Systemic Immunomodulatory Effects of Codonopsis pilosula Glucofructan on S180 Solid-Tumor-Bearing Mice
Abstract
:1. Introduction
2. Results
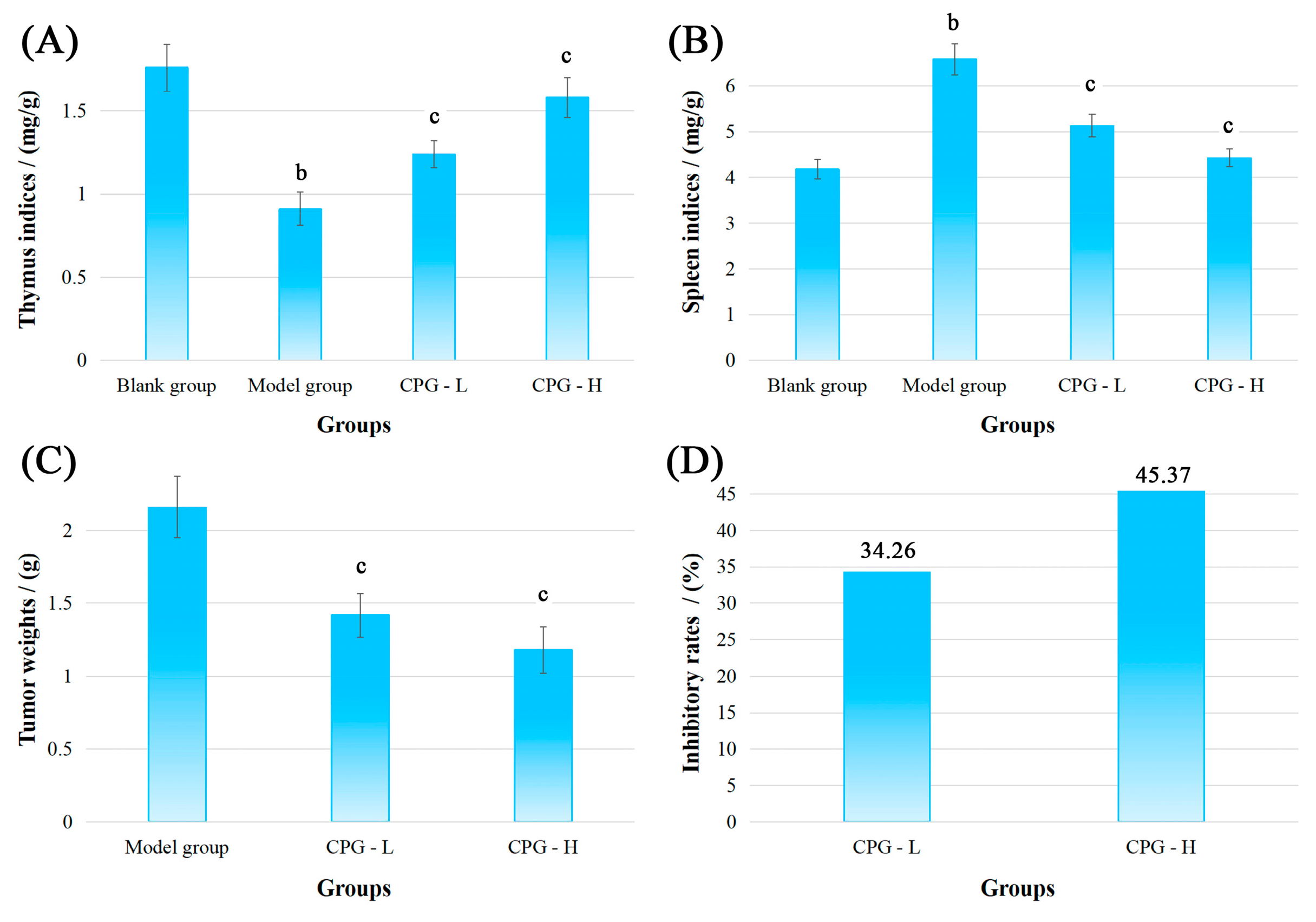
2.1. Physiological Indicators of S180-Bearing Mice
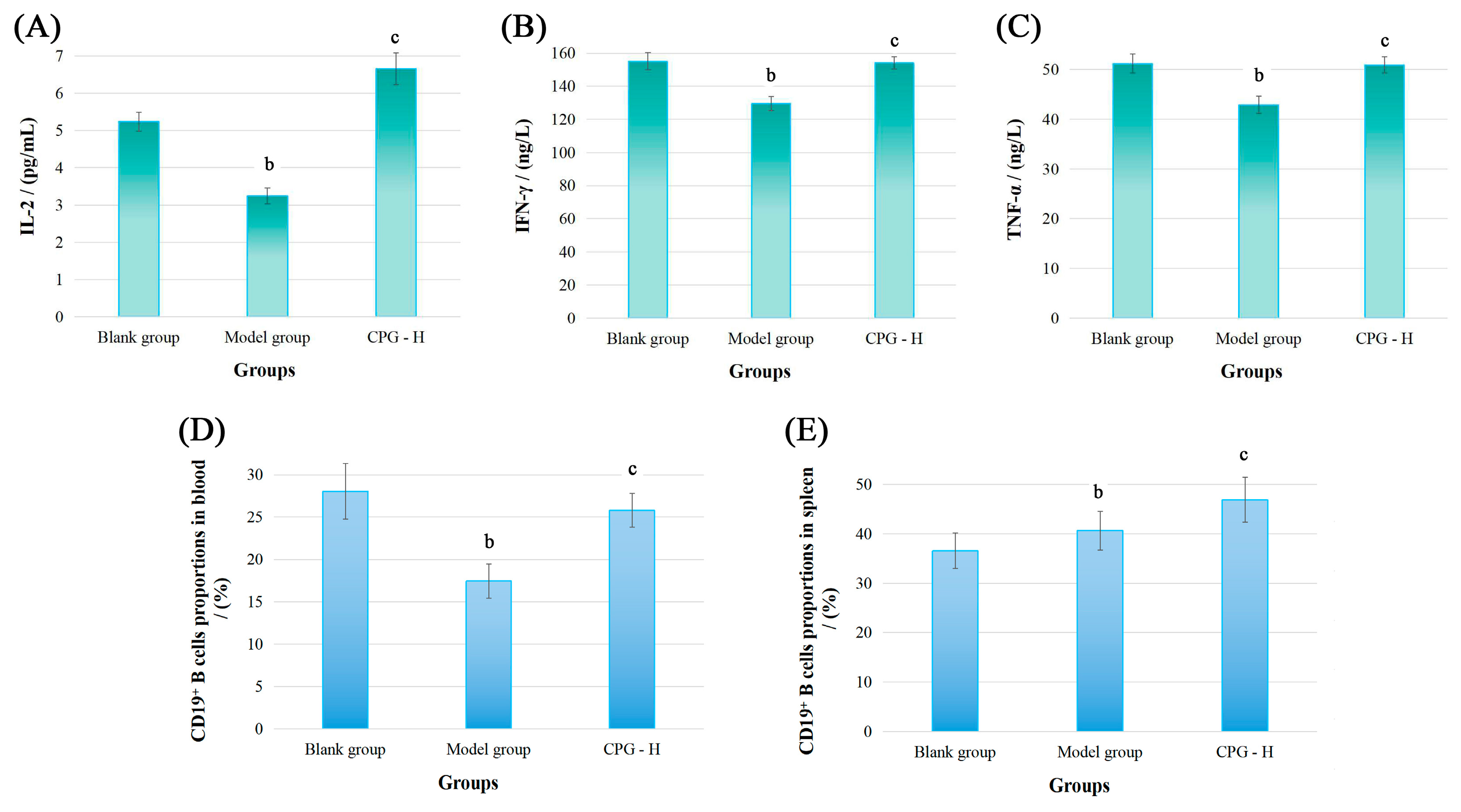
2.2. Cancer-Associated Cytokine Determination
2.3. Distributions of CD19+ B Cells
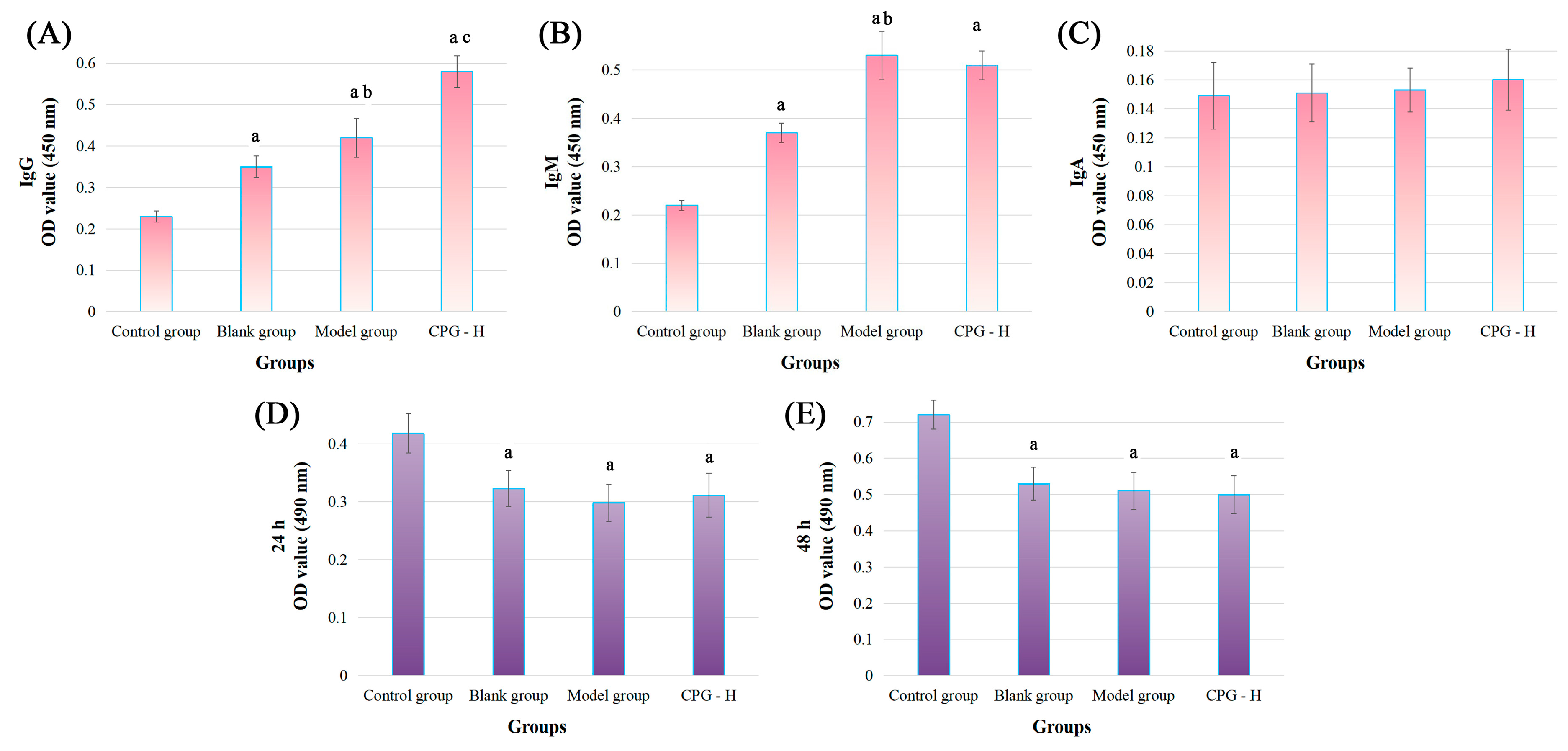
2.4. Tumor-Specific IgG, IgM, and IgA Expression
2.5. Toxic Effects of Mouse Sera on S180 Cells
2.6. Coculture of S180 Cells and Sera
2.7. The Results of Blood Routine Examination
2.8. Immune Cell Activities
2.9. Cell Cycle Distributions and Apoptotic Rates in Tumors
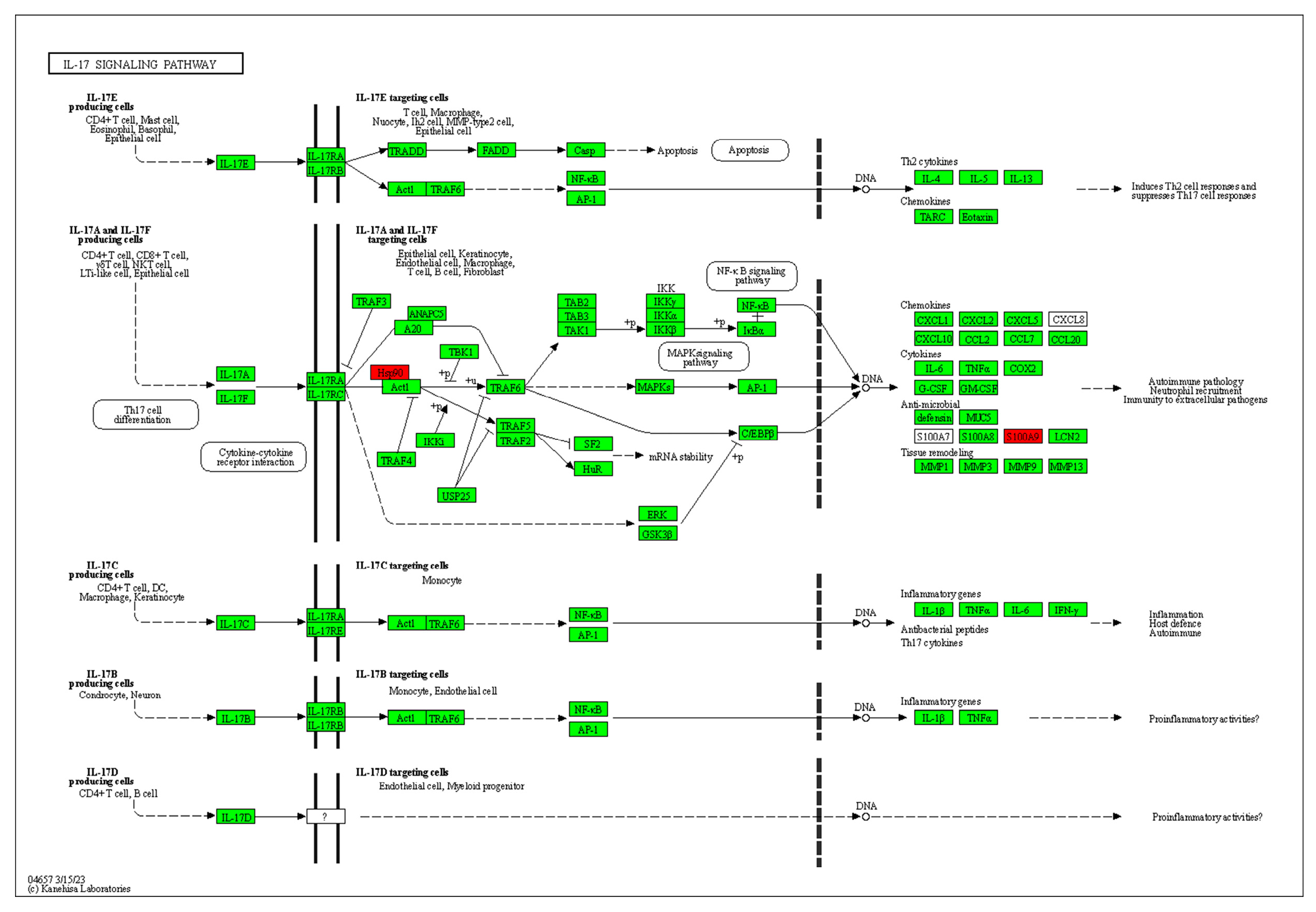
2.10. Differences in Protein Expression of Solid Tumors in Each Group
3. Discussion
3.1. Systemic Immunity and Anti-Tumor Effects
3.2. Systemic Immunity Potential of CPG
3.3. Antitumor Effects of CPG
3.4. CPG-Enhanced Anti-Tumor Humoral Immunity
3.5. CPG-Enhanced Anti-Tumor Cellular Immunity
3.6. Solid Tumor Cell Apoptosis Induced by CPG-Enhanced Immunity
4. Materials and Methods
4.1. Materials
4.2. Animals and Cell Lines
4.3. Immune Organ Indices and Tumor Weights
4.4. IL-2, IFN-γ, and TNF-α Determination
4.5. CD19+ B Cell Distributions
4.6. S180-Specific IgG, IgM, and IgA Level Determination
4.7. Tumor Cell Viability Detection
4.8. Morphology of Sera-Treated S180 Cells
4.9. Blood Routine Parameter Examination
4.10. Immune Cell Activity Detection
4.11. Cell Cycle Distribution Determination
4.12. 2D Gel Electrophoresis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breous, E.; Thimme, R. Potential of immunotherapy for hepatocellular carcinoma. J. Hepatol. 2011, 54, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006, 66, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Chen, Z.; Chen, H. Physicochemical characterization of the oolong tea polysaccharides with high molecular weight and their synergistic effects in combination with polyphenols on hepatocellular carcinoma. Biomed. Pharmacother. 2017, 90, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I.; Waxman, D.J.; Lakka Klement, G. Metronomic chemotherapy: An attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015, 358, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dong, X.-d.; Jiao, J.-s.; Ji, H.-y.; Liu, A.-j. Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 189, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Eswar, K.; Mukherjee, S.; Ganesan, P.; Rengan, A.K. Immunomodulatory natural polysaccharides: An overview of the mechanisms involved. Eur. Polym. J. 2023, 188, 111935. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Yu, J.; Jiao, J.-S.; Dong, X.-D.; Yu, S.-S.; Liu, A.-J. Ultrasonic-Assisted Extraction of Codonopsis pilosula Glucofructan: Optimization, Structure, and Immunoregulatory Activity. Nutrients 2022, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Suckow, M.A. Cancer vaccines: Harnessing the potential of anti-tumor immunity. Vet. J. 2013, 198, 28–33. [Google Scholar] [CrossRef]
- Rossowska, J.; Pajtasz-Piasecka, E.; Rysnik, O.; Wojas, J.; Krawczenko, A.; Szyda, A.; Dus, D. Generation of antitumor response by IL-2-transduced JAWS II dendritic cells. Immunobiology 2011, 216, 1074–1084. [Google Scholar] [CrossRef]
- Kursunel, M.A.; Esendagli, G. The untold story of IFN-gamma in cancer biology. Cytokine Growth Factor. Rev. 2016, 31, 73–81. [Google Scholar] [CrossRef]
- Mausner-Fainberg, K.; Regev, K.; Kolb, H.; Vaknin-Dembinsky, A.; Karni, A. Increased neutralization capacity of TNF-alpha in sera of relapsing remitting multiple sclerosis patients is not related to soluble TNF-alpha receptors or anti-TNF-alpha autoantibody levels. J. Neuroimmunol. 2015, 286, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Basappa; Murugan, S.; Kavitha, C.V.; Purushothaman, A.; Nevin, K.G.; Sugahara, K.; Rangappa, K.S. A small oxazine compound as an anti-tumor agent: A novel pyranoside mimetic that binds to VEGF, HB-EGF, and TNF-alpha. Cancer Lett. 2010, 297, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, T.; Xu, H.; Zhang, R.; Zhao, S.; Kong, L.; Yang, C.; Zhang, Z. Dying tumor cells-inspired vaccine for boosting humoral and cellular immunity against cancer. J. Control. Release 2023, 359, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Rana, P.S. Multilevel ensemble model for prediction of IgA and IgG antibodies. Immunol. Lett. 2017, 184, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.D.; Pucic-Bakovic, M.; Vuckovic, F.; Reiding, K.; Trbojevic-Akmacic, I.; Srajer Gajdosik, M.; Cook, M.I.; Lopez, M.J.; Wuhrer, M.; Camara, L.M.; et al. IgG and IgM glycosylation patterns in patients undergoing image-guided tumor ablation. Biochim. Biophys. Acta 2016, 1860, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.K.; Chattopadhyay, S.; Tripathy, S.; Dash, S.S.; Das, B.; Mandal, D.; Mahapatra, S.K.; Bag, B.G.; Roy, S. Self-assembled betulinic acid augments immunomodulatory activity associates with IgG response. Biomed. Pharmacother. 