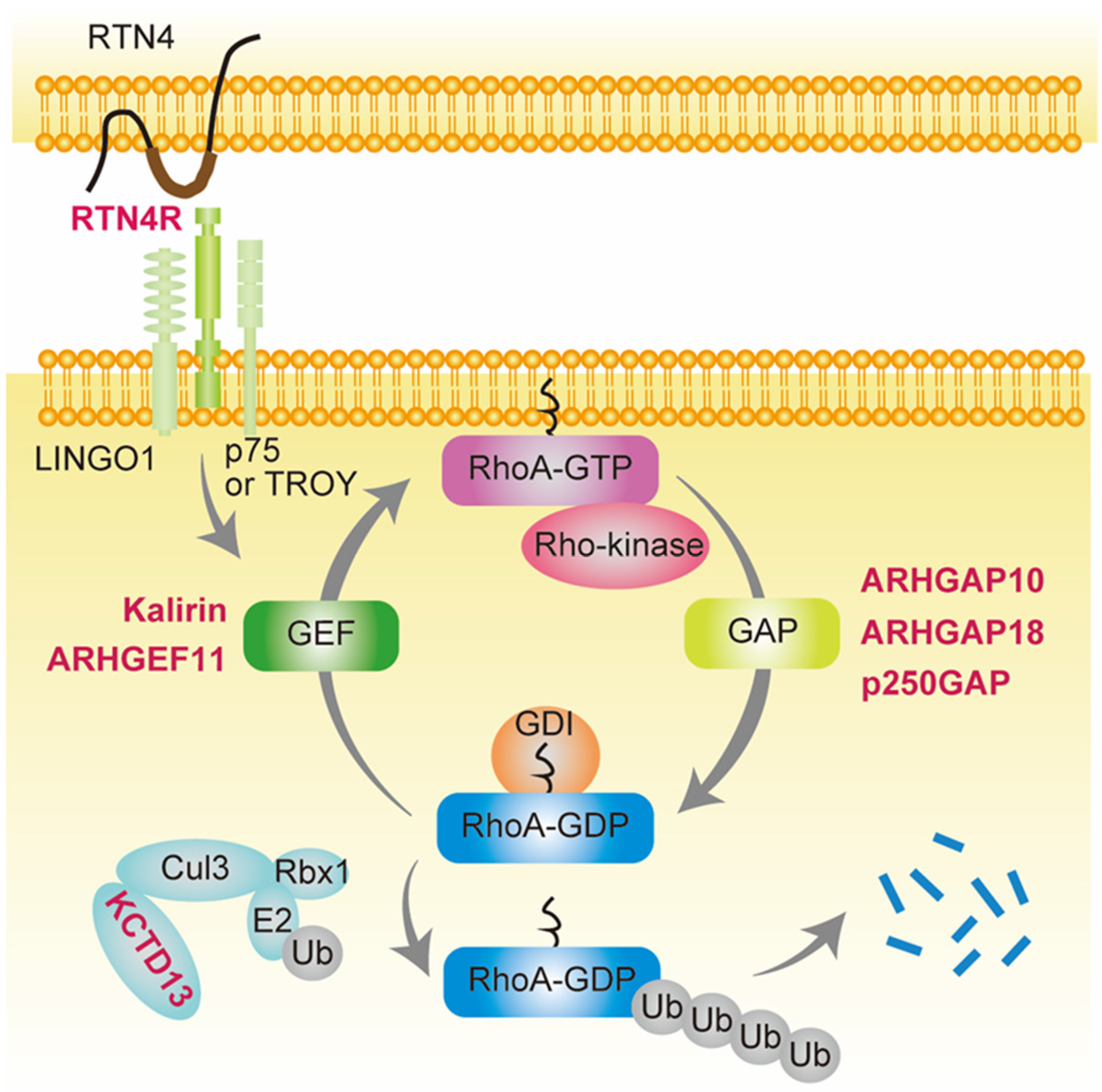
Figure 1.
Schizophrenia-associated genes involved in small GTPase RhoA signaling. Genes shown in red are schizophrenia-associated genes involved in small GTPase RhoA signaling. RhoA contains a conserved GDP/GTP binding domain, and its activity cycles between GDP-bound (inactive) and GTP-bound (active) states [32,33]. ARHGAP10, ARHGAP18, and p250GAP are GTPase-activating proteins (GAPs) that convert RhoA from the GTP- to GDP-bound form, thereby suppressing its activity. In contrast, Kalirin and ARHGEF11 are guanine nucleotide exchange factors (GEFs) that accelerate the exchange of tightly bound GDP for GTP, resulting in RhoA activation. GDIs form soluble complexes with GDP-bound RhoA and control its cycling between the cytosol and membrane [32,34,35]. Reticulon 4 receptor (RTN4R) (also called Nogo-66 receptor, NgR1), a RTN4 receptor subunit, is activated by RTN4 and binds leucine-rich repeat and immunoglobulin domain-containing protein (Lingo-1) and either the p75 neurotrophin receptor or tumor necrosis factor (TNF) receptor orphan Y (TROY), resulting in RhoA activation by GEF [62,63]. Potassium channel tetramerization domain-containing 13 (KCTD13) is the only identified signaling protein capable of inducing the microcephaly phenotype associated with 16p11.2 duplication, which is associated with schizophrenia [61,64]. KCTD13 is functionally related to cullin 3 (Cul3), a core component of E3 ubiquitin-protein ligase complexes that mediates the ubiquitination and subsequent proteasomal degradation of target proteins such as RhoA [65,66,67].
4.1. GAPs
4.1.1. ARHGAP10
The ARHGAP10 gene, which encodes Rho GTPase-activating protein 10 (ARHGAP10), is located on chromosome 4q31.23 and exhibits GAP activity for RhoA and Cdc42 [32,68]. ARHGAP10 is expressed in the brain [68,69], and its mRNA levels rise in the cerebellum, striatum, and frontal cortex from E4 to P56 in mice [69].
CNVs in ARHGAP10 were identified in seven patients with schizophrenia (six with deletions and one with duplication) but not in controls, and there was a significant association of ARHGAP10 CNVs with schizophrenia in Japanese patients (OR = 12.3, p = 0.015) [48]. Most ARHGAP10 CNVs were exonic deletions at the Bin1/amphiphysin/Rvs167 domain, the RhoGAP domain, or both. The relative expression levels of ARHGAP10 mRNA in lymphoblastoid cell lines established from the peripheral blood of patients with exonic ARHGAP10 CNVs were significantly decreased compared to those in patients with schizophrenia without ARHGAP10 CNVs and in a control group [48]. One of the patients (case #5) with ARHGAP10 CNVs had a missense variant (p.S490P) in exon 17 that overlapped with the exonic deletion on the other allele [48]. ARHGAP10 p.S490P showed weaker binding to active RhoA compared to wild-type ARHGAP10, suggesting that this single-nucleotide variation (SNV) exhibits the loss of function of ARHGAP10. Of note, clinical data of these seven patients with ARHGAP10 variants showed that treatment response was poor in most individuals, including case #5 [48].
4.1.2. ARHGAP18
ARHGAP18 is ubiquitously expressed throughout the body, including the brain, and shows GAP activity for RhoA but not for Rac1 or Cdc42 [70,71,72]. Through RhoA/Rho-kinase signaling, ARHGAP18 regulates cell spreading and migration and also the formation of stress fibers and focal adhesions [70]. ARHGAP18 knockdown, which leads to RhoA activation, causes significantly increased formation of stress fibers and focal adhesions in HeLa cells, while these changes are abolished by a Rho-kinase inhibitor or by dominant-negative RhoA transfection [70]. In contrast, the overexpression of wild-type ARHGAP18, but not GAP-defective ARHGAP18, suppresses the formation of stress fibers and focal adhesions in HeLa cells [70]. ARHGAP18 also contributes to cell migration [70]. The knockdown or knockout of ARHGAP18 impairs migration and cellular polarity in breast cancer cells (MDA-MB-231 or SUN-159 cells) [70,72].
