Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration
Abstract
:1. Introduction
2. Results
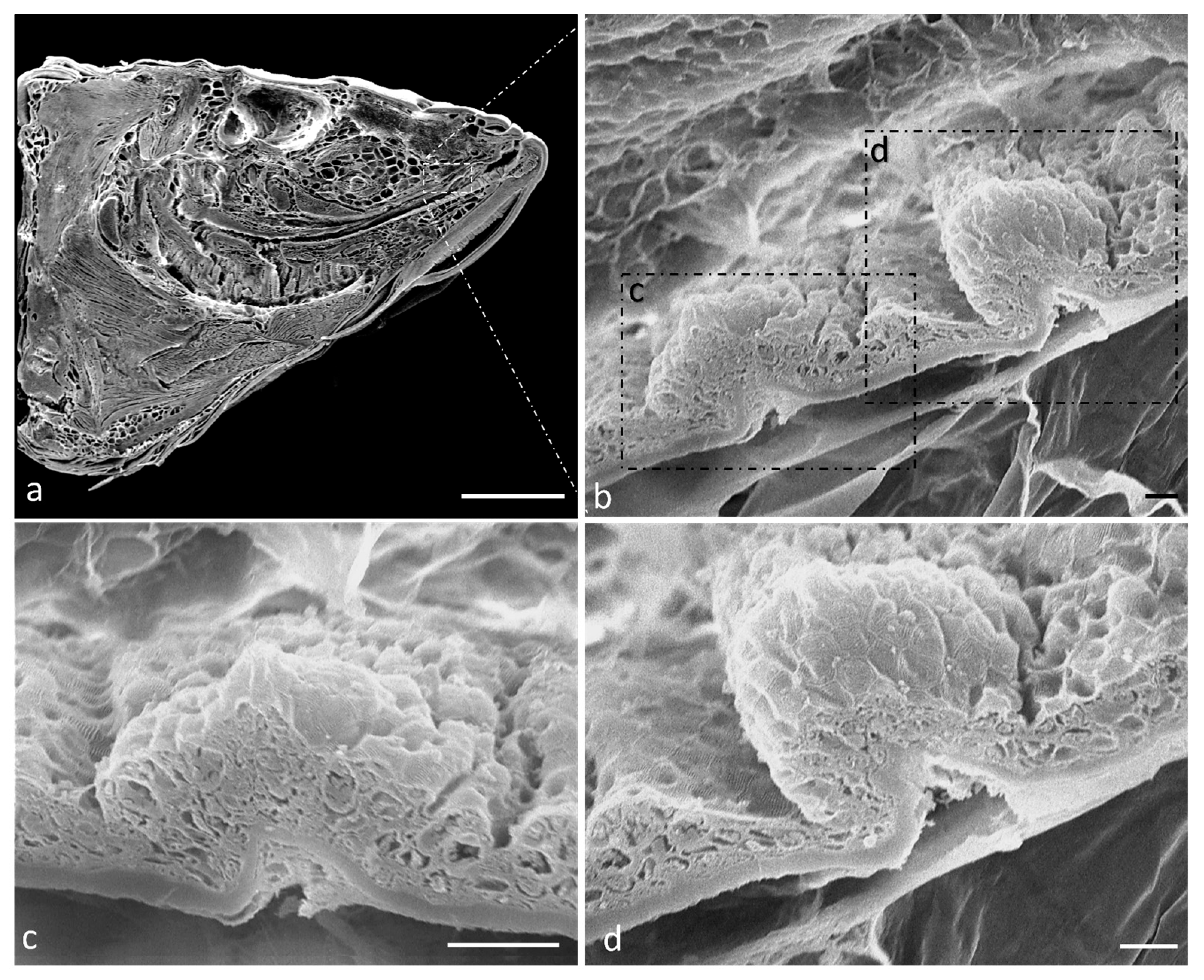
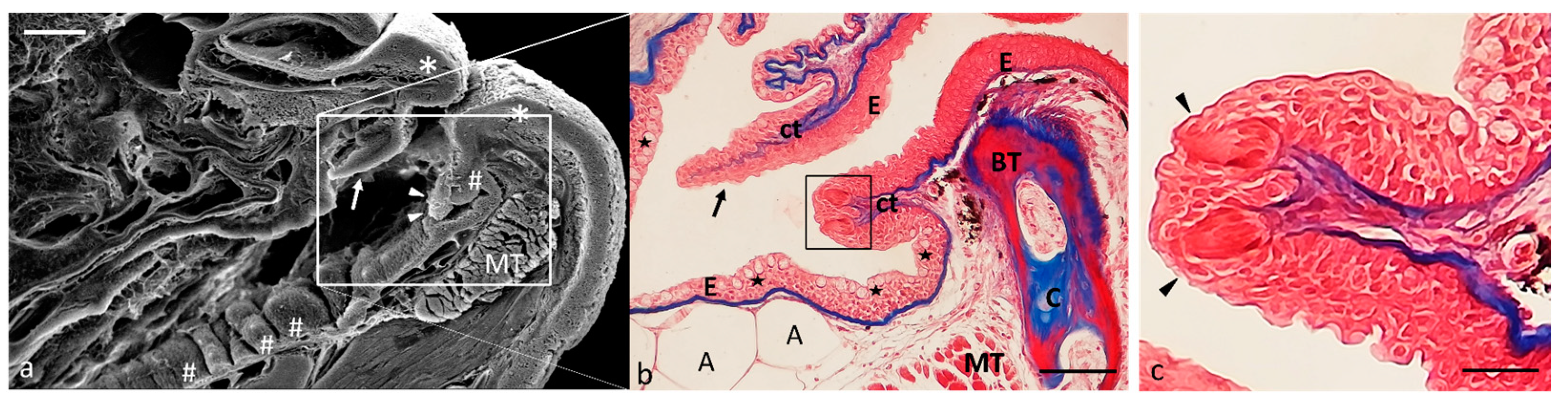
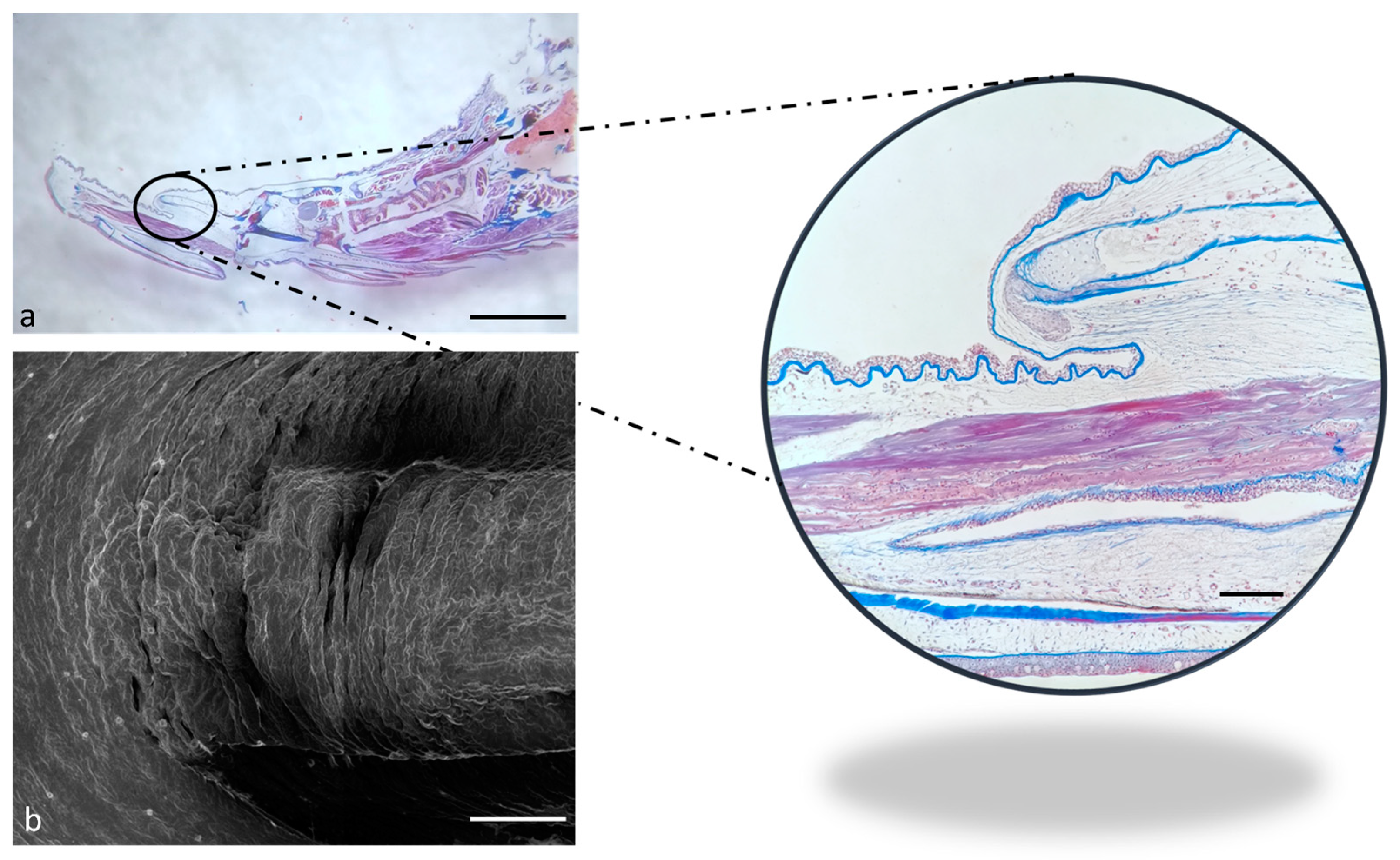
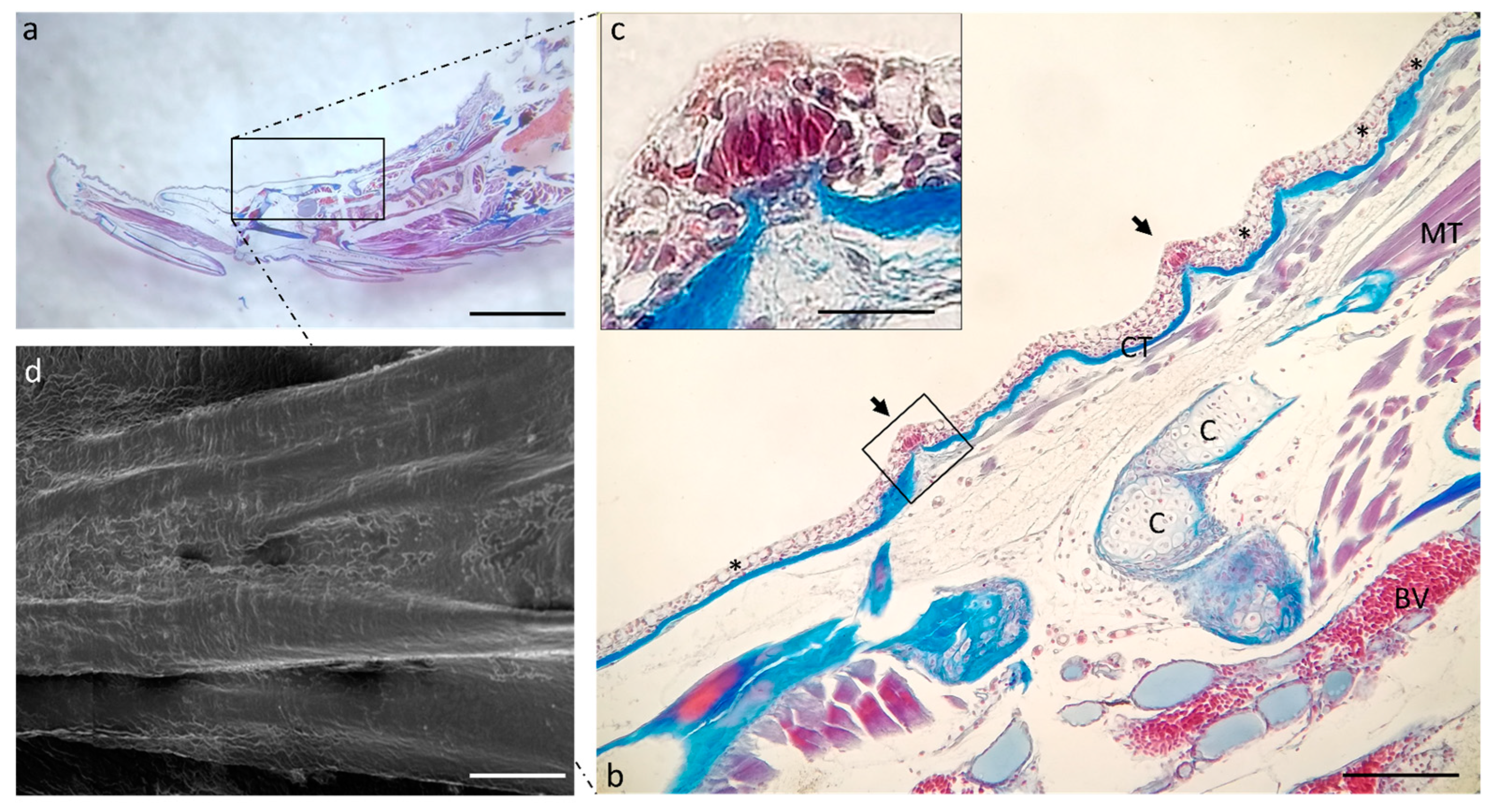
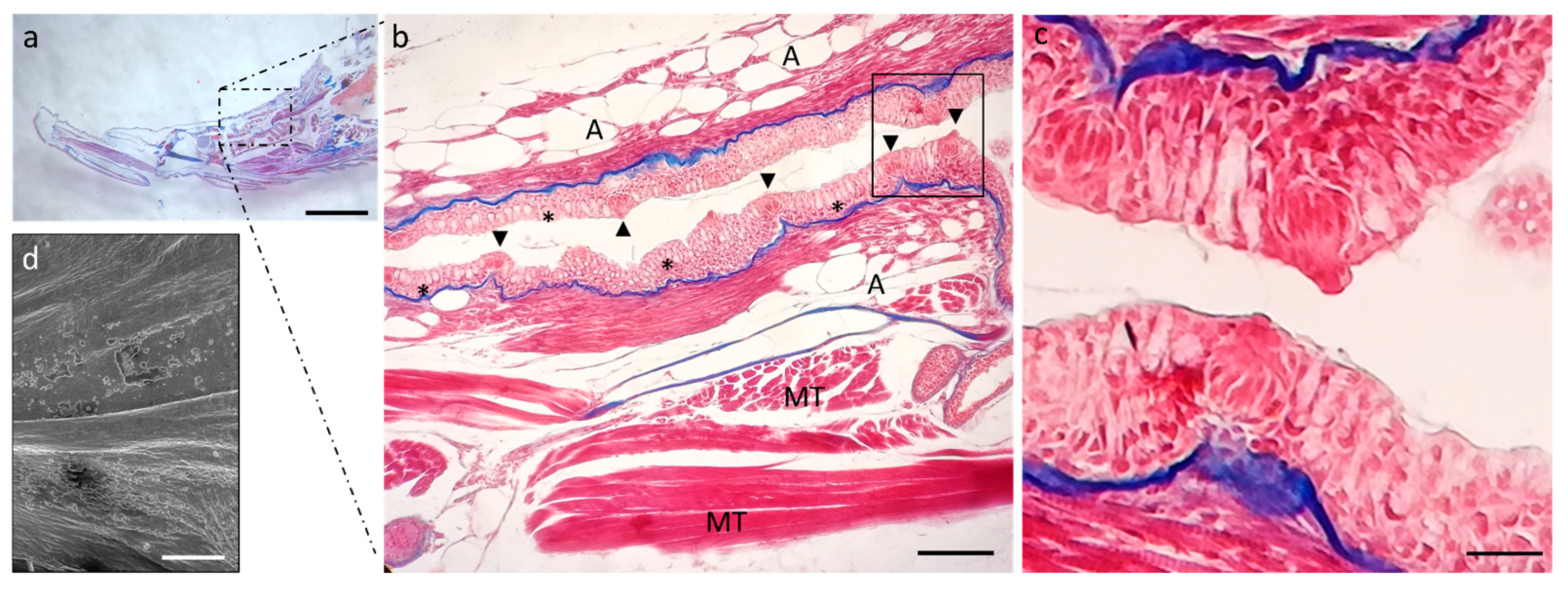
2.1. Ultrastructure and Histology of Taste Buds in the Zebrafish Model
2.2. Calretinin (N-18) and Vimentin RV202 Specificity in Zebrafish
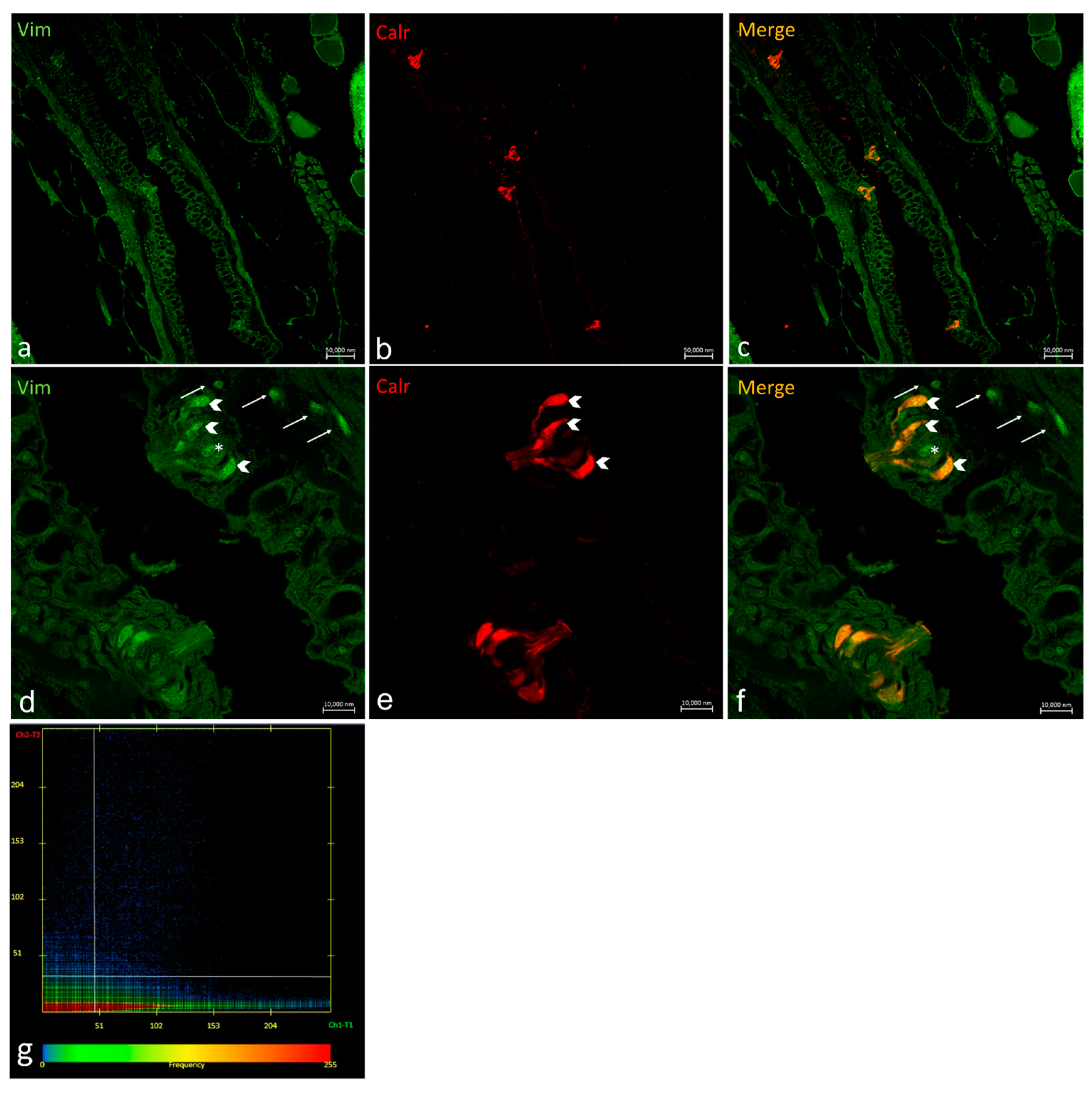
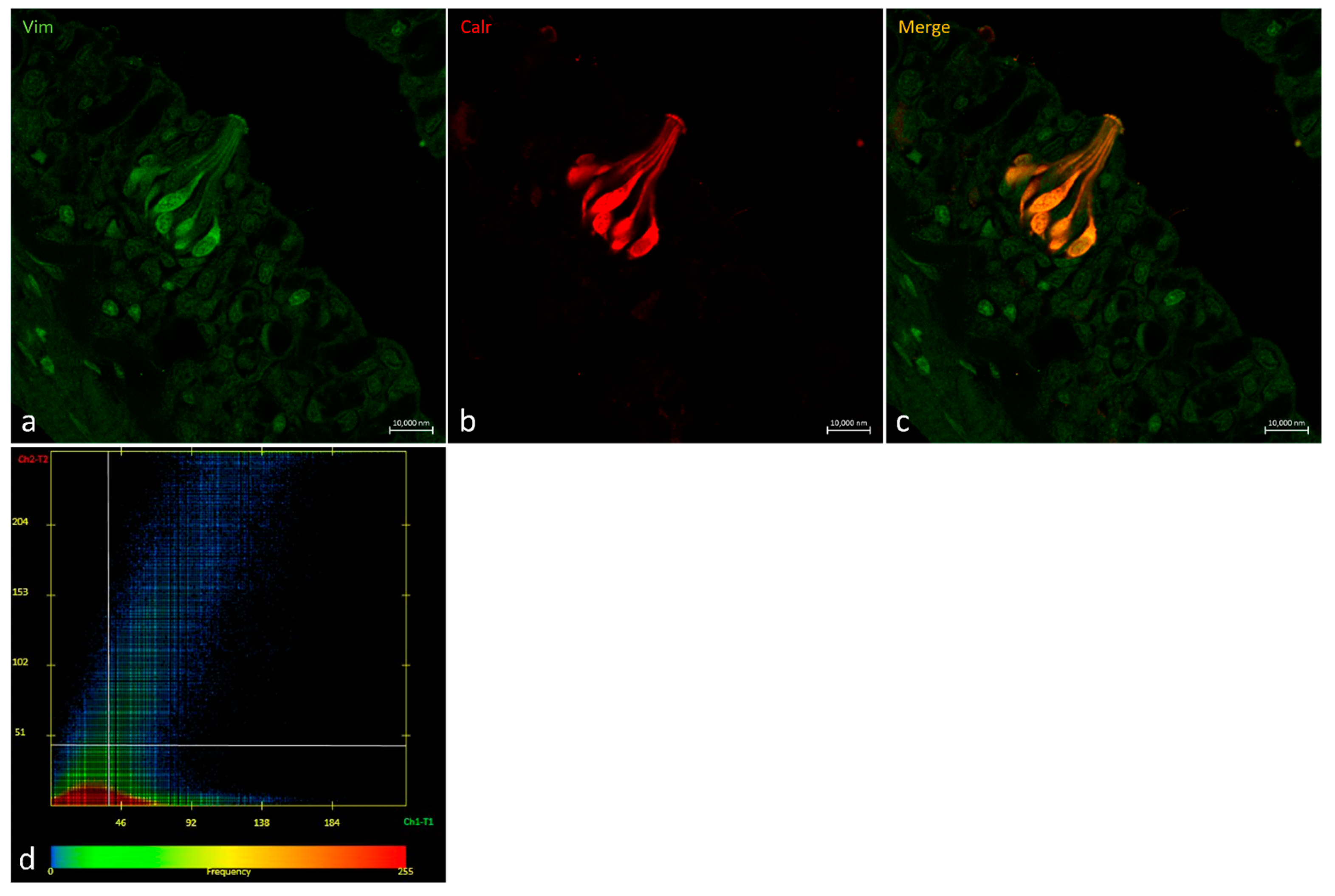
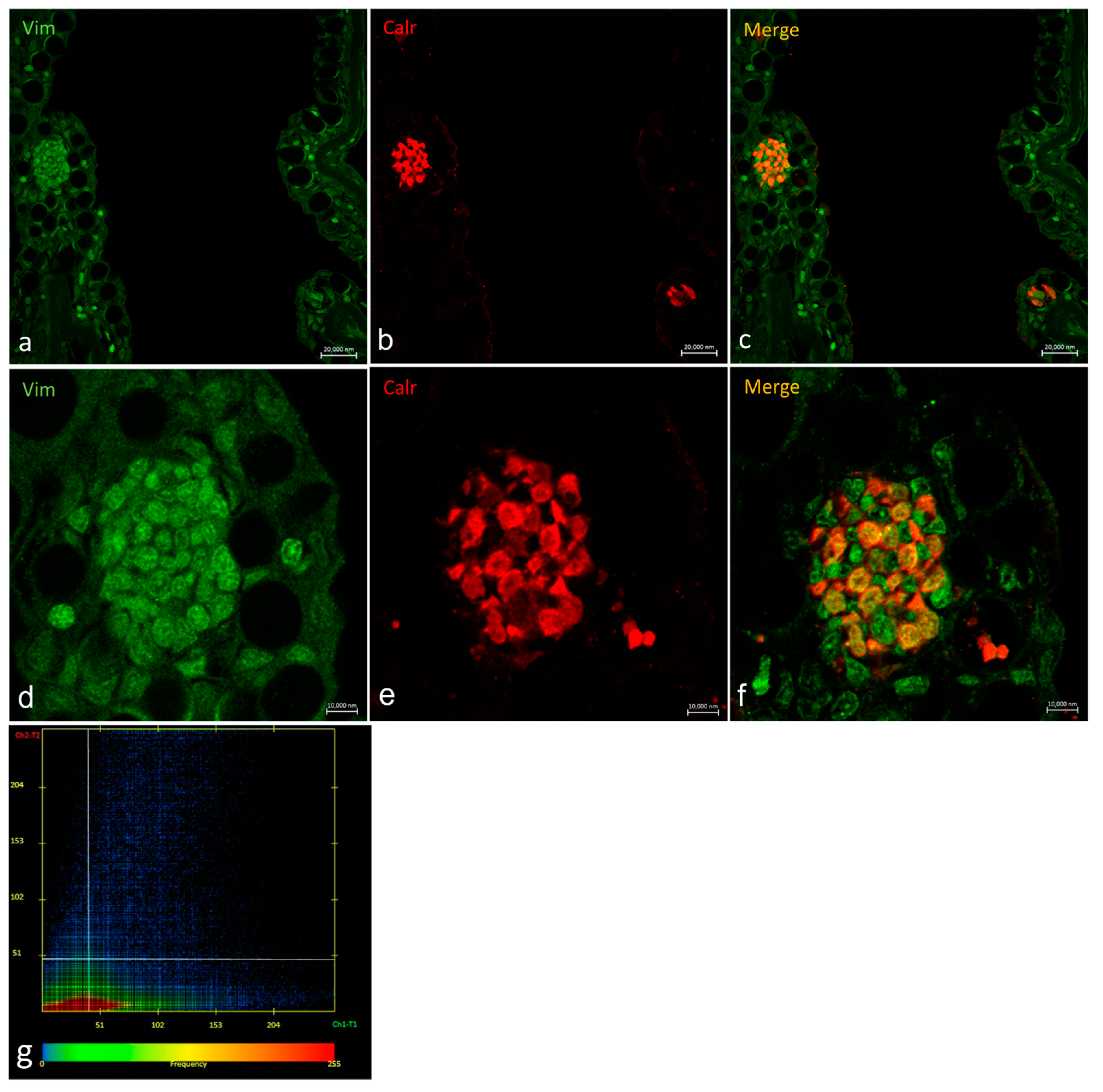
2.3. Vimentin RV202 and Calretinin N-18 Colocalize in Some Taste Buds of Zebrafish
2.4. Ubiquitin-Immunoreaction in Taste Buds
3. Discussion
4. Materials and Methods
4.1. Optical Microscopy
4.2. Scanning Electron Microscopy
4.3. Calretinin (N-18) and Vimentin RV202 Specificity in Zebrafish
4.4. Confocal Immunofluorescence
4.5. Immunoperoxidase Method
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buddington, R.K.; Kuz’mina, V. Chapter 23—Digestive System. In The Laboratory Fish; Ostrander, G.K., Ed.; Academic Press: London, UK, 2000; pp. 379–384. [Google Scholar] [CrossRef]
- Abbate, F.; Germana, G.; De Carlos, F.; Montalbano, G.; Laura, R.; Levanti, M.; Germana, A. The oral cavity of the adult zebrafish (Danio rerio). Anat. Histol. Embryol. 2006, 35, 299–304. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Aragona, M.; Mhalhel, K.; Montalbano, G.; Germanà, A. Anatomical, histological and immunohistochemical study of the tongue in the rainbow trout (Oncorhynchus mykiss). Anat. Histol. Embryol. 2020, 49, 848–858. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Aragona, M.; Mhalhel, K.; Montalbano, G.; Germanà, A. Morphological characteristics of the blackspot seabream (Pagellus bogaraveo) tongue: A structural and immunohistochemical study. Anat. Histol. Embryol. 2022, 51, 103–111. [Google Scholar] [CrossRef]