2015, 75, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chapey, E.; Wallon, M.; Peyron, F. Evaluation of the LDBIO point of care test for the combined detection of toxoplasmic IgG and IgM. Clin. Chim. Acta 2017, 464, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Neovius, M.; Ye, W.; Hammarström, L. IgA Deficiency and Risk of Cancer: A Population-Based Matched Cohort Study. J. Clin. Immunol. 2015, 35, 182–188. [Google Scholar] [CrossRef]
- Vos, Q.; Lees, A.; Wu, Z.Q.; Snapper, C.M.; Mond, J.J. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 2000, 176, 154–170. [Google Scholar] [CrossRef]
- Selter, R.C.; Biberacher, V.; Grummel, V.; Buck, D.; Eienbroker, C.; Oertel, W.H.; Berthele, A.; Tackenberg, B.; Hemmer, B. Natalizumab treatment decreases serum IgM and IgG levels in multiple sclerosis patients. Mult. Scler. 2013, 19, 1454–1461. [Google Scholar] [CrossRef]
- Diaz-Zaragoza, M.; Hernandez-Avila, R.; Govezensky, T.; Mendoza, L.; Meneses-Ruiz, D.M.; Ostoa-Saloma, P. Comparison patterns of 4 T1 antigens recognized by humoral immune response mediated by IgG and IgM antibodies in female and male mice with breast cancer using 2D-immnunoblots. Immunobiology 2015, 220, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Galvez-Cancino, F.; Oyarce, C.; Contreras, F.; Prado, C.; Valeria, C.; Cruz, S.; Lladser, A.; Pacheco, R. Inhibition of dopamine receptor D3 signaling in dendritic cells increases antigen cross-presentation to CD8+ T-cells favoring anti-tumor immunity. J. Neuroimmunol. 2017, 303, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ochioni, A.C.; Imbroisi Filho, R.; Esteves, A.M.; Leandro, J.G.B.; Demaria, T.M.; do Nascimento Júnior, J.X.; Pereira-Dutra, F.S.; Bozza, P.T.; Sola-Penna, M.; Zancan, P. Clotrimazole presents anticancer properties against a mouse melanoma model acting as a PI3K inhibitor and inducing repolarization of tumor-associated macrophages. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166263. [Google Scholar] [CrossRef] [PubMed]
- Naeimi Kararoudi, M.; Tullius, B.P.; Chakravarti, N.; Pomeroy, E.J.; Moriarity, B.S.; Beland, K.; Colamartino, A.B.L.; Haddad, E.; Chu, Y.; Cairo, M.S.; et al. Genetic and epigenetic modification of human primary NK cells for enhanced antitumor activity. Semin. Hematol. 2020, 57, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-y.; Dai, K.-y.; Liu, C.; Yu, J.; Liu, A.-j.; Chen, Y.-f. The ethanol-extracted polysaccharide from Cynanchum paniculatum: Optimization, structure, antioxidant and antitumor effects. Ind. Crops Prod. 2022, 175, 114243. [Google Scholar] [CrossRef]