Single-nucleotide polymorphisms (SNPs) in ARHGAP18 are associated with schizophrenia [49,50]. The genotypes and allelic frequencies of two SNPs, rs7758025 and rs9483050, were significantly different between patients and controls in a Chinese-Han population (genotype: rs7758025, p = 0.0002, and rs9483050, p = 7.54 × 10−6; allelic frequencies: rs7758025, p = 4.36 × 10−5, and rs9483050, p = 5.98 × 10−7). In addition, the AG haplotype in rs7758025-rs9385502 and the CG haplotype in rs11753915-9483050 were associated with schizophrenia (AG haplotype in rs7758025-rs9385502: p = 0.0012, 95% confidence interval [CI] = 0.48–0.93; CG haplotype in rs11753915-9483050: p = 9.6 × 10−6, 95% CI = 0.44–0.78) [49]. Another group reported an association between SNPs in ARHGAP18 and schizophrenia in Caucasian people [50,51]. They also performed a combined analysis with GWAS and functional magnetic resonance imaging scanning and demonstrated that these SNPs were significantly correlated with neuronal activity in the dorsolateral prefrontal cortex during a working memory task [50].
4.1.3. p250GAP (ARHGAP32)
P250GAP (ARHGAP32) is expressed in the brain [73] and exerts GAP activity for RhoA but not Cdc42 and Rac1 in mouse primary hippocampus neurons [74]. P250GAP regulates spine morphology in primary hippocampus neurons and neurogenesis in Neuro-2A cells through the regulation of RhoA activity [74,75]. In addition, p250GAP interacts with the NR2B subunit of N-methyl-D-aspartate (NMDA) receptors and is involved in NMDA receptor-mediated RhoA activation [74,75].
An SNP in p250GAP (rs2298599) was shown to be associated with schizophrenia in a Japanese cohort (p = 0.00015) [52]. The minor genotype frequency was higher in patients with schizophrenia (18%) than in healthy controls (9%) (p = 0.000083) [52]. rs2298599 is located 2.9 kb downstream of p250GAP and showed no significant association with p250GAP expression levels in immortalized lymphoblasts in in silico analysis (p = 0.28) [52]. Thus, further study is needed to clarify the mechanism of the association between p250GAP and schizophrenia.
4.2. GEFs
4.2.1. KALRN
Kalirin has two GEF domains, with activity targeting Rac1 (GEF1) and RhoA (GEF2), respectively [27,76]. The alternative splicing of KALRN gives rise to several isoforms, including Kalirin-4, Kalirin-5, Kalirin-7, Kalirin-8, Kalirin-9 (Kal9), and Kalirin-12 (Kal12) [27,76]. Kal9 and Kal12 contain both the GEF1 and GEF2 domains, while other isoforms contain only the GEF1 domain [27,76]. Kalirin is involved in neurite and dendritic outgrowth and in dendritic arborization in the brain [76]. Expression levels of Kal9 and Kal12 in the brain were found to be higher during early postnatal development than in adulthood [77]. Knockout of Kal9 and Kal12 by shRNA decreased the complexity of rat hippocampal neurons on days in vitro (DIV) 4 and 7 (immature neurons) [78]. In rat cortical neurons, Kal9 overexpression on DIV 2 (immature neurons) resulted in neurite elongation [77], while that on DIV 28 (mature neurons) reduced dendritic length and complexity [79]. These reports indicate that the role of Kal9 in neurons might change depending on the developmental stage.
A transcriptome-wide association study in patients with schizophrenia revealed an increase in exon skipping immediately prior to the GEF2 domain in KALRN transcripts [53]. In addition, some missense variants in KALRN, such as P2255T and T1207M, showed a higher frequency in schizophrenia cases compared to control cases [54,56]. In particular, P2255T in KALRN was significantly associated with schizophrenia (OR = 2.09, p = 0.048) in a Japanese population [54]. Of note, a P2255 residue exists near the RhoA-GEF2 domain [55]. The P2255T variant in Kal9 (Kal9-P2255T) leads to highly stable Kal9 mRNA, resulting in increased protein levels of Kal9 [55], which, for instance, were detected in the auditory cortex of patients with schizophrenia by post-mortem analysis [79]. Furthermore, overexpression of Kal9-P2255T in rat primary neurons and HEK 293 cells increased RhoA activity but had no effect on Rac1 activity [55,80]. These data indicate that Kal9-P2255T increases the expression levels of Kal9, leading to the activation of RhoA. From the viewpoint of neuronal morphology, Kal9-P2255T overexpression in cortical primary neurons led to a significant reduction in proximal dendritic complexity and dendritic spine size compared to wild-type Kal9 (Kal9-WT) [55]. On the other hand, it is known that reticulon 4 receptor (RTN4R) activates Kal9 and subsequently leads to the activation of RhoA [63,81]. The RTN4R/Kal9/RhoA pathway is known to modulate neurite outgrowth [81]. Myelin-associated inhibitors such as oligodendrocyte-myelin glycoprotein (OMGp) have been identified as additional RTN4R (NGR1) ligands and these also suppress neurite outgrowth [80,82]. Pharmacologic inhibition of RhoA with the RhoA inhibitor CT04 prevented the OMGp-induced decrease in neuronal complexity [80]. The overexpression of Kal9-P2255T in cortical primary neurons made them more sensitive to OMGp and decreased both the length and complexity of dendritic arbors [80]. These results suggest that Kal9-P2255T-induced RhoA activation causes morphological changes in neurons.
4.2.2. ARHGEF11
ARHGEF11, also referred to as KIAA0380 or GTRAP48, shows GEF activity for RhoA but not Rac1 or Cdc42 [83]. ARHGEF11 is expressed in the brain [84,85] and in cortical neurons, including dendrites and spines [86]. ARHGEF11 regulates glutamate transport activity by direct binding of excitatory amino acid transporter 4 [85]. The overexpression of ARHGEF11 was shown to decrease spine density in rat cortical neurons [86,87].
ARHGEF11 haplotypes such as C-C of rs6427340-rs6427339 and A-C-C of rs822585-rs6427340-rs6427339 were shown to be associated with schizophrenia (p = 0.0010 and 0.0018, respectively), but the ARHGEF11 SNPs were not [57]. The functions of ARHGEF11 haplotypes associated with schizophrenia have not been clarified. On the other hand, in situ hybridization analysis indicated that ARHGEF11 mRNA levels in the thalamus of patients with schizophrenia were higher than those in healthy controls [88]. These findings raise the possibility that ARHGEF11 activation is associated with schizophrenia pathology.
4.3. Others
4.3.1. RTN4R
RTN4R (also called Nogo-66 receptor, NgR1) is a RTN4 receptor subunit located at chr22q11.2, and it has been shown that deletion of chr22q11.2 is associated with a high risk of developing schizophrenia [89]. RTN4R binds leucine-rich repeat and immunoglobulin domain-containing protein (Lingo-1) and either the p75 neurotrophin receptor or tumor necrosis factor (TNF) receptor orphan Y (TROY) and activates RhoA through Kal9; this results in the collapse of growth cones, which prevents further axonal growth and inhibits myelination [62,63].
Several SNPs in RTN4R are associated with schizophrenia [58,59]. In samples from individuals of Afrikaner origin, significant associations with schizophrenia were seen for SNP rs696880 in women (OR = 0.73, p = 0.046) and for rs701427 (OR = 1.21, p = 0.019), rs696880 (OR = 1.18, p = 0.029), and rs854971 (OR = 1.20, p = 0.021) in men [59]. Diffusion tensor imaging revealed that the SNP rs701428 was associated with white matter abnormalities in 22q11.2 deletion syndrome [90]. Other groups identified several rare missense variants in patients with schizophrenia, specifically p.R68H (rs145773589), p.R119W (rs74315508), p.R196H (rs74315509), p.D259N (rs3747073), p.R292H (rs1432033565), and p.V363M (rs149231717), and p.R292H was significantly associated with schizophrenia (OR = 3.9, p = 0.048) [58,60]. RTN4R-R292H is located in the ligand binding site, and its overexpression in E5.5 chick retinal neurons significantly decreased growth cone collapse induced by treatment with RTN4, a ligand of RTN4R, compared to that resulting from treatment with RTN4R-WT [58]. In addition, a glutathione S-transferase binding assay showed that RTN4R-R292H exhibited reduced interaction with LINGO1 compared to RTN4R-WT [58]. Although these data suggest that RTN4R-R292H has impaired function, the effect of RTN4R-R292H on RhoA signaling remains obscure. In a post-mortem brain analysis, the expression levels of RTN4R were decreased in the dorsolateral prefrontal cortex but increased in the hippocampal CA3 region of patients with schizophrenia compared to healthy controls [91].