- Germanà, A.; Paruta, S.; Germanà, G.P.; Ochoa-Erena, F.J.; Montalbano, G.; Cobo, J.; Vega, J.A. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio). Brain Res. 2007, 1162, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Finger, T.E.; Barlow, L.A. Cellular diversity and regeneration in taste buds. Curr. Opin. Physiol. 2021, 20, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Barlow, L.A. The sense of taste: Development, regeneration, and dysfunction. WIREs Mech. Dis. 2022, 14, e1547. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Cometa, M.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; et al. The BDNF/TrkB Neurotrophin System in the Sensory Organs of Zebrafish. Int. J. Mol. Sci. 2022, 23, 2621. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; Calapai, F.; et al. Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 4696. [Google Scholar] [CrossRef]
- Germana, A.; González-Martínez, T.; Catania, S.; Laura, R.; Cobo, J.; Ciriaco, E.; Vega, J. Neurotrophin receptors in taste buds of adult zebrafish (Danio rerio). Neurosci. Lett. 2004, 354, 189–192. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Janmey, P.; Shah, J.; Janssen, K.; Schliwa, M. Viscoelasticity of intermediate filament networks. Sub-Cell. Biochem. 1998, 31, 381–397. [Google Scholar]
- Janmey, P.A.; Euteneuer, U.; Traub, P.; Schliwa, M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 1991, 113, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef]
- Witt, M.; Kasper, M. Distribution of cytokeratin filaments and vimentin in developing human taste buds. Anat. Embryol. 1999, 199, 291–299. [Google Scholar] [CrossRef]
- Cerdà, J.; Conrad, M.; Markl, J.; Brand, M.; Herrmann, H. Zebrafish vimentin: Molecular characterization, assembly properties and developmental expression. Eur. J. Cell Biol. 1998, 77, 175–187. [Google Scholar] [CrossRef]
- Herrmann, H.; Eckelt, A.; Brettel, M.; Grund, C.; Franke, W.W. Temperature-sensitive Intermediate Filament Assembly: Alternative Structures of Xenopus laevis Vimentin In Vitro and In Vivo. J. Mol. Biol. 1993, 234, 99–113. [Google Scholar] [CrossRef]
- Herrmann, H.; Munick, M.D.; Brettel, M.; Fouquet, B.; Markl, J. Vimentin in a cold-water fish, the rainbow trout: Highly conserved primary structure but unique assembly properties. J. Cell Sci. 1996, 109, 569–578. [Google Scholar] [CrossRef]
