Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies
Abstract
:1. Introduction
| Fasting Paradigms | Characteristics |
|---|---|
| Alternate Day Fasting (ADF) | Ad libitum feeding cycles for 24 h alternated with 24 h of total fasting (no caloric intake). Modified alternate day fasting (25% of caloric needs maintained; consumption of approximately 500 kcal on “fasting” days) is the predominant type of IF in the literature [30,31] |
| Two Days Fasting | Two consecutive or non-consecutive “fast” days, followed by five days of ad libitum food (a 2-day fast is popularly known as a 5:2 fast) [32]. Fasting days can be either full fasting or keeping to 25% of caloric needs. Also, they can be consecutive or non-consecutive [31,32]. |
| Time-Restricted Feeding (TRF) | This type of fast limits food intake to a daily eating window of 4–10 h, promoting a fasting period of 14–18 h. This intervention reduces the opportunity to eat, tending to reduce food intake. However, it does not necessarily imply caloric restriction [33]. An early restriction of the feeding window involves shifting the feeding window to an earlier part of the day, from morning to mid- or late afternoon, aligning feeding periods with the body’s circadian rhythm [32]. |
| Religious Fasting | Fasting is an ancestral practice, present in different religions. Although religious fasts have spiritual purposes, they can also impact physical and mental health in different ways [33]. The most extensively studied religious fast for health effects is the Ramadan fast (JR). Ramadan is one of the five pillars of Islam and occurs during the ninth month of the Islamic lunar calendar, in which healthy adult Muslims abstain from the consumption of food and fluids from dawn (el fajr) until sunset, for approximately 30 days. The duration of the daytime fast varies and is significantly impacted by location and season. In general, the typical duration of the fasting period is 10 h, but it can exceed 18 h [34]. |
2. Methods
2.1. Search Strategy
2.2. Study Selection
- Included clinical intervention studies on human populations of any age group in which a diagnosis of depression or the presence of depressive symptoms had been assessed using standardized instruments.
- Prospective or retrospective cohort population studies evaluating the association between feeding windows and/or mealtimes and affective symptoms, and their possible underlying biological markers.
- Preclinical studies evaluating the physiological effect of intermittent fasting strategies in animal models of depression or behavioral activation.
2.3. Data Extraction
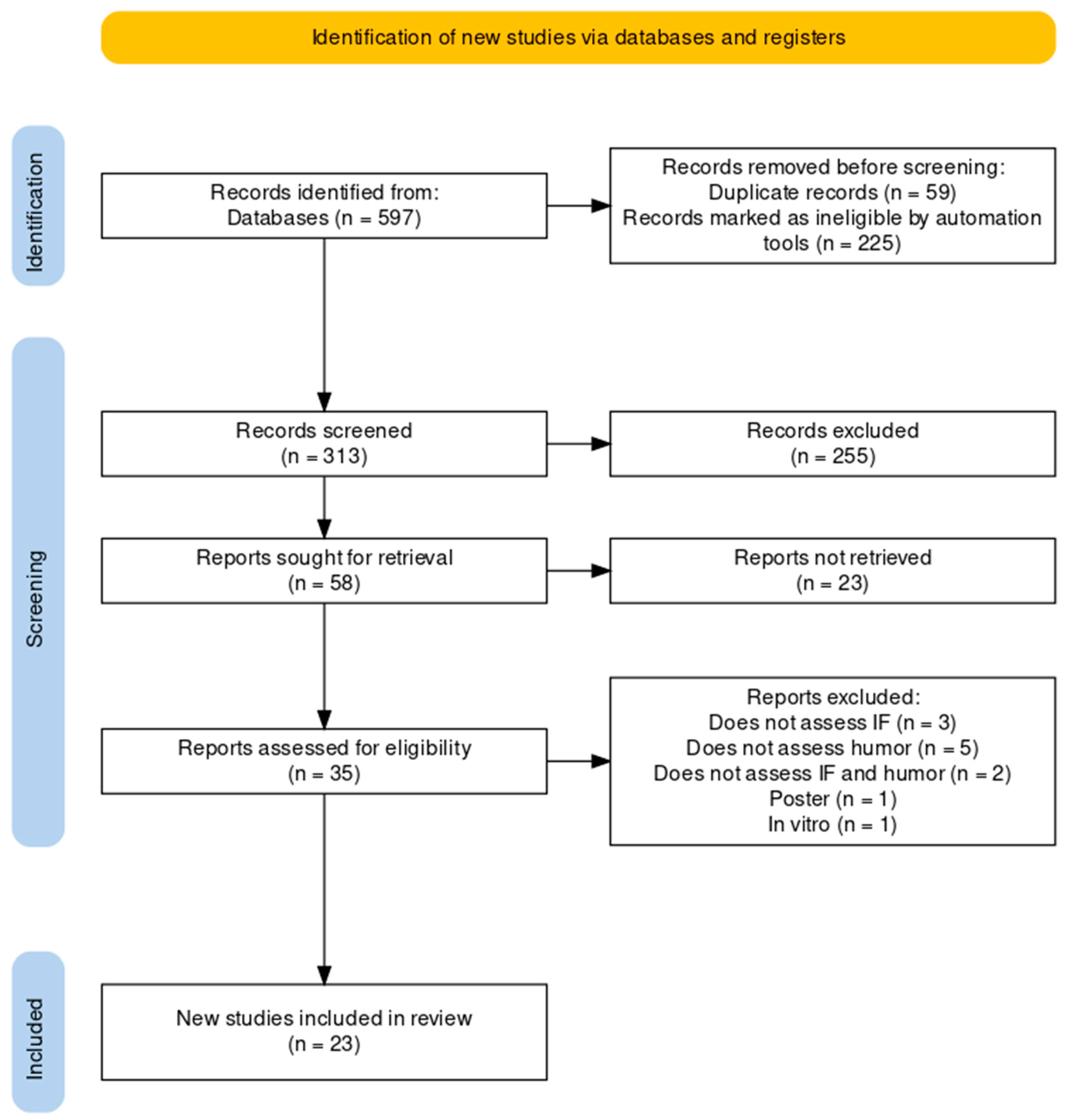
3. Results
- a.
- Description of animal models studies included
- b.
- Description of humans studies included
| Study | Animal | Behavior Models | Experimental Design | Additional Measures | Main Results |
|---|---|---|---|---|---|
| [36] | Male mice | Run to exhaustion test | Six groups: ADF DF, CR, HFD ad libitum, standardized rodent chow (control) for 8 weeks. | Glucose tolerance, OGTT test, MRI | Except for the HFD group, all other groups showed improvement in body composition, physical disposition (longer running time until exhaustion), and insulin sensitivity (reduced fasting glucose and HOMA-IR). |