In addition to the above, genetic variations in components of the RTN4R signaling pathway, such as RTN4 (p = 0.047 and 0.037 for rs11894868 and rs2968804, respectively) and myelin-associated glycoprotein (p = 0.034 and 0.029 for rs7249617 and rs16970218, respectively), were shown to be associated with schizophrenia [92].
4.3.2. 16p11.2 CNVs and the KCTD13-Cul3-RhoA Pathway
16p11.2 microduplication was associated with schizophrenia in two large cohorts (OR = 25.8, p = 1.2 × 10−5; and OR = 8.3, p = 0.022) [61]. In a zebrafish model, potassium channel tetramerization domain-containing 13 (KCTD13) was identified as the sole signaling protein capable of inducing the microcephaly phenotype associated with 16p11.2 duplication [64]. A spatiotemporal protein–protein interaction network analysis showed that KCTD13 is functionally related to cullin 3 (Cul3) [67]. Cul3 is a core component of E3 ubiquitin–protein ligase complexes and mediates the ubiquitination and subsequent proteasomal degradation of target proteins such as RhoA, but not Rac1 and Cdc42 [65,66]. Although it is estimated that 16p11.2 microduplication is associated with decreased RhoA protein levels, organoids derived from patients with autism spectrum disorder who had 16p11.2 duplications showed RhoA activation and slightly increased KCTD13 expression [93]. Therefore, further research should analyze 16p11.2 microduplication in patients with schizophrenia.
5. Crosstalk between Ras and Rho Signaling in Schizophrenia
In cancer, p120RasGAP inhibits the RhoGAP activity of Deleted in liver cancer 1 (DLC1, i.e., STARD12, ARHGAP7), which is a tumor suppressor [94]. Crossveinless-c, the Drosophila homolog of DLC1, regulates the elongation of dendritic branches via RhoA activity [95]. On the other hand, integrin-mediated activation of Abl2/Arg and Src-family kinases increases p190RhoGAP phosphorylation to drive its association with p120RasGAP at the cell membrane, resulting in inhibition of RhoA activity and stabilizing the dendrite structure [96]. However, there are few reports about the crosstalk between Ras and Rho signaling, including p120RasGAP, DLC1, and p190RhoGAP gene variants, in schizophrenia. Only one group reported the association of p190RhoGAP with the pathway of acid phosphatase 1 (ACP1), which was associated with suicide attempts in Caucasians with primary diagnoses of schizophrenia and schizoaffective disorder [97]. Thus, further studies are required to discuss the crosstalk between Ras and Rho signaling in schizophrenia.
6. Genetic Mouse Models of Schizophrenia with Associated Genetic Variants Involved in Small GTPase RhoA Signaling
Mouse models have been developed based on schizophrenia-associated genetic variants involved in small GTPase RhoA signaling, including variants affecting the Arhgap10, Kalrn, and Rtn4r genes, and their phenotypic characterization has been performed [48,55,59,69,80,98] (Table 2).

Table 2.
Animal models with schizophrenia-associated gene variants in genes related to RhoA GAPs/GEFs.
6.1. Arhgap10 S490P/NHEJ Mice
Arhgap10 S490P/NHEJ mice carry double variants of the Arhgap10 gene that mimic the ARHGAP10 variations discovered in a Japanese patient with schizophrenia (case #5). One allele contains a missense variant (p.S490P), while the other contains a frameshift variant caused by non-homologous end joining (NHEJ) [48]. Compared to wild-type littermates, these mice exhibit significantly increased levels of both phosphorylated MYPT1 at Thr696 in the medial PFC (mPFC), striatum, and nucleus accumbens (NAc), and of phosphorylated p21-activated kinase (PAK) (PAK1 at Ser144 and PAK2 at Ser141) in the striatum and NAc. These results suggest that Rho family RhoA and Cdc42 signaling is abnormally activated in the mPFC, striatum, and NAc of Arhgap10 S490P/NHEJ mice [69].
A neuropathological analysis showed that spine density in Arhgap10 S490P/NHEJ mice was decreased in the mPFC but increased in the striatum [48,69]. Of note, repeated oral administration of fasudil, a Rho-kinase inhibitor, at a dose of 20 mg/kg for 7 days rescued the decreased spine density in the mPFC in Arhgap10 S490P/NHEJ mice but had no effect in wild-type mice [99]. These results suggest that abnormal activation of RhoA/Rho-kinase signaling in Arhgap10 S490P/NHEJ mice causes a reduction in spine density in the mPFC. Furthermore, the group that performed the aforementioned study established induced pluripotent stem cells (iPSCs) from patients with schizophrenia and differentiated them into tyrosine hydroxylase (TH)-positive neurons in order to analyze their morphological phenotypes. The TH-positive neurons differentiated from the iPSCs of the patient identified as case #5 exhibited severe defects in both neurite length and branch number, which were restored by the addition of the Rho-kinase inhibitor Y-27632 [48]. These findings suggest that Rho-kinase plays significant roles in the neuropathological changes in spine morphology caused by ARHGAP10 variants (Table 3).

Table 3.
Effects of Rho-kinase inhibitors in genetic and pharmacologic models of schizophrenia.
Comprehensive behavioral analyses revealed increased anxiety and vulnerability to methamphetamine-induced impairment in locomotion and cognitive function in Arhgap10 S490P/NHEJ mice [48,69]. This phenotype is consistent with evidence that psychostimulants, including amphetamine and methamphetamine, cause psychotic symptoms and cognitive dysfunction in patients with schizophrenia at doses that show little effect in healthy controls [104,105,106]. Notably, acute treatment with fasudil rescued the methamphetamine (0.3 mg/kg, i.p.)-induced cognitive impairment in the visual discrimination tasks in Arhgap10 S490P/NHEJ mice [99]. Fasudil also suppressed c-Fos expression in the mPFC that was induced by low-dose methamphetamine in Arhgap10 S490P/NHEJ mice [99] (Table 3).
Collectively, these results suggest that schizophrenia-associated Arhgap10 gene variants result in morphological abnormalities of neurons in the mPFC, and these abnormalities are associated with vulnerability to cognitive deficits induced by methamphetamine treatment. Arhgap10 S490P/NHEJ mice are a unique genetic mouse model of schizophrenia with constructive, phenotypic, and predictive validity.
6.2. Kalrn P2255T Mice
Kalrn P2255T mice harbor the missense variant P2255T at the endogenous locus in Kalrn. In these mice, impaired prepulse inhibition (PPI) is caused by various intervals between prepulse and startle-eliciting noise (Gap-PPI), but not by various noises at a lower sound pressure level than the startle-eliciting noise (Noise-PPI) [80]. Noise-PPI depends on subcortical auditory processing [107], while Gap-PPI has been shown to require the primary auditory cortex [108]. These findings suggest that P2255T in Kalrn affects primary auditory cortex functioning but not subcortical auditory processing. Indeed, Kalrn P2255T mice showed reductions in both dendritic length and the complexity of layer 3 pyramidal neurons in primary auditory neurons at 12 weeks old [80]. These results suggest that Kalrn P2255T mice constitute a genetic model that reflects schizophrenia pathology. Further research should investigate RhoA activity in the brains of these mice and the effect of RhoA/Rho-kinase inhibition on their phenotypes in order to clarify the pathomechanism underlying the Kalrn P2255T variant.
6.3. Rtn4r Knockout Mice
One research group generated Rtn4r knockout mice in which exon 2 in the Rtn4r gene was deleted, and therefore RTN4R expression was selectively abolished [109]. These mice showed delayed learning of a spatial memory task in a water maze test [98]. Another group reported a decrease in both distance traveled and rearing activity in an open-field test in different Rtn4r knockout mice [59]. Because schizophrenia is associated with deletion of 22q11.2 [89], where RTN4R is located, these reports suggest that RTN4R expression may contribute to the etiology of schizophrenia.