- Morbach, H.; Schickel, J.N.; Cunningham-Rundles, C.; Conley, M.E.; Reisli, I.; Franco, J.L.; Meffre, E. CD19 controls Toll-like receptor 9 responses in human B cells. J. Allergy Clin. Immunol. 2016, 137, 889–898.E6. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, M.C.; Bartol, S.J.; Driessen, G.J.; Mascart, F.; Reisli, I.; Franco, J.L.; Wolska-Kusnierz, B.; Kanegane, H.; Boon, L.; van Dongen, J.J.; et al. Human CD19 and CD40L deficiencies impair antibody selection and differentially affect somatic hypermutation. J. Allergy Clin. Immunol. 2014, 134, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ostoa-Saloma, P. Chapter 6—The IgM as a tool for recognition of early tumoral antigens. In Immunotherapy in Resistant Cancer: From the Lab Bench Work to Its Clinical Perspectives; Morales-Montor, J., Segovia-Mendoza, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 2, pp. 91–101. [Google Scholar]
- Yang, S.; Cui, M.; Liu, Q.; Liao, Q. Glycosylation of immunoglobin G in tumors: Function, regulation and clinical implications. Cancer Lett. 2022, 549, 215902. [Google Scholar] [CrossRef]
- Jones, P.C. Does a “thiol shield” protect tumors from natural IgM antibody, and, if so, how can it be suppressed? Med. Hypotheses 2013, 80, 425–430. [Google Scholar] [CrossRef]
- Humar, R.; Schaer, D.J.; Vallelian, F. Erythrophagocytes in hemolytic anemia, wound healing, and cancer. Trends Mol. Med. 2022, 28, 906–915. [Google Scholar] [CrossRef]
- Khusnurrokhman, G.; Wati, F.F. Tumor-promoting inflammation in lung cancer: A literature review. Ann. Med. Surg. 2022, 79, 104022. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-d.; Liu, Y.-n.; Zhao, Y.; Liu, A.-j.; Ji, H.-y.; Yu, J. Structural characterization of a water-soluble polysaccharide from Angelica dahurica and its antitumor activity in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 193, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Lou, X.; Jiao, J.; Li, Y.; Dai, K.; Jia, X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules 2023, 28, 1561. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, R.; Wei, C.; Li, D.; Gao, X. The role of IL-17 in lung cancer growth. Cytokine 2023, 169, 156265. [Google Scholar] [CrossRef] [PubMed]