| [37] | Healthy and DM2 male rats | FST and EPMT | Four groups: Group I (healthy rats) ad libitum diet (controls); Group II (healthy rats) 18 h daily fasting (14:00–8:00 h) for 3 months; Group III (DM2 induction) with a high-fat diet for 4 weeks followed by a single intraperitoneal injection of streptozotocin; and Group IV DM2 rats exposed to IF for 3 months. | BDNF, neurotrophin 3 (NT3), serotonin, dopamine, and glutamate levels in the hippocampus | It was observed that IF reduced anxiety and depression and increased neurogenesis markers BDNF and NT3 in the hippocampus, both in the control group and in DM2 rats; IF improved metabolic dysfunction and reduced corticosterone levels in rats with DM2. |
| [38] | Male mice | FST and OFT | Five groups: without fasting (negative control) versus 3 h of fasting versus 9 h of fasting versus 18 h of fasting, with the use of imipramine. | Brain levels of the transcription factor CREB and its phosphorylated form (CREB-p); possible involvement of 5-HT2 receptors was examined using the HT2A/2C receptor agonist DOI | Fasting for 9 h, but not for 3 h or 18 h, significantly reduced the immobility time without changing the locomotor activity in the co-administration of 9 h of fasting with imipramine produced an additive antidepressant effect, in addition to an increase in the p-CREB/CREB ratio; these effects were partially reversed using DOI. |
| [39] | Male mice | MWM | Three groups: ADF, CR or ad libitum (control), for three months. | MWM (for assessment of learning and spatial memory); neuroblast count (doublecortin-positive cells); and Klotho gene expression in the hippocampal dentate gyrus of mice | It was observed that ADF was superior to CR in increasing hippocampal neurogenesis and inducing Klotho gene expression and was also superior to CR in improving spatial memory. |
| [40] | Male rats | SLA, drinking and eating behavior | Three groups: rats fed ad libtum diet or 2 h/d during light (TRF) and with or without colchicine induced VMH disruption. | Rhythmicity of variations in temperature, food, water consumption, corticosterone level, and cellular expression of c-Fos in the hypothalamus during the light/dark phase | There was a reduction in SLA, an increase in food consumption, water consumption, body weight, and insulin levels in the group with VMH disruption by colchicine; TRF induced the VMH rhythm (assessed by increased c-Fos activity) and reduced food intake and weight in the group with VMH disruption by colchicine; it was observed that the VMH acts as an amplifier of stimuli coming from the SCN. |
| [41] | Male rats | SLA | Two groups: rats with hereditary microphthalmia and normal rats (control), submitted to TRF (6 h during the light period). | Pineal serotonin N-acetyltransferase | Rats with hereditary microphthalmia maintained the ability to alternate and synchronize circadian phases when subjected to FRT, even with loss of the optic nerve (and light-induced circadian clock desynchronization); a peak of pineal enzyme activity was observed during the feeding period in the blind rats. |
| [43] | Male rats | OFT, sucrose preference, novel object recognition test | Two groups: shiftwork (wakefulness and forced activity through rotating drums, to which the rats were submitted for 8 h) and ad libitum access to food (W-AL) and shift-work and TRF during the active phase (W-TRF). | Body weight, food consumption, cell markers of astrocytes (GFAP) and microglia (IBA-1) in different brain structures | It was observed that TRF prevented the occurrence of depressive and anxious behaviors in addition to reducing neuroinflammation markers (induced by the shift-work experimental condition). |