7. RhoA/Rho-Kinase Signaling in a Pharmacological Model of Schizophrenia
So far, we have reviewed genomic/genetic and reverse translational studies that suggest a role of RhoA signaling in the pathogenesis of schizophrenia. Furthermore, potential antipsychotic-like effects of RhoA/Rho-kinase inhibitors have been demonstrated in genetic mouse models harboring schizophrenia-associated variants of genes related to RhoA signaling [48,49,50,51,52,54,56,57,58,59,60,61,99]. In particular, rare ARHGAP10 variants are genetically and biologically associated with schizophrenia, and Rho-kinase may represent a promising drug target for schizophrenia treatment in patients with variants of ARHGAP10 and possibly other genes related to the RhoA/Rho-kinase pathway [99].
In terms of drug development, it is important to assess whether Rho-kinase inhibitors exhibit antipsychotic effects in patients with schizophrenia who carry no variants in ARHGAP10 or related genes. In the field of pain therapy, inhibitors of the voltage-gated sodium channel Nav1.7 are currently in phase II/III clinical trials (NCT02935608 and NCT02365636) for the treatment of chronic pain [110]. A genomic analysis identified a missense variant in SCN9A, which encodes the α-subunit of the voltage-gated sodium channel Nav1.7 in a Chinese family with a rare autosomal dominant form of erythromelalgia, which led to the discovery of this drug target [110]. Nav1.7 inhibitors were developed and their effectiveness has been evaluated in animal models of pain without SCN9A variants (i.e., wild-type) [111]. Based on these evidences, Nav1.7 blockers are being developed as novel treatments for chronic pain, regardless of the presence or absence of SCN9A variants.
From the viewpoint of drug development, we have reported the effects of Rho-kinase inhibitors in pharmacological models of schizophrenia that lack variants of RhoA-related genes (Table 3).
7.1. Dopamine Hypothesis-Based Model (Methamphetamine Treatment Model)
A disturbance of dopamine function is considered to be one of the primary factors underlying schizophrenia (i.e., the dopamine hypothesis) [1]. Methamphetamine and amphetamine are widely used to induce schizophrenia-like behavior in rodents [1,112,113], and there is evidence that both activate RhoA activity [100,114,115,116,117]. Acute methamphetamine treatment (1 or 2 mg/kg, i.p.) was shown to increase the phosphorylation levels of MYPT1 and myosin light chain 2, both of which are substrates of Rho-kinase, in the mPFC and dorsomedial striatum in wild-type mice [100]. In addition, in acute slices of mouse midbrain, as well as in the neuroblastoma cell line SK-N-SH, RhoA and Rac were activated 5 min after amphetamine treatment (10 μM) but their total expression levels were not altered [114]. On the other hand, chronic methamphetamine treatment at a neurotoxic dose increased RhoA and Rho-kinase expression levels in rat hippocampi (15 mg/kg, i.p., eight times at 12 h intervals) [115], rat brain microvascular endothelial cells (1.5 mM for 6 h or 10 nM for 24 h) [115,116], and PC12 cells (0.5–2.5 mM for 24 h) [117]. The mechanism by which methamphetamine and amphetamine activate RhoA remains unclear. It is known that both drugs increase cAMP levels and activate protein kinase A (PKA) [114,118]. PKA phosphorylates RhoA at Ser188, leading to inactivation of RhoA by enhancing its binding affinity with Rho GDP dissociation inhibitor [118,119,120]. Indeed, D1/D5 Gs-coupling receptor agonist SKF38393 suppressed amphetamine-induced RhoA activation in acute slices of mouse midbrain [114]. Therefore, it is unlikely that PKA activation by D1/D5 receptors is associated with amphetamine- or methamphetamine-induced RhoA activation.
There is some evidence that Rho-kinase inhibitors rescue methamphetamine-induced abnormal behaviors. Acute systemic treatment with fasudil (10–20 mg/kg, i.p. or 20 mg/kg, p.o.) rescued methamphetamine (1 mg/kg, i.p.)-induced cognitive impairment in visual discrimination tasks in wild-type mice [100]. NAc pretreatment with the Rho-kinase inhibitor Y-27632 suppressed methamphetamine-induced behaviors such as rearing and sniffing in rats [101]. One possible mechanism of Rho-kinase inhibitors is the inhibition of methamphetamine-induced dopamine elevation in the NAc. Pretreatment with Y-27632 in the NAc of rats suppressed the methamphetamine (1 mg/kg, subcutaneously)-induced elevation of extracellular dopamine levels, but had no effect on the methamphetamine-induced decrease in two major dopamine metabolites, 3,4-dihydroxyphenylacetic acid and homovanillic acid [101]. Another group reported that RhoA mediated the amphetamine-induced internalization of the dopamine transporter and suppressed dopamine uptake in acute slices of mouse midbrain and in SK-N-SH cells [114]. These results are consistent with the previous finding that pre-treatment with Y-27632 in the NAc of rats had no effect on the increase in extracellular dopamine levels induced by treatment with either tetrodotoxin, an inhibitor of voltage-dependent Na+ channels, or GBR-12909, a dopamine re-uptake inhibitor [101]. Alternatively, Rho-kinase inhibitors may suppress methamphetamine-induced neuronal activation. It was reported that fasudil (20 mg/kg, i.p.) suppressed methamphetamine (1 mg/kg, i.p.)-induced c-Fos expression in the mPFC and dorsomedial striatum [100]. Thus, this evidence suggests that RhoA/Rho-kinase signaling is abnormally activated under conditions in which dopaminergic neuronal activity is increased (i.e., schizophrenia). Accordingly, Rho-kinase is a potential novel target of drug discovery and development in schizophrenia.
7.2. Glutamate Hypothesis-Based Model (MK-801 Treatment Model)
Antagonists of NMDA receptors have also been shown to induce schizophrenia-like behaviors [1,121]. Previous studies reported that blocking NMDA receptor signaling by treatment with MK-801 or ketamine results in increased RhoA activity [122,123]. At 24–48 h after repeated MK-801 treatment (0.2 mg/kg/day, i.p.) for 14 days, adolescent rats showed impaired spatial memory and a lower proportion of mature spines, and also, in the hippocampus, increased mRNA levels of RhoA and decreased mRNA levels of Rac1 and Cdc42 [122]. MK-801 treatment of B35 neuronal cells and C6 glial cells (25 μM for 14 days) increased RhoA expression and myosin light chain 2 phosphorylation but decreased Cdc42 expression and PAK1 phosphorylation [123]. Another group reported that ketamine treatment (300 μM for 6 h) of rat hippocampal neurons on DIV 5 increased RhoA and Rho-kinase expression [103]. Treatment with Y-27632 rescued the ketamine-induced decrease in spine density in rat hippocampal neurons on DIV5 [103]. These data suggest that inhibition of NMDA receptor activity in neurons activates RhoA/Rho-kinase signaling, leading to decreased spine density.
Rho-kinase inhibition was also shown to rescue MK-801-induced abnormal behaviors in mice [102]. Fasudil rescued several MK-801-induced conditions, including hyperlocomotion (fasudil dose: 10–20 mg/kg, i.p.), social interaction impairment (10 mg/kg, i.p.), novel object recognition impairment (10–20 mg/kg, i.p.), and PPI deficits (20 mg/kg, i.p.) [102]. These data suggest that RhoA/Rho-kinase signaling contributes to schizophrenia-like behaviors in an MK-801 treatment model in animals.
8. Perspectives
As discussed in this review, RhoA/Rho-kinase is a potential therapeutic target in schizophrenia. In particular, Rho-kinase inhibitors exhibited anti-psychotic-like effects not only in Arhgap10 S490P/NHEJ mice but also in pharmacologic models of schizophrenia (methamphetamine- and MK-801-treated mice). It is therefore expected that regardless of the presence or absence of genetic variants in the small GTPase signaling pathway, these inhibitors will have antipsychotic effects, in addition to their ability to ameliorate cognitive deficits in schizophrenia.