- Markl, J.; Schechter, N. Fish intermediate filament proteins in structure, evolution, and function. Sub-Cell. Biochem. 1998, 31, 1–33. [Google Scholar]
- Collin, S.P.; Marshall, N.J.; Witt, M.; Reutter, K.; Ganchrow, D.; Ganchrow, J.R. Fingerprinting taste buds: Intermediate filaments and their implication for taste bud formation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1233–1237. [Google Scholar] [CrossRef]
- Pattabiraman, S.; Azad, G.K.; Amen, T.; Brielle, S.; Park, J.E.; Sze, S.K.; Meshorer, E.; Kaganovich, D. Vimentin protects differentiating stem cells from stress. Sci. Rep. 2020, 10, 19525. [Google Scholar] [CrossRef]
- Finley, D.; Varshavsky, A. The ubiquitin system: Functions and mechanisms. Trends Biochem. Sci. 1985, 10, 343–347. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta BBA Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Manford, A.G.; Rape, M. Ubiquitin-Dependent Regulation of Stem Cell Biology. Trends Cell Biol. 2017, 27, 568–579. [Google Scholar] [CrossRef]
- Reutter, K. Morphology of vertebrate taste organs and their nerve supply. In Mechanisms of Taste Transduction; CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bang, P.I.; Sewell, W.F.; Malicki, J.J. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio). J. Compart. Neurol. 2001, 438, 173–190. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S.I.; Argo, S.; Campenhausen, H.; Friedrich, R.W.; Rummrich, A.; Weth, F. Olfaction in zebrafish: What does a tiny teleost tell us? Semin. Cell Dev. Biol. 1997, 8, 181–187. [Google Scholar] [CrossRef]
- Oka, Y.; Korsching, S.I. Shared and Unique G Alpha Proteins in the Zebrafish Versus Mammalian Senses of Taste and Smell. Chem. Senses 2011, 36, 357–365. [Google Scholar] [CrossRef]
- Whitfield, T.T. Zebrafish as a model for hearing and deafness. J. Neurobiol. 2002, 53, 157–171. [Google Scholar] [CrossRef]
- Ton, C.; Parng, C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear. Res. 2005, 208, 79–88. [Google Scholar] [CrossRef]
- Babin, P.J.; Goizet, C.; Raldúa, D. Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog. Neurobiol. 2014, 118, 36–58. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498. [Google Scholar] [CrossRef]