| [43] | Male rats | OFT, EPMT, Splash Test | Four groups: sedentary controls (SC), trained controls (TC), sedentary + IF (SIF), and trained + IF (TIF), evaluated for four weeks; intervention: 3 p.m. TRF (5 p.m.–8 a.m.); controls: ad libitum diet for 9 h (8 h–17 h), for 28 days. | Adiposity index, analysis of fecal microbiota, quantification of organic acids in the intestine (colon) and feces, and dosage of IL-1 β in the hippocampus | There was a reduction in anxious behavior and an increase in IL-1β markers in the TIF group. IF and aerobic exercises, associated or not, modulated parameters related to GMBA, such as an improvement in anxiety and depression and quantification of organic acids in the intestine and feces, but they did not act synergistically. |
| Study | Design | Segment | Participants | Intervention or IF Paradigm | Assessment Tools | Main Results |
|---|---|---|---|---|---|---|
| [44] | CS | 28 days | 10 healthy men (age: 20–28 years) | RF | Oral temperature (as a circadian assessment measure); CFF; VAS; Choice Reaction Time, administered 6 times a day: at 09:00, 11:00, 13:00, 16:00, 20:00, and 23:00 on the 6th, 15th, and 28th days | There was a reduction in oral temperature during the day and an increase at night; there was a worsening of the subjective state of alertness and mood (VAS) during the RF. |
| [45] | CS | 7 weeks | 10 healthy men (age: 22.06 ± 1.98 years) | RF | VAL, POMS, one week before the RF, in the 3rd week, 4th week, and 2 months after | No differences in neuromuscular efficiency and resting twitch potential; higher depression, fatigue, and anxiety (POMS) scores were observed during RF. |
| [46] | CS | 6 weeks | 34 healthy subjects (19 men, age 24.8 ± 1.0 years; 15 women, age: 25.5 ± 1.20 years) | RF | FSS, ESS, BDI-II, HADS | An improvement in morning fatigue was observed in both genders; improvement in mood and depressive symptoms in men (HADS and BDI-II); and significant reduction in muscle mass and body water in women. |
| [47] | CS | 1 week of usual diet followed by 4 weeks of intervention (RF) and reassessment 1 month after the end of the intervention | 34 healthy subjects (19 men, age 24.8 ± 1.0; 15 women: age 25.5 ± 1.2 years) | RF | BDI-II, HADS-A/D, QoL, FSS, ESS scales; Cortisol, BDNF, IL-8, MMP-9, IGF-1, and myoglobin levels in the blood; assessments were performed one week before, during, and up to 1 month after the RF | There was a significant reduction in BDNF levels during and up to 1 week after RF, which correlated positively with the HADS-Dscore; only in women was there a significant reduction in cortisol after 1 week of RF that correlated positively with the score on the BDI-II. |
| [48] | RCT | 4 weeks | 100 men with MDD: Intervention group (n = 50, age: 43.38 ± 10.06 years), control group (n = 50, age: 46.54 ± 8.35) | RF | PHQ-9, IPAQ-SF, plasma lipid levels, and blood pressure | There was no difference in depressive symptoms (PHQ-9); there was a reduction in weight, BMI, and body fat. |
