Identification of Uncaria rhynchophylla in the Potential Treatment of Alzheimer’s Disease by Integrating Virtual Screening and In Vitro Validation
Abstract
:1. Introduction
2. Results
2.1. Network Pharmacology Analysis
2.1.1. Active Compounds
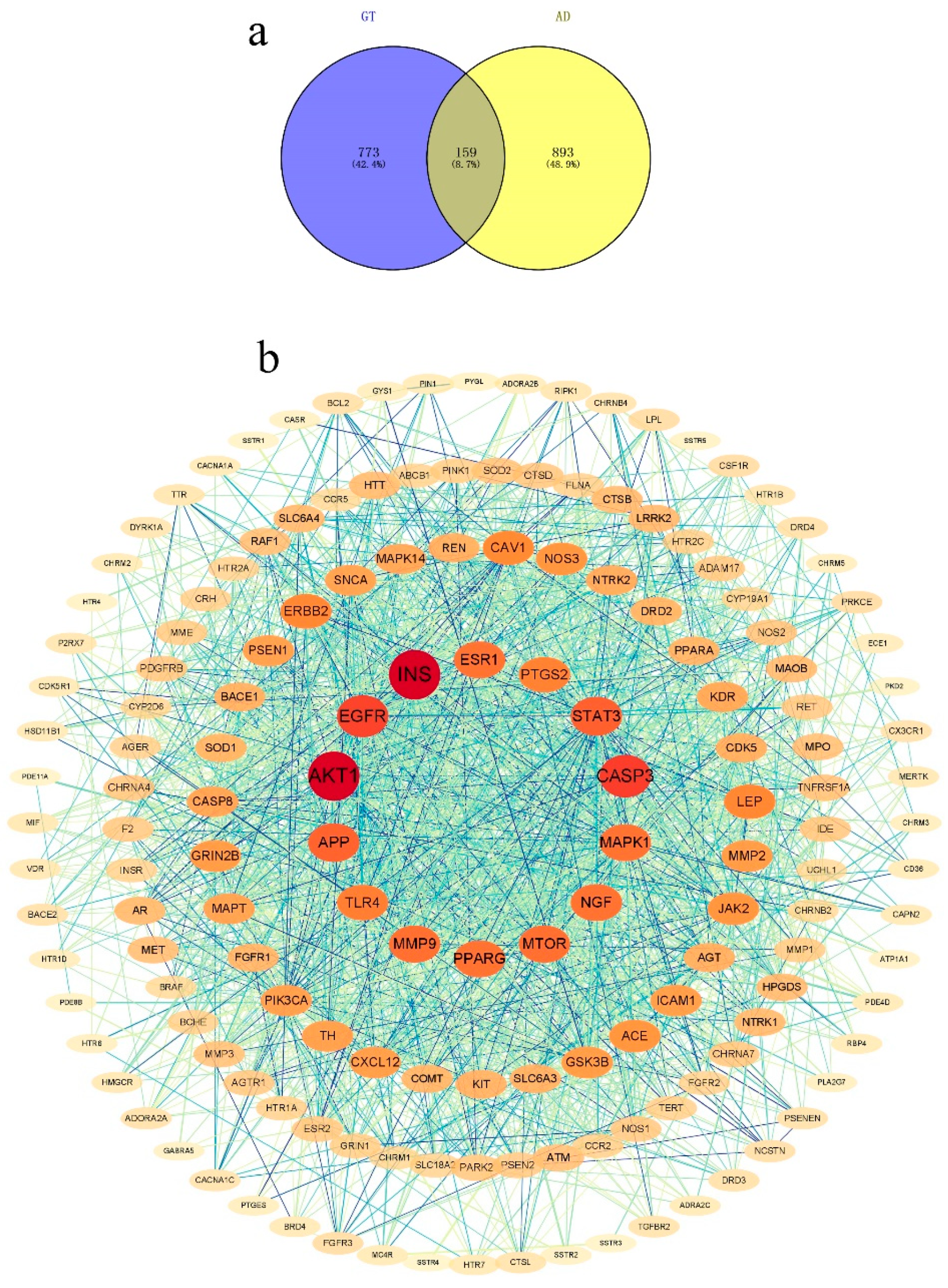
2.1.2. Compounds and Target Screening
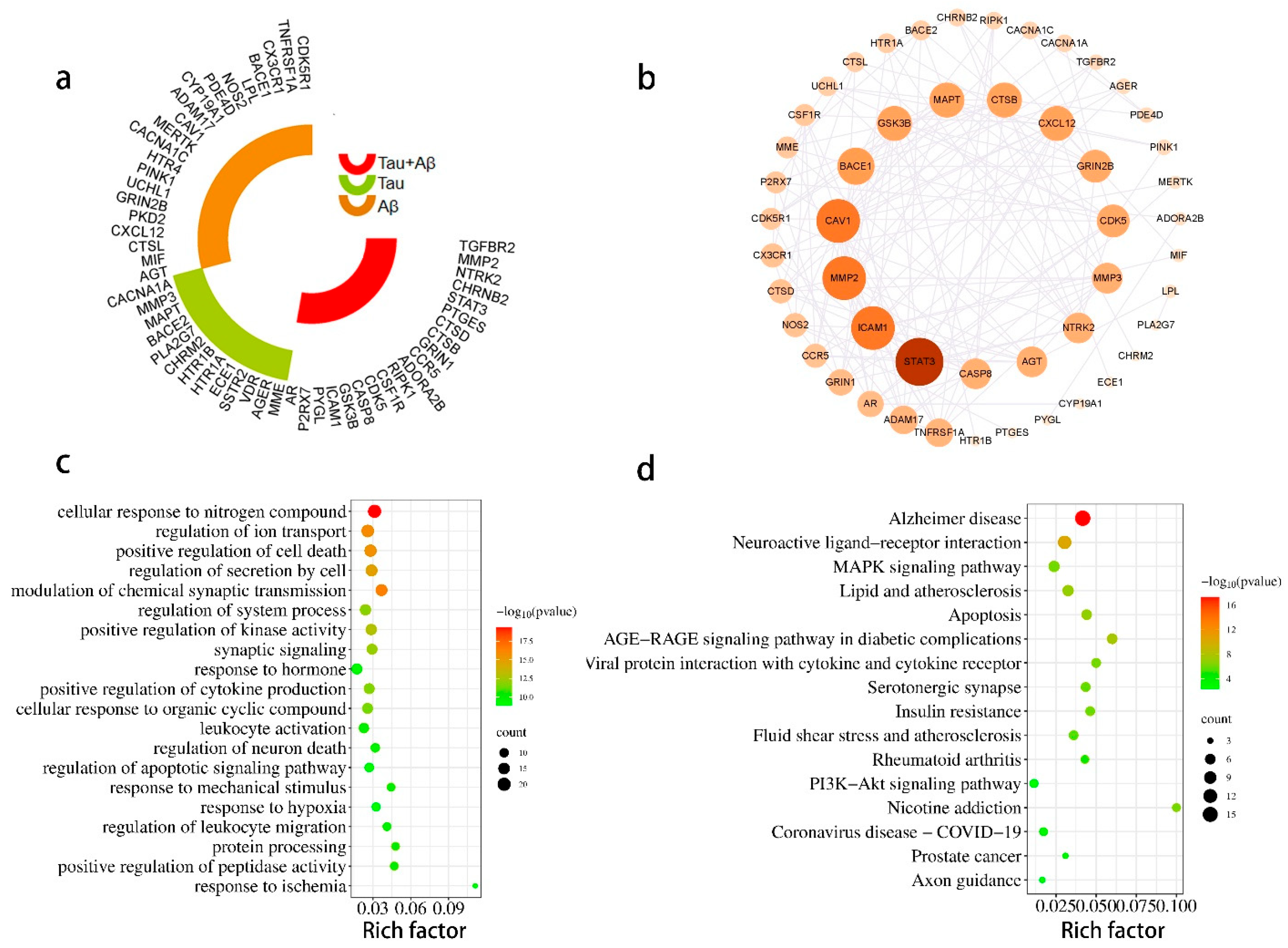
2.1.3. PPI Network
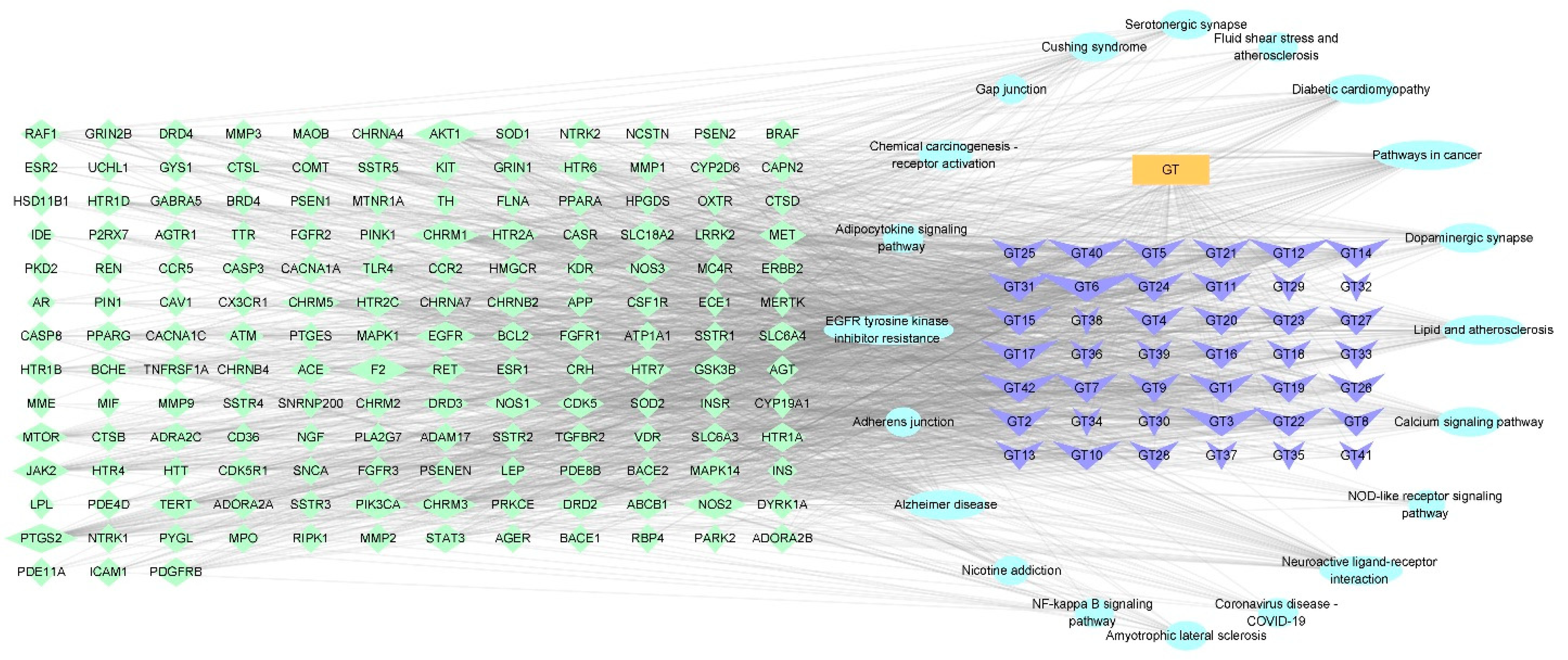
2.1.4. Construction of a Potential Ingredient Target-AD Target-Pathway Network
2.1.5. Enrichment Analysis
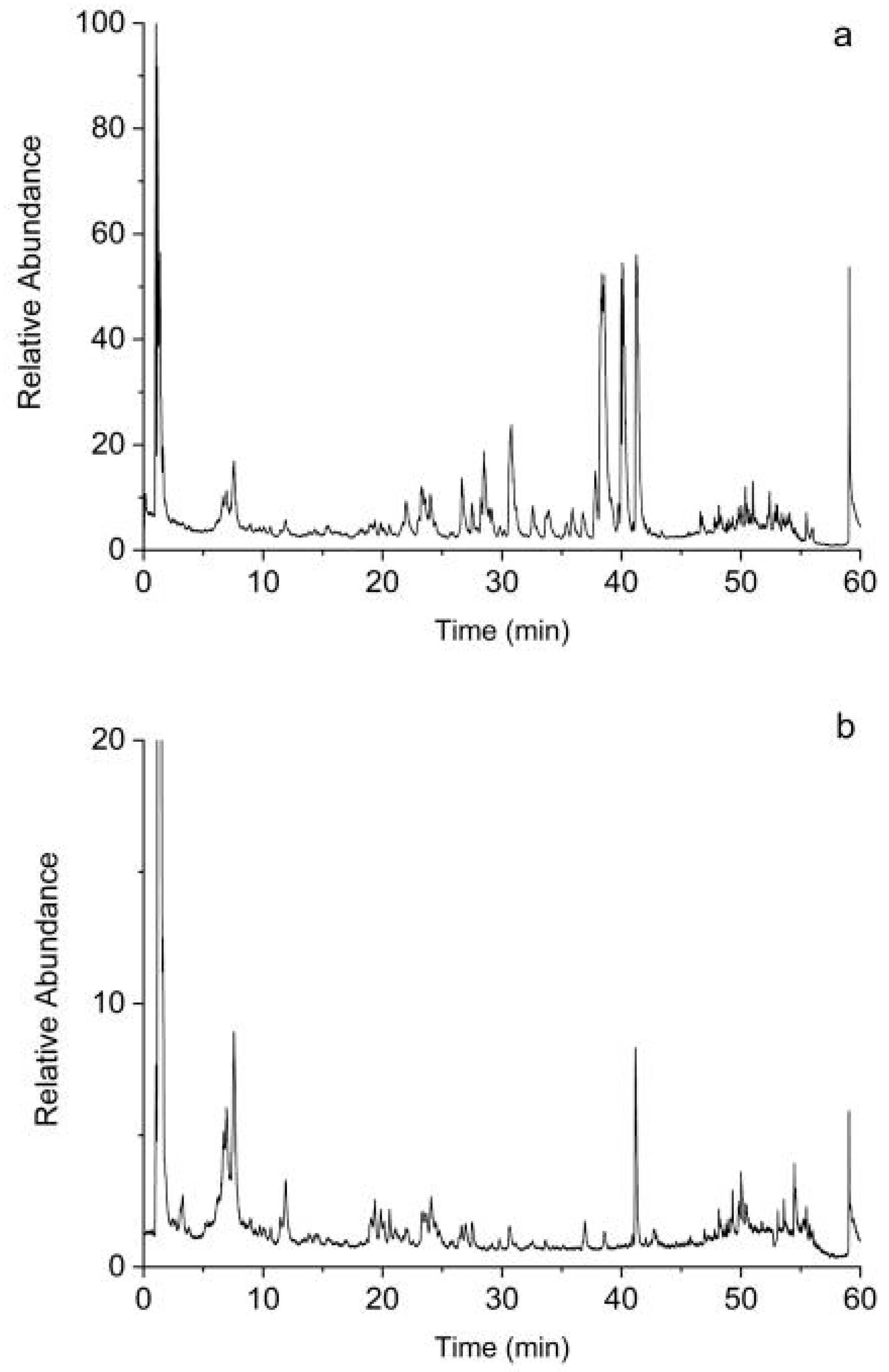
2.1.6. Screening of Bioactive Compounds by UHPLC-Q-Exactive Orbitrap MS Analysis
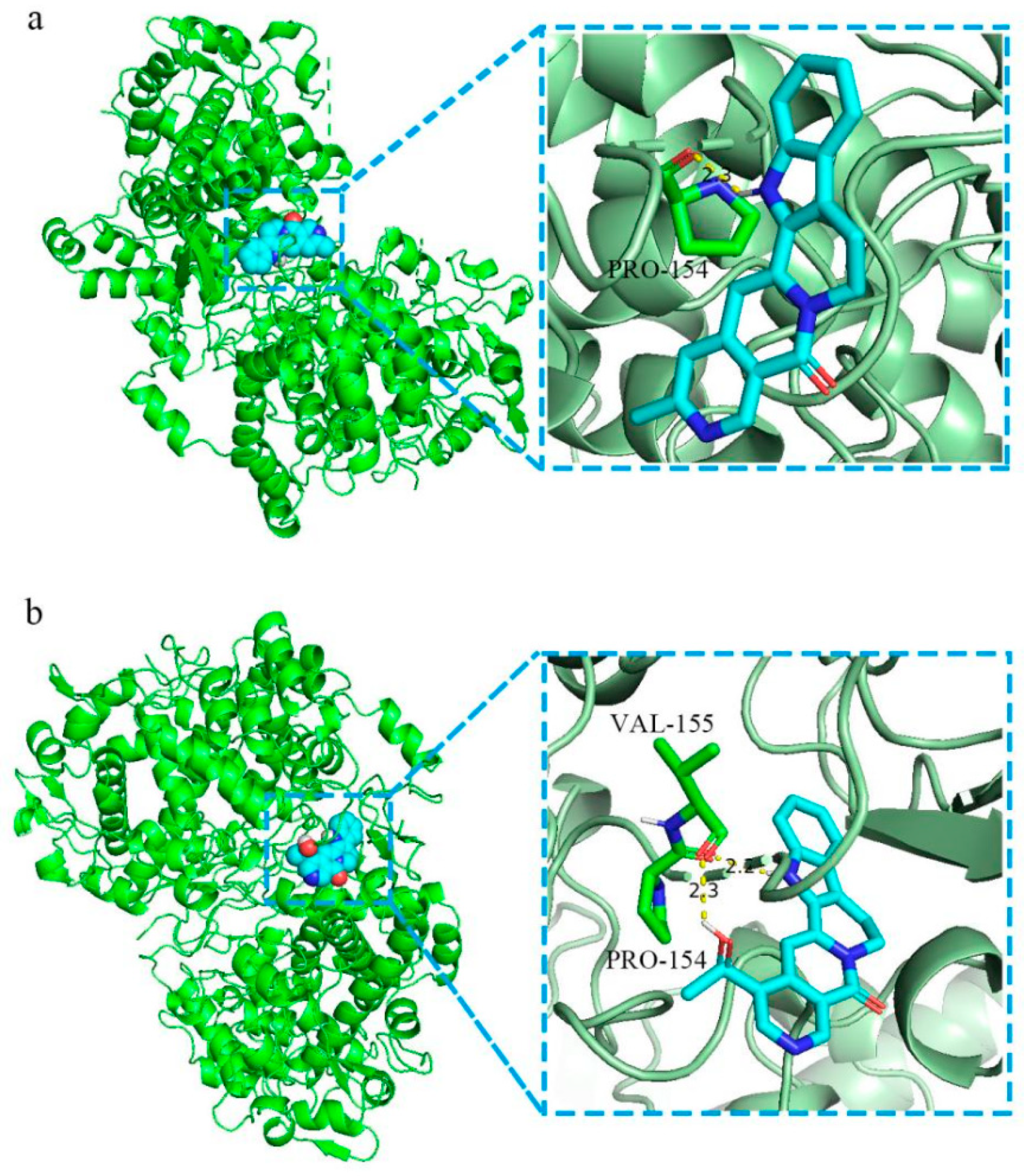
2.1.7. Molecular Docking
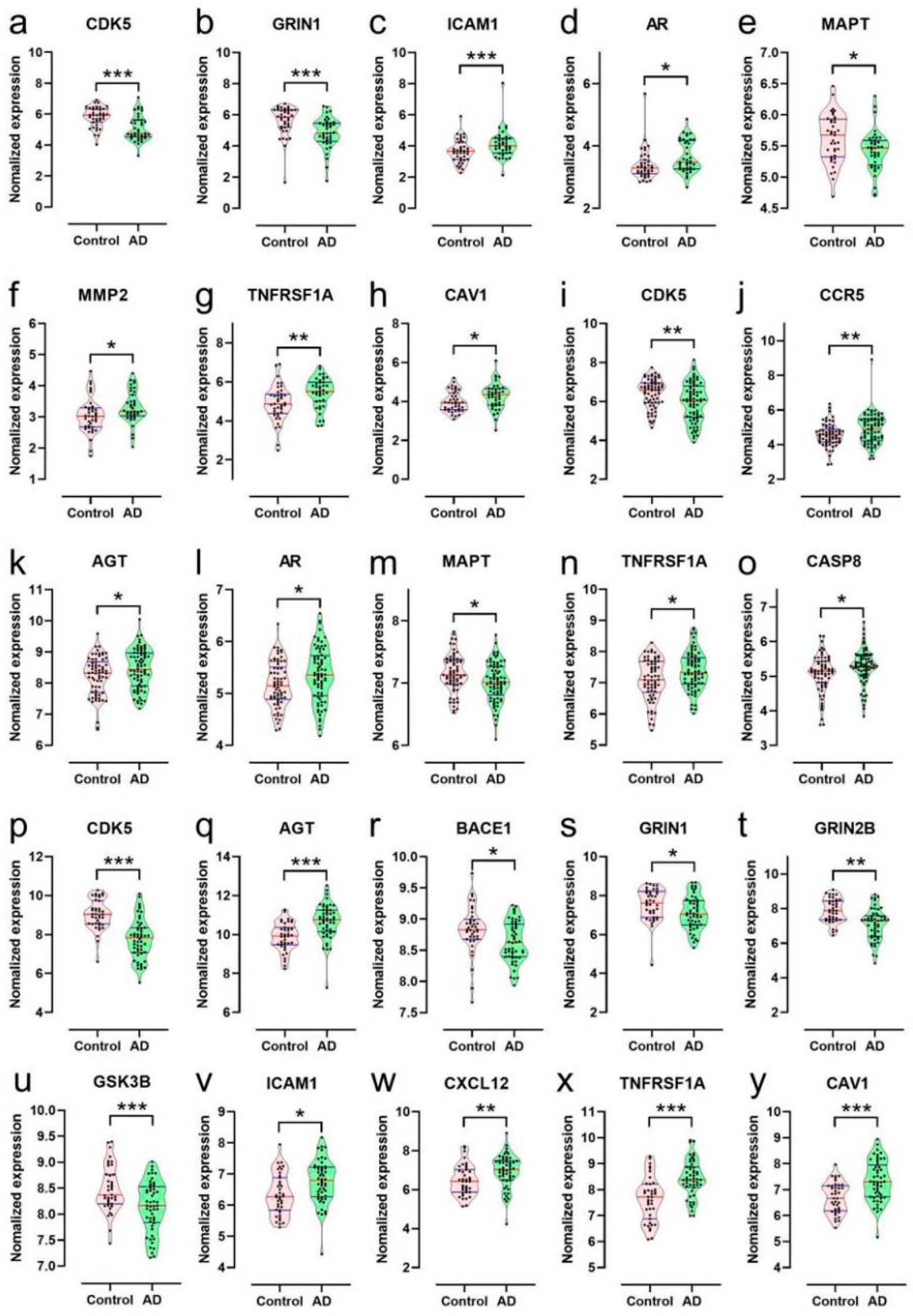
2.1.8. Bioinformatics Analysis of Targets of the Ingredients of GT Related to Aβ and Tau Pathology
2.2. Cell Model Verification
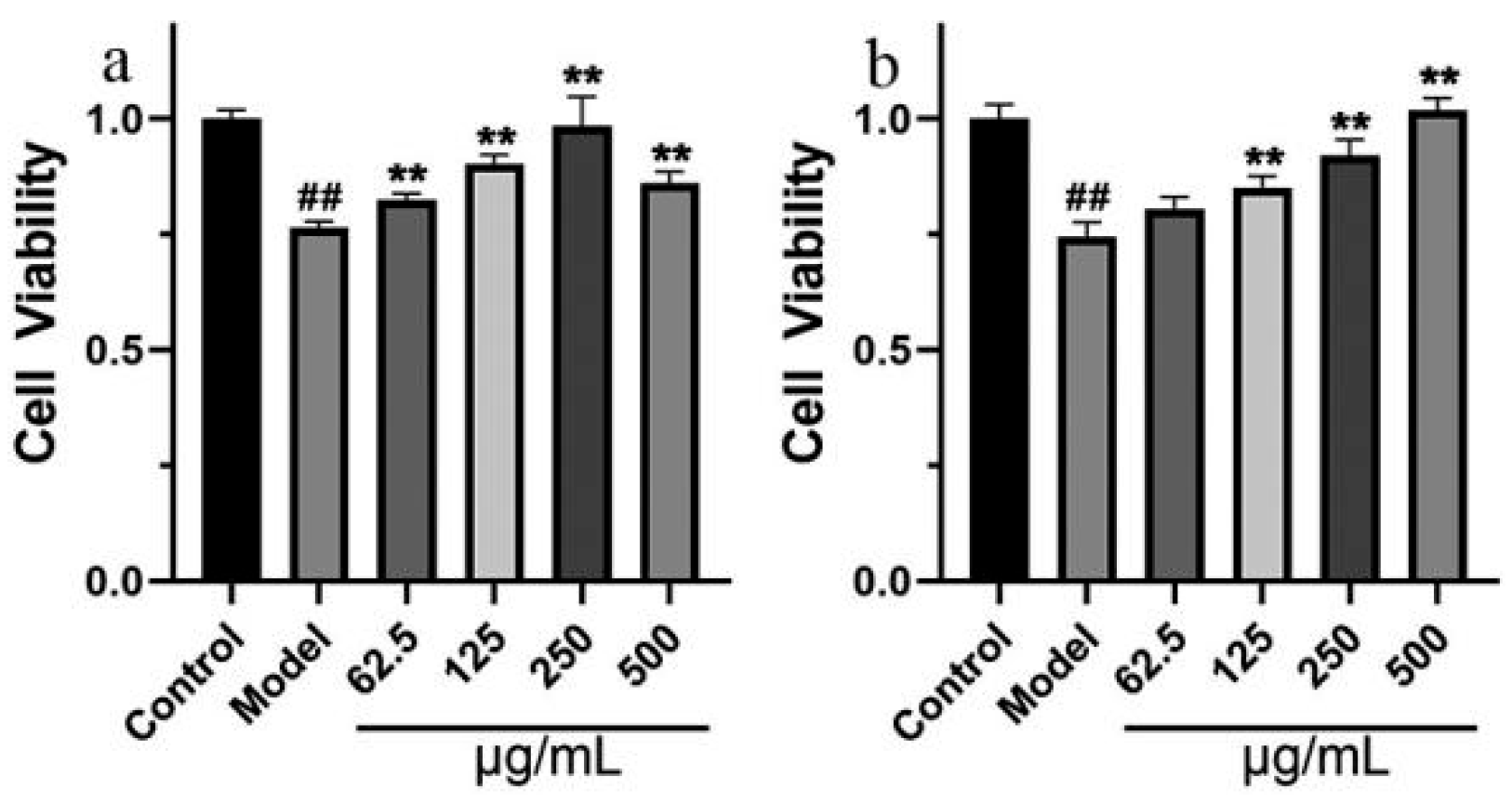
2.2.1. Effects of GT Extract on OA-Treated SH-SY5Y Cells