- Hara, T.J. The diversity of chemical stimulation in fish olfaction and gustation. Rev. Fish Biol. Fish. 1994, 4, 1–35. [Google Scholar] [CrossRef]
- Jakubowski, M.; Whitear, M. Comparative morphology and cytology of taste buds in teleosts. Jahrb. Morphol. Mikrosk. Anat. 2 Abt. Z. Mikrosk. Anat. Forsch. 1990, 104, 529–560. [Google Scholar]
- Reutter, K. Taste organ in the barbel of the bullhead. In Chemoreception in Fishes; Elsevier: Amsterdam, The Netherlands, 1982; pp. 77–91. [Google Scholar]
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Hamamichi, R.; Asano-Miyoshi, M.; Emori, Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience 2006, 141, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Ito, T.; Okudela, K.; Kawabe, R.; Hayashi, H.; Yazawa, T.; Fujita, K.; Kitamura, H. Expression of cyclin-dependent kinase inhibitors in taste buds of mouse and hamster. Tissue Cell 2001, 33, 25–32. [Google Scholar] [CrossRef]
- Miura, H.; Barlow, L.A. Taste bud regeneration and the search for taste progenitor cells. Arch. Ital. Biol. 2010, 148, 107–118. [Google Scholar]
- Nguyen, H.M.; Barlow, L.A. Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci. 2010, 11, 129. [Google Scholar] [CrossRef]
- Fuchs, E.; Weber, K. Intermediate Filaments: Structure, Dynamics, Function and Disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef]
- Franke, W.W.; Schmid, E.; Winter, S.; Osborn, M.; Weber, K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp. Cell Res. 1979, 123, 25–46. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate filaments and their associates: Multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr. Opin. Cell Biol. 2000, 12, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bignami, A.; Raju, T.; Dahl, D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons: In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev. Biol. 1982, 91, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Karsten, U.; Stoslek, P.; Moll, R. Distribution of intermediate-filament proteins in the human enamel organ: Unusually complex pattern of coexpression of cytokeratin polypeptides and vimentin. Differentiation 1989, 40, 207–214. [Google Scholar] [CrossRef]