| [49] | CT | 8 weeks of intervention and reassessment 4 months after the end of the intervention (6 months of follow-up) | 36 healthy subjects (33 included in the final data); intervention group: n = 22 (women: 15, men: 7; age: 42.45 ± 10.82 years); control group: n = 14 (women: 7, men: 7; age: 41.36 ± 12.63) | Intervention: 24 h fasting once a week, with abstinence from solid food and maximum caloric intake of 300 kcal; control: advice on healthy diet | Insulin, glucose, HOMA, BDNF, HbA1C, LDL-C, HDL-C, triglycerides), coagulation markers, liver enzymes, IGF-1, BDNF, blood pressure, waist circumference, BMI, % fat; WHO-5, HADS, POMS, FS, and VAS | There was an improvement in general symptoms of mood and depression (HADS)—as well as in insulin response—over the 8 weeks of intervention in both groups, with no significant difference between them; no differences were observed in BDNF levels; the IF group only showed a greater reduction in BF and alkaline phosphatase compared to the controls. |
| [50] | RCT | 8 weeks | 46 healthy overweight or obese women (age 50 ± 9 years, BMI 32.9 ± 4.4 kg/m2 | 24 h IF every other day (3×/week) associated with CR (70% reduction in energy requirement) compared to CR without IF (control group) | TFEQ, DASS, PSQI, AQoL, and SF-36 | Weight reduction was observed in the IF + CR group; no differences were observed between groups regarding perceived food consumption (TFEQ), mood (DASS), AQoL, sleep quality (PSQI), and cognition (SF-36). |
| [51] | RCT | 5 days | 11 sedentary men (Age 38 ± 5 years; BMI: 32.1 ± 2.1 kg/m2) | 5 days of an isoenergetic diet, with 3 meals a day between 10 a.m. and 5 p.m. (TRF; 8 h eating window) or between 7 a.m. and 9 p.m. (diet with no restriction on eating window) | PANAS and VAS | No differences in mood were observed; an increase in fatigue was observed in both conditions (PANAS and VAS). |
| [52] | CS | 2 weeks of usual diet followed by 4 weeks of intervention | 19 participants with DM2 (9 men, age 48.7 ± 10 years; 10 women, 51.6 ± 8 years) | TRF (9 h ad libitum feeding window) | DASS, AQol, PSQI, CBB, and CIA | No difference in measures of depression, anxiety, and perceived stress (DASS and AQol); there was an improvement in executive functions, specifically in the Groton Maze Learning Task. |
| [53] | RCT | 14 weeks | 90 obese subjects (age 43 ± 1 years; BMI, 39.6 ± 6.7 kg/m2); intervention with 35 women and 10 men versus control with 37 women and 8 men | TRF (8 h/day, from 7 a.m. to 3 p.m.); control: 12 h feeding window (7 a.m.–7 p.m.) | BPAQ, POMS-SF, PHQ-9, MCTQ, PSQI, and 5-point Likert scale; fasting blood pressure, glucose levels, insulin, insulin resistance (HOMA-IR), β-cell HOMA, hemoglobin A1c level, and plasma lipid levels; 3 days of food recall | There was an improvement in fatigue and depressive symptoms in the intervention group (POMS-SF); early TRF was more efficient in weight loss (with an additional loss of 2.3 kg). |
| [54] | RCT | 12 weeks | Healthy men: intervention group (n = 16, age: 59.7 ± 6.6 years; BMI = 26.7 ± 1.8) versus control (n = 15 (Age = 59.7 ± 6.2 years; BMI = 26.5 ± 2.7) | 24 h fasting (on Mondays and Thursdays) associated with restriction of 300 to 500 kcal/day in relation to the participants’ baseline energy intake | POMS, BDI-II, GDS-15, food recall, and fasting record at the beginning of the intervention and at weeks 06 and 12 | A reduction in levels of tension, irritability, and confusion, and subjective improvement in mood (POMS) were observed; there was no difference in scores on the BDI-II and GDS-15 scales; weight, BMI, and fat percentage were reduced by 3.8%, 3.7%, and 5.7%, respectively. |