- Cebi, M.; Cakar, A.; Erdogdu, E.; Durmus-Tekce, H.; Yegen, G.; Ozkan, B.; Parman, Y.; Saruhan-Direskeneli, G. Thymoma patients with or without myasthenia gravis have increased Th17 cells, IL-17 production and ICOS expression. J. Neuroimmunol. 2023, 381, 578129. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Torki, Z.; Zalpoor, H.; Habibi, M.; Pornour, M. Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives. Mol. Ther.—Oncolytics 2023, 28, 132–157. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.L.; Temesgen, A.; Lynch, L. Diet, nutrient supply, and tumor immune responses. Trends Cancer 2023, 9, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, J.; Chen, J.; Wang, Z.; Song, B.; Li, R.; Zhong, S.; Cheong, K.-L. The effect mechanism of polysaccharides inhibit tumor immune escape: A review. J. Funct. Foods 2023, 107, 105638. [Google Scholar] [CrossRef]
- Jiménez-Chávez, Á.d.J.; Nava-García, B.K.; Bustos-Jaimes, I.; Moreno-Fierros, L. B19-VLPs as an effective delivery system for tumour antigens to induce humoral and cellular immune responses against triple negative breast cancer. Immunol. Lett. 2021, 239, 77–87. [Google Scholar] [CrossRef]
- Yu, J.; Dong, X.-d.; Jiao, J.-s.; Yu, S.-s.; Ji, H.-y.; Liu, A.-j.; Chen, Y. Extraction, purification, and biological activities in vivo of a novel fructose-rich polysaccharide from Codonopsis pilosula. Ind. Crops Prod. 2022, 176, 114309. [Google Scholar] [CrossRef]
- Li, N.; Xiong, Y.X.; Ye, F.; Jin, B.; Wu, J.J.; Han, M.M.; Liu, T.; Fan, Y.K.; Li, C.Y.; Liu, J.S.; et al. Isolation, Purification, and Structural Characterization of Polysaccharides from Codonopsis pilosula and Their Anti-Tumor Bioactivity by Immunomodulation. Pharmaceuticals 2023, 16, 895. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-B.; Zhang, Y.-J.; Fan, J.-M.; Jia, X.-S.; Li, D.; Wang, Y.-P.; Zhou, J.; Yan, Q.; Hu, F.-D. Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food Funct. 2020, 11, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-L.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Dong, C.-X.; Du, J. Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 130, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, X.; Ji, H.; Yu, J.; Liu, A. Preparation and structural characterization of acid-extracted polysaccharide from Grifola frondosa and antitumor activity on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2023, 234, 123302. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Nam, M.