In general, optimizing drug target selection is an important step in drug discovery and development. Rho-kinase has many downstream molecules and plays various roles in the body [124]. Thus, Rho-kinase inhibitors may have unwanted side effects in clinical use. In this regard, there are two isoforms of Rho-kinase, namely Rho-kinase 1 and Rho-kinase 2. Rho-kinase 1 is expressed mainly in the lungs, liver, testis, blood, and immune system, while Rho-kinase 2 is found primarily in the brain, heart, and smooth muscle cells [124,125,126]. In the brain, Rho-kinase 1 is expressed in glial cells, whereas Rho-kinase 2 is expressed in neurons [126]. Thus, selective inhibition of Rho-kinase 2 may prevent unwanted peripheral side effects such as reduced blood pressure. Fasudil and Y-27632 are both dual inhibitors of Rho-kinase 1 and Rho-kinase 2. Selective inhibitors of Rho-kinase 2, such as KD025, have been developed [127,128], and it will be valuable to examine whether they have antipsychotic-like effects resembling those of fasudil, but with fewer side effects. Another potential means of avoiding unwanted side effects is to discover molecules downstream of Rho-kinase that are selectively expressed in the brain, especially in neurons. Therefore, the detailed mechanism of RhoA/Rho-kinase signaling in schizophrenia should be further clarified to facilitate the development of safe and effective therapeutic drugs for this disorder.
Author Contributions
Conceptualization, R.T. and K.Y.; writing—original draft preparation, R.T.; writing—review and editing, K.Y.; supervision, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following funding sources: AMED Grant Numbers JP21wm0425007 from the Japan Agency for Medical Research and Development; KAKENHI Grant Numbers JP23H02669 and JP23K19425 from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
This study was funded in part by Sumitomo Pharma Co., Ltd.
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Gogtay, N.; Vyas, N.S.; Testa, R.; Wood, S.J.; Pantelis, C. Age of onset of schizophrenia: Perspectives from structural neuroimaging studies. Schizophr. Bull. 2011, 37, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Haijma, S.V.; Van Haren, N.; Cahn, W.; Koolschijn, P.C.M.P.; Pol, H.E.H.; Kahn, R.S. Brain volumes in schizophrenia: A meta-analysis in over 18,000 subjects. Schizophr. Bull. 2012, 39, 1129–1138. [Google Scholar] [CrossRef]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2022, 48, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Glausier, J.; Lewis, D. Dendritic spine pathology in schizophrenia. Neuroscience 2013, 251, 90–107. [Google Scholar] [CrossRef]
- Broadbelt, K.; Byne, W.; Jones, L.B. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr. Res. 2002, 58, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Konopaske, G.T.; Lange, N.; Coyle, J.T.; Benes, F.M. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 2014, 71, 1323–1331. [Google Scholar] [CrossRef]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef]
- Weinberger, D.R.; Berman, K.F.; Zec, R.F. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry 1986, 43, 114–124. [Google Scholar] [CrossRef]
- Haddad, P.M.; Correll, C.U. The acute efficacy of antipsychotics in schizophrenia: A review of recent meta-analyses. Ther. Adv. Psychopharmacol. 2018, 8, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Cipriani, A.; Spineli, L.; Marvidis, D.; Örey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Elkis, H. Treatment-resistant schizophrenia. Psychiatr. Clin. N. Am. 2007, 30, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef]
- Rosenheck, R.; Cramer, J.; Xu, W.; Thomas, J.; Henderson, W.; Frisman, L.; Fye, C.; Charney, D. A Comparison of clozapine and haloperidol in hospitalized patients with Refractory Schizophrenia. N. Engl. J. Med. 1997, 337, 809–815. [Google Scholar] [CrossRef]
- Siskind, D.; Siskind, V.; Kisely, S. Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis. Can. J. Psychiatry 2017, 62, 772–777. [Google Scholar] [CrossRef]
- Blackman, G.M.; Lisshammar, J.E.M.; Zafar, R.M.; Pollak, T.A.; Pritchard, M.M.; Cullen, A.E.; Rogers, J.M.B.; Carter, B.; Griffiths, K.M.; Nour, M.B.B.; et al. Clozapine Response in Schizophrenia and Hematological Changes. J. Clin. Psychopharmacol. 2020, 41, 19–24. [Google Scholar] [CrossRef]
- Stilo, S.A.; Murray, R.M. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Kushima, I.; Nakatochi, M.; Aleksic, B.; Okada, T.; Kimura, H.; Kato, H.; Morikawa, M.; Inada, T.; Ishizuka, K.; Torii, Y.; et al. Cross-Disorder Analysis of Genic and Regulatory Copy Number Variations in Bipolar Disorder, Schizophrenia, and Autism Spectrum Disorder. Biol. Psychiatry 2022, 92, 362–374. [Google Scholar] [CrossRef]
- Kushima, I.; Aleksic, B.; Nakatochi, M.; Shimamura, T.; Okada, T.; Uno, Y.; Morikawa, M.; Ishizuka, K.; Shiino, T.; Kimura, H.; et al. Comparative Analyses of Copy-Number Variation in Autism Spectrum Disorder and Schizophrenia Reveal Etiological Overlap and Biological Insights. Cell Rep. 2018, 24, 2838–2856. [Google Scholar] [CrossRef] [PubMed]
- Kushima, I.; Aleksic, B.; Nakatochi, M.; Shimamura, T.; Shiino, T.; Yoshimi, A.; Kimura, H.; Takasaki, Y.; Wang, C.; Xing, J.; et al. High-resolution copy number variation analysis of schizophrenia in Japan. Mol. Psychiatry 2016, 22, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, T.; Grabrucker, A.M. Rho GTPases in the Amygdala—A Switch for Fears? Cells 2020, 9, 1972. [Google Scholar] [CrossRef] [PubMed]
- Duman, J.G.; Blanco, F.A.; Cronkite, C.A.; Ru, Q.; Erikson, K.C.; Mulherkar, S.; Bin Saifullah, A.; Firozi, K.; Tolias, K.F. Rac-maninoff and Rho-vel: The symphony of Rho-GTPase signaling at excitatory synapses. Small GTPases 2021, 13, 14–47. [Google Scholar] [CrossRef]
- Huang, G.-H.; Sun, Z.-L.; Li, H.-J.; Feng, D.-F. Rho GTPase-activating proteins: Regulators of Rho GTPase activity in neuronal development and CNS diseases. Mol. Cell. Neurosci. 2017, 80, 18–31. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Barr, A.M.; Honer, W.G. Spines, synapses, and schizophrenia. Biol. Psychiatry 2015, 78, 741–743. [Google Scholar] [CrossRef]
- Mould, A.W.; Al-Juffali, N.; von Delft, A.; Brennan, P.E.; Tunbridge, E.M. Kalirin as a Novel Treatment Target for Cognitive Dysfunction in Schizophrenia. CNS Drugs 2021, 36, 1–16. [Google Scholar] [CrossRef]
- Hanifa, M.; Singh, M.; Randhawa, P.K.; Jaggi, A.S.; Bali, A. A focus on Rho/ROCK signaling pathway: An emerging therapeutic target in depression. Eur. J. Pharmacol. 2023, 946, 175648. [Google Scholar] [CrossRef]
- Van Bokhoven, H. Genetic and epigenetic networks in intellectual disabilities. Annu. Rev. Genet. 2011, 45, 81–104. [Google Scholar] [CrossRef]
- Liaci, C.; Camera, M.; Caslini, G.; Rando, S.; Contino, S.; Romano, V.; Merlo, G.R. Neuronal Cytoskeleton in Intellectual Disability: From Systems Biology and Modeling to Therapeutic Opportunities. Int. J. Mol. Sci. 2021, 22, 6167. [Google Scholar] [CrossRef]