| [55] | CT | 8 weeks | 36 individuals with multiple sclerosis divided into 3 groups: control (n = 12; men: 3, women: 9, mean age: 33.3 ± 7); daily CR (n = 12; men: 2, women: 10, mean age: 40.5 ± 5.4); and intermittent CR (n = 12; men: 2, women: 10, mean age: 38.5 ± 7.4) | Control diet (100% of caloric requirement 7 days/week), daily CR (78% of caloric requirement, 7 days/week), and intermittent CR (“5:2” style diet (25% of daily caloric requirement for 2 consecutive days per week; 100% of daily caloric needs 5 days a week) | FAMS and PSQI, at baseline and during the 4th and 8th week | Daily and intermittent CR were associated with better emotional well-being and lower rates of depressive symptoms (FAMS); There was also a marginal benefit in weight loss. |
| [56] | RCT | 12 weeks | 26 pre-diabetic individuals; intervention: 6 men, 9 women (age: 52.9 ± 8.7 years); placebo: 2 males, 9 females, (age: 54.1 ± 6.4 years) | PROFAST: IF 2 days/week (non-consecutive) associated with a daily reduction of 600–650 calories and increased use of the probiotic Lacticaseibacillus rhamnosus HN001 (n = 15) or placebo (n = 11) | PHQ-9, GAD, and EDQ | Improvement in depressive (PHQ-9) but not anxiety (GAD) symptoms was observed in the total sample (both groups combined) over time. |
| [57] | RCT | 12 weeks | 34 young adults: intervention (n = 17; 9 men, 8 women; age: 24.7 ± 4.8 years), control (n = 17; 8 men, 9 women; age: 23.2 ± 3.9); average BMI: 27.0 kg/m2, average age: 23.9 years | Intervention: fasting for two consecutive days per week (5:2) with CR of 80% of energy requirement associated with resistance exercises; control: CR of 20% during the entire intervention period associated with resistance exercises | Diet adherence (self-reported), post-intervention intention survey assessing mood, hunger, and satiety levels; level of plasma lipids, glucose, insulin, CRP, and HOMA-IR | There was a positive correlation between mood and adherence in both groups; improvement in the feeling of hunger and bad mood on non-fasting days; high rate of adherence to diet (~80%) and low levels of hunger in both groups; reduction in LDL-c and HDL-c in both groups. There was no difference in markers of glycemic regulation. |
| [58] | Retrospective cohort | No follow-up | 1572 adults divided into 2 groups: TRF < 8 h (n = 120; men: 47 ± 39.2; women: 73 ± 60.8, age: 55 ± 14.8) vs. > 8 h (n = 1452; men: 613 ± 42.2, mean age: 45.8 ± 17.1; women: 839 ± 57.8; mean age: 45.8 ± 17.1) | TRF | Food frequency questionnaires; PSQI; PSS; and CES-D-10 | There was no significant difference in stress levels (PSS), sleep quality (PSQI), and depressive symptoms (CES-D-10) between groups. |
4. Discussion
4.1. Studies Assessing IF in Animal Models of Mood Disorders
4.2. Studies Assessing IF in Human
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease (GBD). Disease and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (accessed on 25 December 2022).
- World Health Organization (WHO). World Health Organization (WHO). World Mental Health Report. In Transforming Mental Health for All; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240049338 (accessed on 25 December 2022).