- Lane, E.B.; Hogan, B.L.M.; Kurkinen, M.; Garrels, J.I. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 1983, 303, 701–704. [Google Scholar] [CrossRef]
- Venkatesan, N.; Rajapaksha, P.; Payne, J.; Goodfellow, F.; Wang, Z.; Kawabata, F.; Tabata, S.; Stice, S.; Beckstead, R.; Liu, H.-X. Distribution of α-Gustducin and Vimentin in premature and mature taste buds in chickens. Biochem. Biophys. Res. Commun. 2016, 479, 305–311. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Norouzi, M.; McCulloch, C.A. Impact of vimentin on regulation of cell signaling and matrix remodeling. Front. Cell Dev. Biol. 2022, 10, 869069. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-L.; Wang, J.; Zheleznyak, A.; Brown, E.J. Ubiquitin-Related Proteins Regulate Interaction of Vimentin Intermediate Filaments with the Plasma Membrane. Mol. Cell 1999, 4, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Raderman-Little, R. The Effect of Temperature on the Turnover of Taste Bud Cells in Catfish. Cell Prolif. 1979, 12, 269–280. [Google Scholar] [CrossRef]
- Dumortier, J.; Daemi, N.; Pourreyron, C.; Anderson, W.; Bellaton, C.; Jacquier, M.-F.; Bertrand, S.; Chayvialle, J.-A.; Remy, L. Loss of epithelial differentiation markers and acquisition of vimentin expression after xenograft with laminin-1 enhance migratory and invasive abilities of human colon cancer cells LoVo C5. Differentiation 1998, 63, 141–150. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Thompson, E.W.; Williams, E.D. Mesenchymal to Epithelial Transition in Development and Disease. Cells Tissues Organs 2007, 185, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Spooner-Harris, M.; Kerns, K.; Zigo, M.; Sutovsky, P.; Balboula, A.; Patterson, A.L. A re-appraisal of mesenchymal-epithelial transition (MET) in endometrial epithelial remodeling. Cell Tissue Res. 2022, 185, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Antonello, Z.A.; Reiff, T.; Dominguez, M. Mesenchymal to epithelial transition during tissue homeostasis and regeneration: Patching up the Drosophila midgut epithelium. Fly 2015, 9, 132–137. [Google Scholar] [CrossRef]