-W.; Go, R.-E.; Koo, J.; Kim, T.H.; Park, J.-E.; Choi, K.-C. TGF-β2 antisense oligonucleotide enhances T-cell mediated anti-tumor activities by IL-2 via attenuation of fibrotic reaction in a humanized mouse model of pancreatic ductal adenocarcinoma. Biomed. Pharmacother. 2023, 159, 114212. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, J.; Wang, Q.; He, Z.; Sun, T.; Yao, Y.; Wang, W.; Shen, P. Pretreatment of umbilical cord derived MSCs with IFN-γ and TNF-α enhances the tumor-suppressive effect on acute myeloid leukemia. Biochem. Pharmacol. 2022, 199, 115007. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.J.; Avery, D.T.; Ma, C.S.; Moens, L.; Deenick, E.K.; Bustamante, J.; Boisson-Dupuis, S.; Wong, M.; Adelstein, S.; Arkwright, P.D.; et al. IL-21 signalling via STAT3 primes human naïve B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood 2013, 122, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Zeng, Q.; Xu, Z.; Zhang, H.; Qin, S.; Liu, C.; Xu, C.; Qian, Z.; Zhang, S.; Huang, S.; et al. IL-2, IL-4, IFN-γ or TNF-α enhances BAFF-stimulated cell viability and survival by activating Erk1/2 and S6K1 pathways in neoplastic B-lymphoid cells. Cytokine 2016, 84, 37–46. [Google Scholar] [CrossRef]
- Wang, R.; Hua, Y.; Wu, H.; Wang, J.; Xiao, Y.-c.; Chen, X.; Ao, Q.; Zeng, Q.; Zhu, X.; Zhang, X. Hydroxyapatite nanoparticles promote TLR4 agonist-mediated anti-tumor immunity through synergically enhanced macrophage polarization. Acta Biomater. 2023, 164, 626–640. [Google Scholar] [CrossRef]
- Li, G.-Y.; Feng, Y.-Q.; Jia, Y.-F.; Wang, K.-F.; Li, Y.; Zhang, S.-J.; Han, S.-X.; Wang, J.-C. Metformin enhances T lymphocyte anti-tumor immunity by increasing the infiltration via vessel normalization. Eur. J. Pharmacol. 2023, 944, 175592. [Google Scholar] [CrossRef]
- Song, A.; Ding, T.; Wei, N.; Yang, J.; Ma, M.; Zheng, S.; Jin, H. Schisandrin B induces HepG2 cells pyroptosis by activating NK cells mediated anti-tumor immunity. Toxicol. Appl. Pharmacol. 2023, 472, 116574. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2021, 268, 120537. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, X.; Shang, Z.; Ji, Y.; Wang, H.; Wang, Z.; Wang, P.; Zhang, Y.; Xiao, H. N-glycan structures of target cancer biomarker characterized by two-dimensional gel electrophoresis and mass spectrometry. Anal. Chim. Acta 2020, 1123, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.-d.