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Howland, R.H. Sequenced Treatment Alternatives to Relieve Depression (STAR*D). Part 2: Study outcomes. J. Psychosoc. Nurs. Ment. Health Serv. 2008, 46, 21–24. [Google Scholar] [CrossRef]
- Moriarty, A.S.; Castleton, J.; Gilbody, S.; McMillan, D.; Ali, S.; Riley, R.D.; A Chew-Graham, C. Predicting and preventing relapse of depression in primary care. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2020, 70, 54–55. [Google Scholar] [CrossRef]
- Sarris, J.; Thomson, R.; Hargraves, F.; Eaton, M.; de Manincor, M.; Veronese, N.; Solmi, M.; Stubbs, B.; Yung, A.R.; Firth, J. Multiple lifestyle factors and depressed mood: A cross-sectional and longitudinal analysis of the UK Biobank (N = 84,860). BMC Med. 2020, 18, 354. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2020, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Shadrina, M.; Bondarenko, E.A.; Slominsky, P.A. Genetics Factors in Major Depression Disease. Front. Psychiatry 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, E.; Lichtenstein, P.; Larsson, H.; Song, J.; Agrawal, A.; Børglum, A.D.; Bulik, C.M.; Daly, M.J.; Davis, L.K.; Demontis, D.; et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol. Med. 2019, 49, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Amare, A.T.; O Schubert, K.; Klingler-Hoffmann, M.; Cohen-Woods, S.; Baune, B.T. The genetic overlap between mood disorders and cardiometabolic diseases: A systematic review of genome wide and candidate gene studies. Transl. Psychiatry 2017, 7, e1007. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Rev. Bras. Psiquiatr. 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- E Gangwisch, J.; Hale, L.; Garcia, L.; Malaspina, D.; Opler, M.G.; E Payne, M.; Rossom, R.C.; Lane, D. High glycemic index diet as a risk factor for depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015, 102, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; Van Der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. The effect of a Mediterranean diet on the symptoms of depression in young males (the AMMEND: A Mediterranean Diet in MEN with Depression study): A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 572–580. [Google Scholar] [CrossRef]
- Dietch, D.M.; Kerr-Gaffney, J.; Hockey, M.; Marx, W.; Ruusunen, A.; Young, A.H.; Berk, M.; Mondelli, V. Efficacy of low carbohydrate and ketogenic diets in treating mood and anxiety disorders: Systematic review and implications for clinical practice. BJPsych Open 2023, 9, e70. [Google Scholar] [CrossRef]
- Vemuganti, R.; Arumugam, T.V. Molecular Mechanisms of Intermittent Fasting-induced Ischemic Tolerance. Cond. Med. 2020, 3, 9–17. [Google Scholar] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef]
- Prada, G.; Díaz-Gomes, J.L. Effects of Intermittent Fasting on Health, Aging, And Disease. N. Engl. J. Med. 2020, 381, 2541–2551. [Google Scholar] [CrossRef]
- Berthelot, E.; Etchecopar-Etchart, D.; Thellier, D.; Lancon, C.; Boyer, L.; Fond, G. Fasting Interventions for Stress, Anxiety and Depressive Symptoms: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, R.; Martínez-Vizcaíno, V.; E Mesas, A.; Notario-Pacheco, B.; Medrano, M.; Heilbronn, L.K. Does intermittent fasting impact mental disorders? A systematic review with meta-analysis. Crit. Rev. Food Sci. Nutr. 2022; in press. [Google Scholar] [CrossRef]
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Eckel, R.H.; Kahn, R.; Robertson, R.M.; Rizza, R.A. Preventing cardiovascular disease and diabetes: A call to action from the American Diabetes Association and the American Heart Association. Circulation 2006, 113, 2943–2946. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Does Timing Matter? A Narrative Review of Intermittent Fasting Variants and Their Effects on Bodyweight and Body Composition. Nutrients 2022, 14, 5022. [Google Scholar] [CrossRef]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Bloomer, R.J. The impact of religious fasting on human health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef]