- Guo, D.; Yang, X.; Shi, L. Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics. Cells 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Symons, M.; Settleman, J. Rho family GTPases: More than simple switches. Trends Cell Biol. 2000, 10, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dvorsky, R.; Ahmadian, M.R. Deciphering the molecular and functional basis of Dbl family proteins: A novel systematic approach toward classification of selective activation of the Rho family proteins. J. Biol. Chem. 2013, 288, 4486–4500. [Google Scholar] [CrossRef] [PubMed]
- Kreider-Letterman, G.; Carr, N.M.; Garcia-Mata, R. Fixing the GAP: The role of RhoGAPs in cancer. Eur. J. Cell Biol. 2022, 101, 151209. [Google Scholar] [CrossRef]
- Bustelo, X.R.; Sauzeau, V.; Berenjeno, I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. BioEssays 2007, 29, 356–370. [Google Scholar] [CrossRef]
- Amin, E.; Dubey, B.N.; Zhang, S.-C.; Gremer, L.; Dvorsky, R.; Moll, J.M.; Taha, M.S.; Nagel-Steger, L.; Piekorz, R.P.; Somlyo, A.V.; et al. Rho-kinase: Regulation, (dys)function, and inhibition. Biol. Chem. 2013, 394, 1399–1410. [Google Scholar] [CrossRef]
- Amano, M.; Kanazawa, Y.; Kozawa, K.; Kaibuchi, K. Identification of the Kinase-Substrate Recognition Interface between MYPT1 and Rho-Kinase. Biomolecules 2022, 12, 159. [Google Scholar] [CrossRef]
- Grassie, M.E.; Moffat, L.D.; Walsh, M.P.; MacDonald, J.A. The myosin phosphatase targeting protein (MYPT) family: A regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch. Biochem. Biophys. 2011, 510, 147–159. [Google Scholar] [CrossRef]
- Seccia, T.M.; Rigato, M.; Ravarotto, V.; Calò, L.A. ROCK (RhoA/Rho Kinase) in Cardiovascular–Renal Pathophysiology: A Review of New Advancements. J. Clin. Med. 2020, 9, 1328. [Google Scholar] [CrossRef]
- Luo, L. RHO GTPASES in neuronal morphogenesis. Nat. Rev. Neurosci. 2000, 1, 173–180. [Google Scholar] [CrossRef]
- Civiero, L.; Greggio, E. PAKs in the brain: Function and dysfunction. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 444–453. [Google Scholar] [CrossRef]
- Dickson, B.J. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 2001, 11, 103–110. [Google Scholar] [CrossRef]
- Burridge, K.; Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 2015, 343, 14–20. [Google Scholar] [CrossRef]
- Benarroch, E.E. Rho GTPases: Role in dendrite and axonal growth, mental retardation, and axonal regeneration. Neurology 2007, 68, 1315–1318. [Google Scholar] [CrossRef]
- Newey, S.E.; Velamoor, V.; Govek, E.-E.; Van Aelst, L. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 2005, 64, 58–74. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Sobue, A.; Kushima, I.; Wang, C.; Arioka, Y.; Kato, H.; Kodama, A.; Kubo, H.; Ito, N.; Sawahata, M.; et al. ARHGAP10, which encodes Rho GTPase-activating protein 10, is a novel gene for schizophrenia risk. Transl. Psychiatry 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cai, Y.; Zhang, H.; Yang, Y.; Yang, G.; Wang, X.; Zhao, J.; Lin, J.; Zhu, J.; Li, W.; et al. Association of ARHGAP18 polymorphisms with schizophrenia in the Chinese-Han population. PLoS ONE 2017, 12, e0175209. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Turner, J.A.; Fallon, J.A.; Lakatos, A.; Keator, D.B.; Guffanti, G.; Macciardi, F. Gene discovery through imaging genetics: Identification of two novel genes associated with schizophrenia. Mol. Psychiatry 2008, 14, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Macciardi, F.; Guffanti, G.; Fallon, J.H.; Wang, Q.; Turner, J.A.; Lakatos, A.; Miles, M.F.; Lander, A.; Vawter, M.P.; et al. Identifying gene regulatory networks in schizophrenia. NeuroImage 2010, 53, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Ohi, K.; Hashimoto, R.; Nakazawa, T.; Okada, T.; Yasuda, Y.; Yamamori, H.; Fukumoto, M.; Umeda-Yano, S.; Iwase, M.; Kazui, H.; et al. The p250GAP gene is associated with risk for schizophrenia and schizotypal personality traits. PLoS ONE 2012, 7, e35696. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; Van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef] [PubMed]
- Kushima, I.; Nakamura, Y.; Aleksic, B.; Ikeda, M.; Ito, Y.; Shiino, T.; Okochi, T.; Fukuo, Y.; Ujike, H.; Suzuki, M.; et al. Resequencing and association analysis of the KALRN and EPHB1 genes and their contribution to schizophrenia susceptibility. Schizophr. Bull. 2010, 38, 552–560. [Google Scholar] [CrossRef]
- Russell, T.A.; Grubisha, M.J.; Remmers, C.L.; Kang, S.K.; Forrest, M.P.; Smith, K.R.; Kopeikina, K.J.; Gao, R.; Sweet, R.A.; Penzes, P. A Schizophrenia-Linked KALRN Coding Variant Alters Neuron Morphology, Protein Function, and Transcript Stability. Biol. Psychiatry 2018, 83, 499–508. [Google Scholar] [CrossRef]
- Gulsuner, S.; Stein, D.J.; Susser, E.S.; Sibeko, G.; Pretorius, A.; Walsh, T.; Majara, L.; Mndini, M.M.; Mqulwana, S.G.; Ntola, O.A.; et al. Genetics of schizophrenia in the South African Xhosa. Science 2020, 367, 569–573. [Google Scholar] [CrossRef]
- Mizuki, Y.; Takaki, M.; Okahisa, Y.; Sakamoto, S.; Kodama, M.; Ujike, H.; Uchitomi, Y. Human Rho guanine nucleotide exchange factor 11 gene is associated with schizophrenia in a Japanese population. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 552–558. [Google Scholar] [CrossRef]
- Kimura, H.; Fujita, Y.; Kawabata, T.; Ishizuka, K.; Wang, C.; Iwayama, Y.; Okahisa, Y.; Kushima, I.; Morikawa, M.; Uno, Y.; et al. A novel rare variant R292H in RTN4R affects growth cone formation and possibly contributes to schizophrenia susceptibility. Transl. Psychiatry 2017, 7, e1214. [Google Scholar] [CrossRef]
- Hsu, R.; Woodroffe, A.; Lai, W.-S.; Cook, M.N.; Mukai, J.; Dunning, J.P.; Swanson, D.J.; Roos, J.L.; Abecasis, G.R.; Karayiorgou, M.; et al. Nogo Receptor 1 (RTN4R) as a candidate gene for schizophrenia: Analysis using human and mouse genetic approaches. PLoS ONE 2007, 2, e1234. [Google Scholar] [CrossRef]
- Sinibaldi, L.; De Luca, A.; Bellacchio, E.; Conti, E.; Pasini, A.; Paloscia, C.; Spalletta, G.; Caltagirone, C.; Pizzuti, A.; Dallapiccola, B. Mutations of the Nogo-66 receptor (RTN4R) gene in schizophrenia. Hum. Mutat. 2004, 24, 534–535. [Google Scholar] [CrossRef]
- McCarthy, S.E.; Makarov, V.; Addington, A.M.; McClellan, J.; Yoon, S.; Perkins, D.O.; Dickel, D.E.; Kusenda, M.; Krastoshevsky, O.; Krause, V.; et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat. Genet. 2009, 41, 1223–1227. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.W.; Li, Q.M.; Tep, C.; Park, J.B.; He, Z.; Yoon, S.O. The role of Kalirin9 in p75/nogo receptor-mediated RhoA activation in cerebellar granule neurons. J. Biol. Chem. 2008, 283, 24690–24697. [Google Scholar] [CrossRef] [PubMed]
- Golzio, C.; Willer, J.; Talkowski, M.E.; Oh, E.C.; Taniguchi, Y.; Jacquemont, S.; Reymond, A.; Sun, M.; Sawa, A.; Gusella, J.F.; et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 2012, 485, 363–367. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Meng, M.; Zhao, Y.; Dong, N.; Yan, H.; Liu, L.; Ding, M.; Peng, H.B.; Shao, F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol. Cell 2009, 35, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Genschik, P.; Sumara, I.; Lechner, E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): Cellular functions and disease implications. EMBO J. 2013, 32, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.N.; Corominas, R.; Lemmens, I.; Yang, X.; Tavernier, J.; Hill, D.E.; Vidal, M.; Sebat, J.; Iakoucheva, L.M. Spatiotemporal 16p11.2 protein network implicates cortical late mid-fetal brain development and KCTD13-Cul3-RhoA pathway in psychiatric diseases. Neuron 2015, 85, 742–754. [Google Scholar] [CrossRef]