- Saez, I.; Koyuncu, S.; Gutierrez-Garcia, R.; Dieterich, C.; Vilchez, D. Insights into the ubiquitin-proteasome system of human embryonic stem cells. Sci. Rep. 2018, 8, 4092. [Google Scholar] [CrossRef]
- Dubiel, W.; Dubiel, D.; Wolf, D.A.; Naumann, M. Cullin 3-Based Ubiquitin Ligases as Master Regulators of Mammalian Cell Differentiation. Trends Biochem. Sci. 2018, 43, 95–107. [Google Scholar] [CrossRef]
- Moore, D.L.; Pilz, G.A.; Araúzo-Bravo, M.J.; Barral, Y.; Jessberger, S. A mechanism for the segregation of age in mammalian neural stem cells. Science 2015, 349, 1334–1338. [Google Scholar] [CrossRef]
- Schwaller, B. Calretinin: From a “simple” Ca2+ buffer to a multifunctional protein implicated in many biological processes. Front. Neuroanat. 2014, 8, 3. [Google Scholar] [CrossRef]
- Castro-Muñozledo, F.; Meza-Aguilar, D.G.; Domínguez-Castillo, R.; Hernández-Zequinely, V.; Sánchez-Guzmán, E. Vimentin as a Marker of Early Differentiating, Highly Motile Corneal Epithelial Cells. J. Cell. Physiol. 2017, 232, 818–830. [Google Scholar] [CrossRef]
- Germanà, A.; Guerrera, M.C.; Laurà, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germanà, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Zaccone, G.; Cupello, C.; Fernandes, J.M.O.; Viswanath, K.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Icardo, J.M.; et al. Expression of acetylcholine, its contribution to regulation of immune function and O2 sensing and phylogenetic interpretations of the African butterfly fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish Immunol. 2021, 111, 189–200. [Google Scholar] [CrossRef] [PubMed]


| Taxa | Max Score | Total Score | Query Cover | E Value | Per. Ident | Acc. Len | Accession |
|---|---|---|---|---|---|---|---|
| Calretinin N-18 | |||||||
| Homo sapiens | - | - | - | - | - | 271 | CAA39991.1 |
| Rattus norvegicus | 547 | 547 | 100% | 0.0 | 98.52% | 271 | Query_41808 |
| Mus musculus | 546 | 546 | 100% | 0.0 | 98.15% | 271 | Query_41807 |
| Danio rerio | 400 | 400 | 100% | 7 × 10−147 | 71.96% | 269 | AAH59467.1 |