- Cherif, A.; Roelands, B.; Meeusen, R.; Chamari, K. Effects of Intermittent Fasting, Caloric Restriction, and Ramadan Intermittent Fasting on Cognitive Performance at Rest and During Exercise in Adults. Sports Med. 2016, 46, 35–47. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.G.; Caldwell, J.L.; van der Merwe, M.; Sharma, S.; Butawan, M.; Puppa, M.; Bloomer, R.J. A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice. Nutrients 2019, 11, 350. [Google Scholar] [CrossRef]
- Elesawy, B.H.; Raafat, B.M.; Al Muqbali, A.; Abbas, A.M.; Sakr, H.F. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sci. 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, J.; Lv, J.; Tang, F.; Liu, L.; Sun, Z.; Wang, L.; Siwela, S.P.; Wang, Y.; Song, Y.; et al. Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Murphy, T.; Stangl, D.; Ahmet, S.; Morisse, B.; Nix, A.; Aimone, L.J.; Aimone, J.B.; Kuro-O, M.; Gage, F.H.; et al. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol. Psychiatry 2021, 26, 6365–6379. [Google Scholar] [CrossRef]
- Choi, S.; Wong, L.S.; Yamat, C.; Dallman, M.F. Hypothalamic Ventromedial Nuclei Amplify Circadian Rhythms: Do They Contain a Food-Entrained Endogenous Oscillator? J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 3843–3852. [Google Scholar] [CrossRef]
- Shim, S.; Tanaka, H. Effects of restricted food access on circadian fluctuation of serotonin N-acetyltransferase activities in hereditary microphthalmic rats. Physiol. Behav. 2000, 71, 477–483. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.N.; Zárate-Mozo, C.; Guzmán-Ruiz, M.A.; Cárdenas-Rivera, A.; Escobar, C. Time-restricted feeding prevents depressive-like and anxiety-like behaviors in male rats exposed to an experimental model of shift-work. J. Neurosci. Res. 2021, 99, 604–620. [Google Scholar] [CrossRef]
- Soares, N.L.; Dorand, V.A.M.; Cavalcante, H.C.; Batista, K.S.; de Souza, D.M.; Lima, M.d.S.; Salvadori, M.G.d.S.S.; Magnani, M.; Alves, A.F.; Aquino, J.d.S. Does intermittent fasting associated with aerobic training influence parameters related to the gut-brain axis of Wistar rats? J. Affect. Disord. 2021, 293, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Roky, R.; Iraki, L.; HajKhlifa, R.; Ghazal, N.L.; Hakkou, F. Daytime Alertness, Mood, Psychomotor Performances, and Oral Temperature during Ramadan Intermittent Fasting. Ann. Nutr. Metab. 2000, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gueldich, H.; Zghal, F.; Borji, R.; Chtourou, H.; Sahli, S.; Rebai, H. The effects of Ramadan intermittent fasting on the underlying mechanisms of force production capacity during maximal isometric voluntary contraction. Chrono-International 2019, 36, 698–708. [Google Scholar] [CrossRef]
- Nugraha, B.; Riat, A.; Ghashang, S.K.; Eljurnazi, L.; Gutenbrunner, C. A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition. Nutrients 2020, 12, 2281. [Google Scholar] [CrossRef]
- Riat, A.; Suwandi, A.; Ghashang, S.K.; Buettner, M.; Eljurnazi, L.; Grassl, G.A.; Gutenbrunner, C.; Nugraha, B. Ramadan Fasting in Germany (17–18 h/Day): Effect on Cortisol and Brain-Derived Neurotrophic Factor in Association With Mood and Body Composition Parameters. Front. Nutr. 2021, 8, 697920. [Google Scholar] [CrossRef]
- Jahrami, H.; BaHammam, A.S.; Haji, E.A.; Bragazzi, N.L.; Rakha, I.; Alsabbagh, A.; Nugraha, B.; Pasiakos, S.M. Ramadan Fasting Improves Body Composition without Exacerbating Depression in Males with Diagnosed Major Depressive Disorders. Nutrients 2021, 13, 2718. [Google Scholar] [CrossRef]
- Kessler, C.S.; Stange, R.; Schlenkermann, M.; Jeitler, M.; Michalsen, A.; Selle, A.; Raucci, F.; Steckhan, N. A nonrandomized controlled clinical pilot trial on 8 wk of intermittent fasting (24 h/wk). Nutrition 2018, 46, 143–152.e2. [Google Scholar] [CrossRef]