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Fan, Y.; Gao, X.; Gong, Y.; Dai, K.; Wang, Z.; Xu, B.; Yu, J. The Protective Effects of Water-Soluble Alginic Acid on the N-Terminal of Thymopentin. Molecules 2023, 28, 6445. [Google Scholar] [CrossRef] [PubMed]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-y.; Liu, C.; Dai, K.-y.; Yu, J.; Liu, A.-j.; Chen, Y.-f. The immunosuppressive effects of low molecular weight chitosan on thymopentin-activated mice bearing H22 solid tumors. Int. Immunopharmacol. 2021, 99, 108008. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.-y.; Liu, C.; Liu, A.-j. The structural characteristics of an acid-soluble polysaccharide from Grifola frondosa and its antitumor effects on H22-bearing mice. Int. J. Biol. Macromol. 2020, 158, 1288–1298. [Google Scholar] [CrossRef]


| Measurement | Unit | Reference Value | Blank Group | Model Group | CPG-H Group |
|---|---|---|---|---|---|
| Leucocyte count | 109/L | 4–12 | 6.97 ± 1.26 | 13.53 ± 1.46 b | 9.33 ± 1.53 c |
| Lymphocyte percentage | % | 54–85 | 76.31 ± 5.24 | 34.08 ± 2.21 b | 65.37 ± 4.42 c |
| Intermediate cell percentage | % | 0–9 | 1.35 ± 0.22 | 1.59 ± 0.23 | 1.62 ± 0.24 |
| Neutrophil percentage | % | 12–44 | 22.32 ± 2.13 | 64.34 ± 3.21 b | 33.03 ± 2.65 c |
| Erythrocyte count | 1012/L | 6–12.5 | 8.81 ± 0.53 | 7.42 ± 0.52 b | 8.70 ± 0.63 c |
| Hemoglobin | g/L | 100–190 | 173.35 ± 9.83 | 137.68 ± 8.99 b | 166.43 ± 9.21 c |
| Mean corpuscular volume | fL | 51–65 | 58.70 ± 4.83 | 59.00 ± 4.35 | 58.46 ± 4.13 |
| Hematocrit | L/L | 0.35–0.55 | 0.47 ± 0.03 | 0.40 ± 0.02 | 0.47 ± 0.03 |
| Mean corpuscular hemoglobin | Pg | 12–30 | 21.80 ± 1.83 | 20.13 ± 0.99 | 21.23 ± 1.12 |
| Mean corpuscular hemoglobin concentration | g/L | 230–330 | 373.30 ± 21.12 | 342.95 ± 24.56 b | 364.75 ± 23.47 c |
| Red cell distribution width | fL | 15–55 | 42.41 ± 4.56 | 41.94 ± 3.96 | 42.41 ± 5.12 |
| Platelet count | 109/L | 100–300 | 333.40 ± 14.26 | 455.21 ± 21.34 b | 368.32 ± 22.88 c |
| Mean platelet volume | fL | 6.1–12 | 7.42 ± 1.56 | 7.93 ± 1.52 | 7.98 ± 1.82 |
| Plateletcrit | L/L | 0.1–0.6 | 0.23 ± 0.01 | 0.33 ± 0.03 b | 0.27 ± 0.02 c |
| Platelet distribution width | % | 1–30 | 14.89 ± 1.21 | 14.89 ± 1.42 | 14.87 ± 1.31 |
| Group | Macrophage Pinocytosis | Lymphocyte Proliferation Capacity | NK Cell Killing Activity | |
|---|---|---|---|---|
| OD550 nm Value | Canavalin A (Con A) | Lipopolysaccharide (LPS) | (%) | |
| Blank group | 0.76 ± 0.04 | 4.20 ± 0.35 | 6.09 ± 0.31 | 60.52 ± 4.05 |