- Shibata, H.; Oishi, K.; Yamagiwa, A.; Matsumoto, M.; Mukai, H.; Ono, Y. PKNbeta interacts with the SH3 domains of Graf and a novel Graf related protein, Graf2, which are GTPase activating proteins for Rho family. J. Biochem. 2001, 130, 23–31. [Google Scholar] [CrossRef]
- Hada, K.; Wulaer, B.; Nagai, T.; Itoh, N.; Sawahata, M.; Sobue, A.; Mizoguchi, H.; Mori, D.; Kushima, I.; Nabeshima, T.; et al. Mice carrying a schizophrenia-associated mutation of the Arhgap10 gene are vulnerable to the effects of methamphetamine treatment on cognitive function: Association with morphological abnormalities in striatal neurons. Mol. Brain 2021, 14, 21. [Google Scholar] [CrossRef]
- Maeda, M.; Hasegawa, H.; Hyodo, T.; Ito, S.; Asano, E.; Yuang, H.; Funasaka, K.; Shimokata, K.; Hasegawa, Y.; Hamaguchi, M.; et al. ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol. Biol. Cell 2011, 22, 3840–3852. [Google Scholar] [CrossRef]
- Thompson, W.R.; Yen, S.S.; Uzer, G.; Xie, Z.; Sen, B.; Styner, M.; Burridge, K.; Rubin, J. LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone 2017, 107, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Wang, Z.; Li, Y.; Jhan, J.-R.; Jiang, Y.; Yang, C. ARHGAP18 Downregulation by miR-200b Suppresses Metastasis of Triple-Negative Breast Cancer by Enhancing Activation of RhoA. Cancer Res. 2017, 77, 4051–4064. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Watabe, A.M.; Tezuka, T.; Yoshida, Y.; Yokoyama, K.; Umemori, H.; Inoue, A.; Okabe, S.; Manabe, T.; Yamamoto, T. p250GAP, a novel brain-enriched GTPase-activating protein for Rho family GTPases, is involved in the N-Methyl-d-aspartate receptor signaling. Mol. Biol. Cell 2003, 14, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Kuriu, T.; Tezuka, T.; Umemori, H.; Okabe, S.; Yamamoto, T. Regulation of dendritic spine morphology by an NMDA receptor-associated Rho GTPase-activating protein, p250GAP. J. Neurochem. 2008, 105, 1384–1393. [Google Scholar] [CrossRef]
- Kannan, M.; Lee, S.-J.; Schwedhelm-Domeyer, N.; Nakazawa, T.; Stegmüller, J. p250GAP is a novel player in the Cdh1-APC/Smurf1 pathway of axon growth regulation. PLoS ONE 2012, 7, e50735. [Google Scholar] [CrossRef]
- Paskus, J.D.; Herring, B.E.; Roche, K.W. Kalirin and Trio: RhoGEFs in Synaptic Transmission, Plasticity, and Complex Brain Disorders. Trends Neurosci. 2020, 43, 505–518. [Google Scholar] [CrossRef]
- Penzes, P.; Johnson, R.C.; Kambampati, V.; Mains, R.E.; Eipper, B.A. Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology. J. Neurosci. 2001, 21, 8426–8434. [Google Scholar] [CrossRef]
- Yan, Y.; Eipper, B.A.; Mains, R.E. Kalirin-9 and Kalirin-12 Play Essential Roles in Dendritic Outgrowth and Branching. Cereb. Cortex 2014, 25, 3487–3501. [Google Scholar] [CrossRef]
- Deo, A.J.; Cahill, M.E.; Li, S.; Goldszer, I.; Henteleff, R.; VanLeeuwen, J.-E.; Rafalovich, I.; Gao, R.; Stachowski, E.K.; Sampson, A.R.; et al. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: Its role in dendritic pathology. Neurobiol. Dis. 2012, 45, 796–803. [Google Scholar] [CrossRef][Green Version]
- Grubisha, M.J.; Sun, T.; Eisenman, L.; Erickson, S.L.; Chou, S.-Y.; Helmer, C.D.; Trudgen, M.T.; Ding, Y.; Homanics, G.E.; Penzes, P.; et al. A Kalirin missense mutation enhances dendritic RhoA signaling and leads to regression of cortical dendritic arbors across development. Proc. Natl. Acad. Sci. USA 2021, 118, e2022546118. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.-A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Involvement of the Myelin-Associated Inhibitors and Their Receptors in CNS Plasticity and Injury. Mol. Neurobiol. 2017, 55, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Rümenapp, U.; Blomquist, A.; Schwörer, G.; Schablowski, H.; Psoma, A.; Jakobs, K.H. Rho-specific binding and guanine nucleotide exchange catalysis by KIAA0380, a dbl family member. FEBS Lett. 1999, 459, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Murga, C.; Zohar, M.; Igishi, T.; Gutkind, J.S. A Novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 1999, 274, 5868–5879. [Google Scholar] [CrossRef]
- Jackson, M.; Song, W.; Liu, M.-Y.; Jin, L.; Dykes-Hoberg, M.; Lin, C.-L.G.; Bowers, W.J.; Federoff, H.J.; Sternweis, P.C.; Rothstein, J.D. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 2001, 410, 89–93. [Google Scholar] [CrossRef]
- Mizuki, Y.; Takaki, M.; Sakamoto, S.; Okamoto, S.; Kishimoto, M.; Okahisa, Y.; Itoh, M.; Yamada, N. Human Rho Guanine Nucleotide Exchange Factor 11 (ARHGEF11) Regulates Dendritic Morphogenesis. Int. J. Mol. Sci. 2016, 18, 67. [Google Scholar] [CrossRef]
- Mizuki, Y.; Sakamoto, S.; Okahisa, Y.; Yada, Y.; Hashimoto, N.; Takaki, M.; Yamada, N. Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus. Int. J. Neuropsychopharmacol. 2020, 24, 367–382. [Google Scholar] [CrossRef]
- Davidkova, G.; Mccullumsmith, R.E.; Meador-Woodruff, J.H. Expression of ARHGEF11 mRNA in schizophrenic thalamus. Ann. N. Y. Acad. Sci. 2003, 1003, 375–377. [Google Scholar] [CrossRef]
- Karayiorgou, M.; Simon, T.J.; Gogos, J.A. 22q11.2 microdeletions: Linking DNA structural variation to brain dysfunction and schizophrenia. Nat. Rev. Neurosci. 2010, 11, 402–416. [Google Scholar] [CrossRef]
- Perlstein, M.D.; Chohan, M.R.; Coman, I.L.; Antshel, K.M.; Fremont, W.P.; Gnirke, M.H.; Kikinis, Z.; Middleton, F.A.; Radoeva, P.D.; Shenton, M.E.; et al. White matter abnormalities in 22q11.2 deletion syndrome: Preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr. Res. 2014, 152, 117–123. [Google Scholar] [CrossRef]
- Fernandez-Enright, F.; Andrews, J.L.; Newell, K.A.; Pantelis, C.; Huang, X.F. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl. Psychiatry 2014, 4, e348. [Google Scholar] [CrossRef]
- Jitoku, D.; Hattori, E.; Iwayama, Y.; Yamada, K.; Toyota, T.; Kikuchi, M.; Maekawa, M.; Nishikawa, T.; Yoshikawa, T. Association study of Nogo-related genes with schizophrenia in a Japanese case-control sample. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156, 581–592. [Google Scholar] [CrossRef]
- Urresti, J.; Zhang, P.; Moran-Losada, P.; Yu, N.-K.; Negraes, P.D.; Trujillo, C.A.; Antaki, D.; Amar, M.; Chau, K.; Pramod, A.B.; et al. Cortical organoids model early brain development disrupted by 16p11.2 copy number variants in autism. Mol. Psychiatry 2021, 26, 7560–7580. [Google Scholar] [CrossRef]
- Chau, J.E.; Vish, K.J.; Boggon, T.J.; Stiegler, A.L. SH3 domain regulation of RhoGAP activity: Crosstalk between p120RasGAP and DLC1 RhoGAP. Nat. Commun. 2022, 13, 4788. [Google Scholar] [CrossRef]
- Sato, D.; Sugimura, K.; Satoh, D.; Uemura, T. Crossveinless-c, the Drosophila homolog of tumor suppressor DLC1, regulates directional elongation of dendritic branches via down-regulating Rho1 activity. Genes Cells 2010, 15, 485–500. [Google Scholar] [CrossRef]