- Teong, X.T.; Hutchison, A.T.; Liu, B.; Wittert, G.A.; Lange, K.; Banks, S.; Heilbronn, L.K. Eight weeks of intermittent fasting versus calorie restriction does not alter eating behaviors, mood, sleep quality, quality of life and cognitive performance in women with overweight. Nutr. Res. 2021, 92, 32–39. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Brennan, L.; Hawley, J.A. Effects of time-restricted feeding on mood, hunger and fatigue. Obes. Res. Clin. Pract. 2019, 13, 245. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Hussin, N.M.; Shahar, S.; Teng, N.I.M.F.; Ngah, W.Z.W.; Das, S.K. Efficacy of Fasting and Calorie Restriction (FCR) on mood and depression among ageing men. J. Nutr. Health Aging 2013, 17, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Pringle, H.; Penning, E.; Plank, L.D.; Murphy, R. PROFAST: A Randomized Trial Assessing the Effects of Intermittent Fasting and Lacticaseibacillus rhamnosus Probiotic among People with Prediabetes. Nutrients 2020, 12, 3530. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time-restricted feeding is associated with mental health in elderly Italian adults. Chronobiol. Int. 2021, 38, 1507–1516. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Roiz-Santiañez, R.; Suarez-Pinilla, P.; Crespo-Facorro, B. Brain Structural Effects of Antipsychotic Treatment in Schizophrenia: A Systematic Review. Curr. Neuropharmacol. 2015, 13, 422–434. [Google Scholar] [CrossRef]
- Watson, K.T.; Simard, J.F.; Henderson, V.W.; Nutkiewicz, L.; Lamers, F.; Nasca, C.; Rasgon, N.; Penninx, B.W. Incident Major Depressive Disorder Predicted by Three Measures of Insulin Resistance: A Dutch Cohort Study. Am. J. Psychiatry 2021, 178, 914–920. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Molecular components of the circadian clock in mammals. Diabetes Obes. Metab. 2015, 17, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, A.; Fukuzawa, M.; Iwami, S.; Nishimura, Y.; Motohashi, H.; Tahara, Y.; Shibata, S. Night eating model shows time-specific depression-like behavior in the forced swimming test. Sci. Rep. 2018, 8, 1081. [Google Scholar] [CrossRef]
- Ben-Hamo, M.; Larson, T.A.; Duge, L.S.; Sikkema, C.; Wilkinson, C.W.; de la Iglesia, H.O.; González, M.M.C. Circadian Forced Desynchrony of the Master Clock Leads to Phenotypic Manifestation of Depression in Rats. eNeuro 2017, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [Google Scholar] [CrossRef]
- Teker, H.T.; Ceylani, T. Intermittent fasting supports the balance of the gut microbiota composition. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2023, 26, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef]
- Qasrawi, S.O.; Pandi-Perumal, S.R.; BaHammam, A.S. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017, 21, 577–586. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Madkour, M.; Jahrami, H.; Salahat, D.; Alhasan, F.; BaHammam, A.; Faris, M.A.-I. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: A prospective observational study. PLoS ONE 2020, 15, e0237922. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2020, 157, 53–72. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murta, L.; Seixas, D.; Harada, L.; Damiano, R.F.; Zanetti, M. Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 15551. https://doi.org/10.3390/ijms242115551
Murta L, Seixas D, Harada L, Damiano RF, Zanetti M. Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies. International Journal of Molecular Sciences. 2023; 24(21):15551. https://doi.org/10.3390/ijms242115551
Chicago/Turabian StyleMurta, Laís, Daniela Seixas, Luana Harada, Rodolfo Furlan Damiano, and Marcus Zanetti. 2023. "Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies" International Journal of Molecular Sciences 24, no. 21: 15551. https://doi.org/10.3390/ijms242115551
APA StyleMurta, L., Seixas, D., Harada, L., Damiano, R. F., & Zanetti, M. (2023). Intermittent Fasting as a Potential Therapeutic Instrument for Major Depression Disorder: A Systematic Review of Clinical and Preclinical Studies. International Journal of Molecular Sciences, 24(21), 15551. https://doi.org/10.3390/ijms242115551