| Model group | 0.31 ± 0.02 b | 2.47 ± 0.13 b | 3.95 ± 0.23 b | 36.13 ± 2.08 b |
| CPG-H group | 0.71 ± 0.05 c | 3.87 ± 0.29 c | 5.49 ± 0.37 c | 63.31 ± 3.79 c |
| No. | Accession | Name | Nominal Mass (Mr) | Calculated pI Value | Expression Compared with Model Group |
|---|---|---|---|---|---|
| 1 | P07901 | Heat shock protein HSP 90-alpha | 85,134 | 4.97 | Upregulation |
| 2 | P11499 | Heat shock protein HSP 90-beta | 83,571 | 4.97 | Upregulation |
| 3 | P07724 | Albumin | 70,700 | 5.75 | Upregulation |
| 4 | P63038 | 60 kDa heat shock protein | 61,088 | 5.91 | Upregulation |
| 5 | P20152 | Vimentin | 53,712 | 5.06 | Downregulation |
| 6 | P48036 | Annexin A5 | 35,787 | 4.83 | Downregulation |
| 7 | P16015 | Carbonic anhydrase 3 | 29,633 | 6.89 | Upregulation |
| 8 | O70250 | Phosphoglycerate mutase 2 | 28,980 | 8.65 | Downregulation |
| 9 | Q62422 | Osteoclast-stimulating factor 1 | 23,996 | 5.46 | Upregulation |
| 10 | Q9R0Q7 | Prostaglandin E synthase 3 | 18,995 | 4.36 | Downregulation |
| 11 | Q60605 | Myosin light polypeptide | 16,930 | 4.40 | Upregulation |
| 12 | Q9Z0J0 | NPC intracellular cholesterol transporter 2 | 16,774 | 7.59 | Downregulation |
| 13 | P31725 | Protein S100-A9 | 13,211 | 6.64 | Downregulation |
| 14 | P63166 | Small ubiquitin-related modifier 1 | 11,607 | 5.35 | Downregulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Long, Y.; Gong, Y.; Gao, X.; Zheng, G.; Ji, H. Systemic Immunomodulatory Effects of Codonopsis pilosula Glucofructan on S180 Solid-Tumor-Bearing Mice. Int. J. Mol. Sci. 2023, 24, 15598. https://doi.org/10.3390/ijms242115598
Fan Y, Long Y, Gong Y, Gao X, Zheng G, Ji H. Systemic Immunomodulatory Effects of Codonopsis pilosula Glucofructan on S180 Solid-Tumor-Bearing Mice. International Journal of Molecular Sciences. 2023; 24(21):15598. https://doi.org/10.3390/ijms242115598
Chicago/Turabian StyleFan, Yuting, Yan Long, Youshun Gong, Xiaoji Gao, Guoqiang Zheng, and Haiyu Ji. 2023. "Systemic Immunomodulatory Effects of Codonopsis pilosula Glucofructan on S180 Solid-Tumor-Bearing Mice" International Journal of Molecular Sciences 24, no. 21: 15598. https://doi.org/10.3390/ijms242115598
APA StyleFan, Y., Long, Y., Gong, Y., Gao, X., Zheng, G., & Ji, H. (2023). Systemic Immunomodulatory Effects of Codonopsis pilosula Glucofructan on S180 Solid-Tumor-Bearing Mice. International Journal of Molecular Sciences, 24(21), 15598. https://doi.org/10.3390/ijms242115598