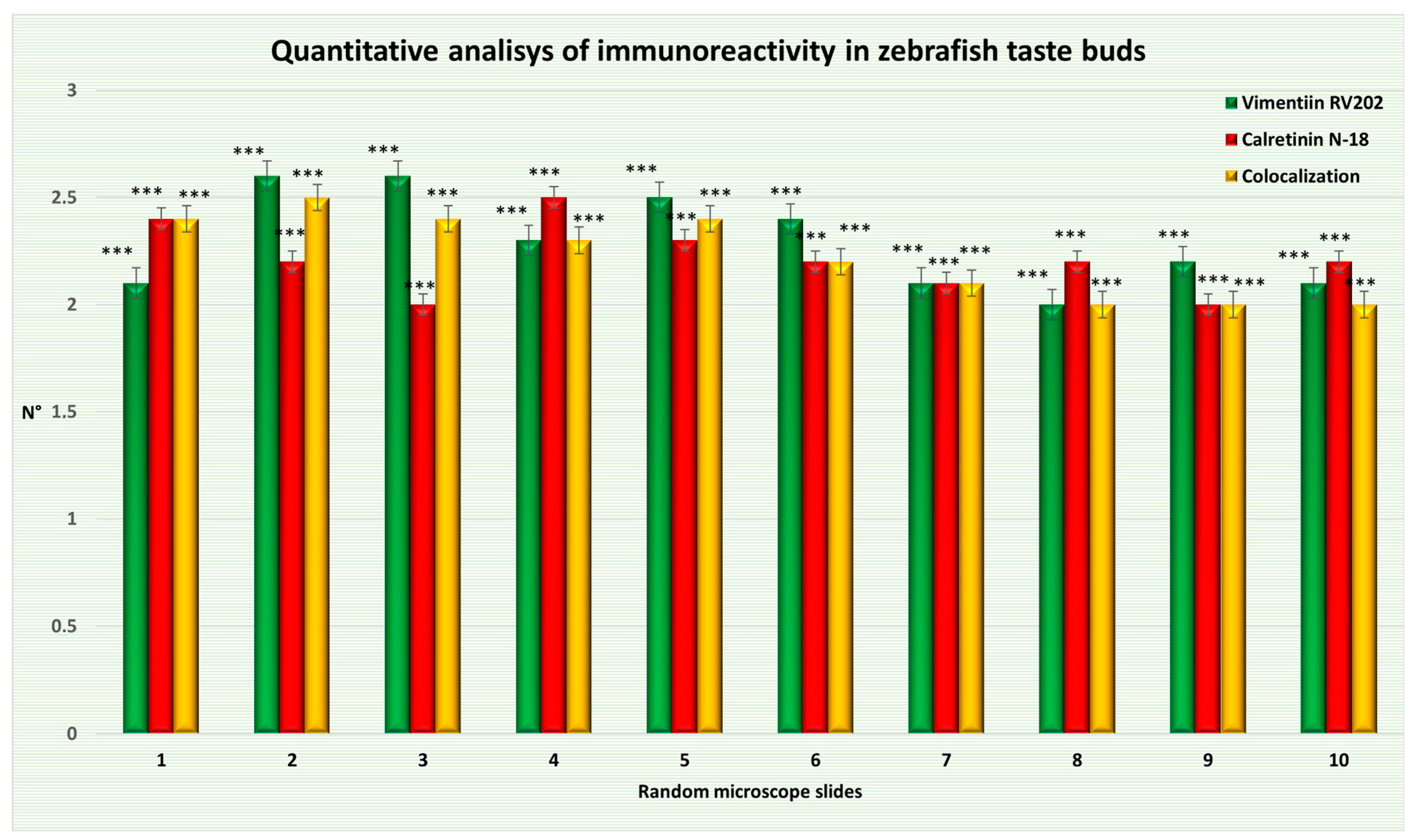
| Random Microscope Slide | Mean ± Δσ of Taste Bud Cells Immunopositive to Vimentin RV202 | Mean ± Δσ of Taste Bud Cells Immunopositive to Calretinin N-18 | Mean ± Δσ of Taste Bud Cells Showing Colocalization |
|---|---|---|---|
| (1) | 2.1 ± 0.7 *** | 2.4 ± 0.66 *** | 2.4 ± 0.8 *** |
| (2) | 2.6 ± 0.48 *** | 2.2 ± 0.6 *** | 2.5 ± 0.67 *** |
| (3) | 2.6 ± 0.48 *** | 2 ± 0.63 *** | 2.4 ± 0.66 *** |
| (4) | 2.3 ± 0.64 *** | 2.5 ± 0.67 *** | 2.3 ± 0.64 *** |
| (5) | 2.5 ± 0.67 *** | 2.3 ± 0.64 *** | 2.4 ± 0.8 *** |
| (6) | 2.4 ± 0.8 *** | 2.2 ± 0.87 *** | 2.2 ± 0.74 *** |
| (7) | 2.1 ± 0.7 *** | 2.1 ± 0.83 *** | 2.1 ± 0.7 *** |
| (8) | 2 ± 0.63 *** | 2.2 ± 0.74 *** | 2 ± 0.63 *** |
| (9) | 2.2 ± 0.4 *** | 2 ± 0.77 *** | 2 ± 0.63 *** |
| (10) | 2.1 ± 0.7 *** | 2.2 ± 0.74 *** | 2 ± 0.77 *** |
| Chemicals | Supplier | Dilution | Catalog Number |
|---|---|---|---|
| Paraformaldehyde | Sigma-Aldrich, Inc., St. Louis, MO, USA | 4% | 158127 |
| Phosphate-buffered saline (PBS) | Sigma-Aldrich, Inc., St. Louis, MO, USA. | manufacturer’s notice | P4417 |
| Triton X100 | Sigma-Aldrich, Inc., St. Louis, MO, USA. | 0.1% | X100 |
| 0.2% | |||
| Hydrogen Peroxide (H2O2) | Sigma-Aldrich, Inc., St. Louis, MO, USA. | 0.3% | 1085971000 |
| Fetal Bovine Serum | Sigma-Aldrich, Inc., St. Louis, MO, USA. | 25% | F7524 |
| 0.1% | |||
| DAB | Sigma-Aldrich, Inc., St. Louis, MO, USA. | manufacturer’s notice | D5905 |
| Trizma® base | Sigma-Aldrich, Inc., St. Louis, MO, USA. | manufacturer’s notice | T1503 |
| Hydrochloric acid (HCl) | Avantor delivered by VWR Radnor, PA, USA | manufacturer’s notice | 35328 |
| Masson trichrome with aniline blue staining | Bio-Optica Milano S.p.a Milan, Italy | manufacturer’s notice | 04-010802 |
| Sorensen’s Phosphate Buffer, 0.2 M | Avantor delivered by VWR Radnor, PA, USA. | manufacturer’s notice | 100496-400 |
| Glutaraldehyde | Sigma-Aldrich, Inc., St. Louis, MO, USA. | 2.5% | G5882 |
| Fluoromount Aqueous Mounting Medium | Sigma-Aldrich, Inc., St. Louis, MO, USA. | manufacturer’s notice | F4680 |
| Primary Antibodies | Supplier | Catalog Number | Source | Dilution | Antibody ID |