- Shapiro, L.P.; Parsons, R.G.; Koleske, A.J.; Gourley, S.L. Differential expression of cytoskeletal regulatory factors in the adolescent prefrontal cortex: Implications for cortical development. J. Neurosci. Res. 2016, 95, 1123–1143. [Google Scholar] [CrossRef]
- Li, J.; Yoshikawa, A.; Meltzer, H.Y. Replication of rs300774, a genetic biomarker near ACP1, associated with suicide attempts in patients with schizophrenia: Relation to brain cholesterol biosynthesis. J. Psychiatr. Res. 2017, 94, 54–61. [Google Scholar] [CrossRef]
- Van Gaalen, M.M.; Relo, A.L.; Mueller, B.K.; Gross, G.; Mezler, M. NOGO-66 receptor deficient mice show slow acquisition of spatial memory task performance. Neurosci. Lett. 2012, 510, 58–61. [Google Scholar] [CrossRef]
- Tanaka, R.; Liao, J.; Hada, K.; Mori, D.; Nagai, T.; Matsuzaki, T.; Nabeshima, T.; Kaibuchi, K.; Ozaki, N.; Mizoguchi, H.; et al. Inhibition of Rho-kinase ameliorates decreased spine density in the medial prefrontal cortex and methamphetamine-induced cognitive dysfunction in mice carrying schizophrenia-associated mutations of the Arhgap10 gene. Pharmacol. Res. 2023, 187, 106589. [Google Scholar] [CrossRef]
- Liao, J.; Dong, G.; Zhu, W.; Wulaer, B.; Mizoguchi, H.; Sawahata, M.; Liu, Y.; Kaibuchi, K.; Ozaki, N.; Nabeshima, T.; et al. Rho kinase inhibitors ameliorate cognitive impairment in a male mouse model of methamphetamine-induced schizophrenia. Pharmacol. Res. 2023, 194, 106838. [Google Scholar] [CrossRef]
- Narita, M.; Takagi, M.; Aoki, K.; Kuzumaki, N.; Suzuki, T. Implication of Rho-associated kinase in the elevation of extracellular dopamine levels and its related behaviors induced by methamphetamine in rats. J. Neurochem. 2003, 86, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Liao, J.; Liu, Y.; Tanaka, R.; Miyagawa, Y.; Sawahata, M.; Sobue, A.; Mizoguchi, H.; Nagai, T.; Kaibuchi, K.; et al. Antipsychotic-like effects of fasudil, a Rho-kinase inhibitor, in a pharmacologic animal model of schizophrenia. Eur. J. Pharmacol. 2022, 931, 175207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hao, Z.; Li, X.; Bo, L.; Zhang, R.; Wang, Y.; Duan, X.; Kang, R.; Huang, L. Ketamine destabilizes growth of dendritic spines in developing hippocampal neurons in vitro via a Rho-dependent mechanism. Mol. Med. Rep. 2018, 18, 5037–5043. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Kane, J.M.; Alvir, J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology 1987, 91, 415–433. [Google Scholar] [CrossRef]
- Curran, C.; Byrappa, N.; McBride, A. Stimulant psychosis: Systematic review. Br. J. Psychiatry 2004, 185, 196–204. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A.; van Dyck, C.H.; Gil, R.; D’Souza, C.D.; Erdos, J.; McCance, E.; Rosenblatt, W.; Fingado, C.; Zoghbi, S.S.; et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA 1996, 93, 9235–9240. [Google Scholar] [CrossRef]
- Koch, M. The neurobiology of startle. Prog. Neurobiol. 1999, 59, 107–128. [Google Scholar] [CrossRef]
- Bowen, G.P.; Lin, D.; Taylor, M.K.; Ison, J.R. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb. Cortex 2003, 13, 815–822. [Google Scholar] [CrossRef]
- Kim, J.-E.; Liu, B.P.; Park, J.H.; Strittmatter, S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004, 44, 439–451. [Google Scholar] [CrossRef]
- Dugger, S.A.; Platt, A.; Goldstein, D.B. Drug development in the era of precision medicine. Nat. Rev. Drug Discov. 2018, 17, 183–196. [Google Scholar] [CrossRef]
- Obeng, S.; Hiranita, T.; León, F.; McMahon, L.R.; McCurdy, C.R. Novel Approaches, Drug Candidates, and Targets in Pain Drug Discovery. J. Med. Chem. 2021, 64, 6523–6548. [Google Scholar] [CrossRef]
- Harro, J. Neuropsychiatric Adverse Effects of Amphetamine and Methamphetamine. Int. Rev. Neurobiol. 2015, 120, 179–204. [Google Scholar] [PubMed]
- Shin, E.-J.; Dang, D.-K.; Tran, T.-V.; Tran, H.-Q.; Jeong, J.H.; Nah, S.-Y.; Jang, C.-G.; Yamada, K.; Nabeshima, T.; Kim, H.-C. Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch. Pharmacal Res. 2017, 40, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Underhill, S.M.; Stolz, D.B.; Murdoch, G.H.; Thiels, E.; Romero, G.; Amara, S.G. Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine. Proc. Natl. Acad. Sci. USA 2015, 112, E7138–E7147. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; He, J.-T.; Zhang, K.-K.; Chen, L.-J.; Wang, Q.; Xie, X.-L. Methamphetamine reduces expressions of tight junction proteins, rearranges F-actin cytoskeleton and increases the blood brain barrier permeability via the RhoA/ROCK-dependent pathway. Biochem. Biophys. Res. Commun. 2019, 509, 395–401. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Aalinkeel, R.; Sykes, D.E.; Reynolds, J.L.; Bindukumar, B.; Adal, A.; Qi, M.; Toh, J.; Xu, G.; Prasad, P.N.; et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008, 1203, 133–148. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Liu, C.; Xie, W.; Huang, E.; Huang, W.; Wang, J.; Chen, L.; Wang, H.; Qiu, P.; et al. Inhibition of ROCK2 expression protects against methamphetamine-induced neurotoxicity in PC12 cells. Brain Res. 2013, 1533, 16–25. [Google Scholar] [CrossRef]
- Kennedy, L.A.; Zigmond, M.J. The behavioral effects of D-amphetamine are correlated with its effects on cAMP in different brain regions. Brain Res. 1979, 168, 408–413. [Google Scholar] [CrossRef]
- Forget, M.A.; Desrosiers, R.R.; Gingras, D.; Béliveau, R. Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 2002, 361 Pt 2, 243–254. [Google Scholar] [CrossRef]
- Ellerbroek, S.M.; Wennerberg, K.; Burridge, K. Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 2003, 278, 19023–19031. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef]
- Han, D.; Xu, L.; Xiao, H.; Schmidt, G.C.P.; Shi, S. Dizocilpine reduces head diameter of dendritic spines in the hippocampus of adolescent rats. Psychiatry Res. 2013, 210, 351–356. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lee, C.-T.; Tsai, F.-M.; Chen, M.-L. The Effects of Poria cocos on Rho Signaling-Induced Regulation of Mobility and F-Actin Aggregation in MK-801-Treated B35 and C6 Cells. Behav. Neurol. 2022, 2022, 8225499. [Google Scholar] [CrossRef]
- Koch, J.C.; Tatenhorst, L.; Roser, A.-E.; Saal, K.-A.; Tönges, L.; Lingor, P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol. Ther. 2018, 189, 1–21. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef]
- Iizuka, M.; Kimura, K.; Wang, S.; Kato, K.; Amano, M.; Kaibuchi, K.; Mizoguchi, A. Distinct distribution and localization of Rho-kinase in mouse epithelial, muscle and neural tissues. Cell Struct. Funct. 2012, 37, 155–175. [Google Scholar] [CrossRef]
- Lee, J.H.; Zheng, Y.; Bornstadt, D.; Wei, Y.; Balcioglu, A.; Daneshmand, A.; Yalcin, N.; Yu, E.; Herisson, F.; Atalay, Y.B.; et al. Selective ROCK2 Inhibition in Focal Cerebral Ischemia. Ann. Clin. Transl. Neurol. 2013, 1, 2–14. [Google Scholar] [CrossRef]
- Xu, X.; Yao, L. Recent advances in the development of Rho kinase inhibitors (2015–2021). Med. Res. Rev. 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).