|---|---|---|---|---|---|
| Vimentin RV202 | Thermo Fisher Scientific Waltham, MA, USA | OMA1-06001 | Mouse | 1:100 | AB_325529 |
| Calretinin-N18 | Santa Cruz Biotechnology Dallas, TX, USA | sc-11644 | Goat | 1:100 | AB_634545 |
| Ubiquitin | Enzo Biochem New York, NY, USA | ADI-SPA-200-D | Rabbit | 1:5000 | AB_2039666 |
| Secondary Antibody | Supplier | Catalogue Number | Source | Dilution | Antibody ID |
| Anti-goat IgG (H+L) Alexa Fluor 594 | Molecular Probes, Invitrogen, Waltham, MA, USA | A-11058 | donkey | 1:300 | AB_142540 |
| Anti-mouse IgG (H+L) Alexa Fluor 488 | Molecular Probes, Invitrogen, Waltham, MA, USA | A-11001 | goat | 1:300 | AB_2534069 |
| Anti-rabbit (IgG) peroxidase-conjugated | Sigma-Aldrich, Inc., St. Louis, MO, USA | A0545 | Goat | 1:50 | AB_257896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, M.; Porcino, C.; Briglia, M.; Mhalhel, K.; Abbate, F.; Levanti, M.; Montalbano, G.; Laurà, R.; Lauriano, E.R.; Germanà, A.; et al. Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration. Int. J. Mol. Sci. 2023, 24, 15619. https://doi.org/10.3390/ijms242115619
Aragona M, Porcino C, Briglia M, Mhalhel K, Abbate F, Levanti M, Montalbano G, Laurà R, Lauriano ER, Germanà A, et al. Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration. International Journal of Molecular Sciences. 2023; 24(21):15619. https://doi.org/10.3390/ijms242115619
Chicago/Turabian StyleAragona, Marialuisa, Caterina Porcino, Marilena Briglia, Kamel Mhalhel, Francesco Abbate, Maria Levanti, Giuseppe Montalbano, Rosaria Laurà, Eugenia Rita Lauriano, Antonino Germanà, and et al. 2023. "Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration" International Journal of Molecular Sciences 24, no. 21: 15619. https://doi.org/10.3390/ijms242115619
APA StyleAragona, M., Porcino, C., Briglia, M., Mhalhel, K., Abbate, F., Levanti, M., Montalbano, G., Laurà, R., Lauriano, E. R., Germanà, A., & Guerrera, M. C. (2023). Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration. International Journal of Molecular Sciences, 24(21), 15619. https://doi.org/10.3390/ijms242115619