2.2.2. The Neuroprotective Effect of GT Extract
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology
4.1.1. Collection of Active Ingredients and Targets of GT
4.1.2. Target Prediction
4.1.3. Biology Functional Analysis
4.1.4. Molecular Docking
4.1.5. Preparation of GT
4.1.6. UHPLC-Q-Exactive Orbitrap MS Analysis
4.1.7. Analysis of GT Targets Related to AD Pathology
4.2. Pharmacodynamic Experiment
4.2.1. Cell Culture and Treatment
4.2.2. Western Blotting Analysis
4.2.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alzheimer’s disease | (AD) |
| Biological process | (BP) |
| Cellular component | (CC) |
| Central nervous system | (CNS) |
| Food and Drug Administration | (FDA) |
| Molecular function | (MF) |
| Neuronal deaths | (NDs) |
| Neuronal fiber tangles | (NFTs) |
| Okadaic acid | (OA) |
| Protein-protein interaction | (PPI) |
| Traditional Chinese medicine | (TCM) |
| Ultra-high-performance liquid chromatography | (UHPLC) |
| Uncaria rhynchophylla | (GT) |
References
- Pan, Y.; Zhang, Y.; Liu, N.; Lu, W.; Yang, J.; Li, Y.; Liu, Z.; Wei, Y.; Lou, Y.; Kong, J. Vitamin D Attenuates Alzheimer-like Pathology Induced by Okadaic Acid. ACS Chem. Neurosci. 2021, 12, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.N.; Tian, J.Z. Recognition of dementia in ancient China. Neurobiol. Aging 2012, 33, 2948.e11–2948.e13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, J.; Wang, Z.; Guo, P.; Liu, A.; Du, G. Identification of Multi-Target Anti-AD Chemical Constituents From Traditional Chinese Medicine Formulae by Integrating Virtual Screening and In Vitro Validation. Front. Pharmacol. 2021, 12, 709607. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Gao, D.; Du, K.; Han, C.; Liu, Y.; Wang, Y. Rhein Ameliorates Cognitive Impairment in an APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease by Relieving Oxidative Stress through Activating the SIRT1/PGC-1α Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2524832. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Su, H.F.; Ye, C.Y.; Qiu, S.W.; Tian, Q. Therapeutic Mechanism and Key Alkaloids of Uncaria rhynchophylla in Alzheimer’s Disease From the Perspective of Pathophysiological Processes. Front. Pharmacol. 2021, 12, 806984. [Google Scholar] [CrossRef]
- Pons, V.; Rivest, S. Targeting Systemic Innate Immune Cells as a Therapeutic Avenue for Alzheimer Disease. Pharmacol. Rev. 2022, 74, 1–17. [Google Scholar] [CrossRef]
- Cai, H.; Luo, Y.; Yan, X.; Ding, P.; Huang, Y.; Fang, S.; Zhang, R.; Chen, Y.; Guo, Z.; Fang, J.; et al. The Mechanisms of Bushen-Yizhi Formula as a Therapeutic Agent against Alzheimer’s Disease. Sci. Rep. 2018, 8, 3104. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, B.; Pang, X.; Yang, R.; Chen, M.; Zhao, J.; Wang, J.; Wang, Z.; Yu, Z.; Wang, Y.; et al. Network Pharmacology-Based Analysis of Xiao-Xu-Ming Decoction on the Treatment of Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 595254. [Google Scholar] [CrossRef] [PubMed]
- Beshir, S.A.; Aadithsoorya, A.M. Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review. Int. J. Alzheimer’s Dis. 2022, 2022, 9343514. [Google Scholar] [CrossRef]
- Barenholtz Levy, H. Accelerated Approval of Aducanumab: Where Do We Stand Now? Ann. Pharmacother. 2022, 56, 736–739. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Yang, S.; Song, D.; Wang, C.; Chen, C.; Li, X.; Wang, Q.; Ge, S.; Yang, R.; et al. Ling-Yang-Gou-Teng-decoction prevents vascular dementia through inhibiting oxidative stress induced neurovascular coupling dysfunction. J. Ethnopharmacol. 2018, 222, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, M.L.; Zhao, J.J.; Hong, H.; Qu, W.; Feng, F.; Liu, W.Y. GTS40, an active fraction of Gou Teng-San (GTS), protects PC12 from H2O2-induced cell injury through antioxidative properties. Chin. J. Nat. Med. 2017, 15, 495–504. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Z.; Sun, L.; Li, W.; Liu, G.; Tang, Y. A Computational Systems Pharmacology Approach to Investigate Molecular Mechanisms of Herbal Formula Tian-Ma-Gou-Teng-Yin for Treatment of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ip, S.P.; Liu, L.; Xian, Y.F.; Lin, Z.X. Uncaria rhynchophylla and its Major Constituents on Central Nervous System: A Review on Their Pharmacological Actions. Curr. Vasc. Pharmacol. 2020, 18, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Hu, Z.; Zhao, M.; Che, C.T.; Ip, S.P. Bioassay-Guided Isolation of Neuroprotective Compounds from Uncaria rhynchophylla against Beta-Amyloid-Induced Neurotoxicity. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 802625. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, D.F.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.L.; Bi, R.; Yao, Y.G. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer’s disease. Alzheimer’s Dement.: J. Alzheimer’s Assoc. 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Zhang, D.F.; Fan, Y.; Xu, M.; Wang, G.; Wang, D.; Li, J.; Kong, L.L.; Zhou, H.; Luo, R.; Bi, R.; et al. Complement C7 is a novel risk gene for Alzheimer’s disease in Han Chinese. Natl. Sci. Rev. 2019, 6, 257–274. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Reddy, P.H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 101191. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Zhang, P.; Li, X.; Lu, L.; Sun, Y.; Zhang, B.; Allen, S.; White, L.; Phillips, J.; et al. Discovery of novel hybrids containing clioquinol-1-benzyl-1,2,3,6-tetrahydropyridine as multi-target-directed ligands (MTDLs) against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 244, 114841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Heo, S.; Kim, D.W.; Kim, I.S.; Ahn, D.; Tae, H.J.; Kim, M.K.; Park, B.Y. Ethanol Extract of Maclura tricuspidata Fruit Protects SH-SY5Y Neuroblastoma Cells against H2O2-Induced Oxidative Damage via Inhibiting MAPK and NF-κB Signaling. Int. J. Mol. Sci. 2021, 22, 6946. [Google Scholar] [CrossRef] [PubMed]
- March-Diaz, R.; Lara-Ureña, N.; Romero-Molina, C.; Heras-Garvin, A.; Ortega-de San Luis, C.; Alvarez-Vergara, M.I.; Sanchez-Garcia, M.A.; Sanchez-Mejias, E.; Davila, J.C.; Rosales-Nieves, A.E.; et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging 2021, 1, 385–399. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 700–789. [CrossRef] [PubMed]
- Xiong, J.; Kang, S.S.; Wang, Z.; Liu, X.; Kuo, T.C. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 2022, 603, 470–476. [Google Scholar] [CrossRef]
- Li, J.; Du, Q.; Li, N.; Du, S.; Sun, Z. Alpiniae oxyphyllae Fructus and Alzheimer’s disease: An update and current perspective on this traditional Chinese medicine. Biomed. Pharmacother. 2021, 135, 111167. [Google Scholar] [CrossRef]
- Kang, S.S.; Meng, L.; Zhang, X.; Wu, Z.; Mancieri, A.; Xie, B.; Liu, X.; Weinshenker, D.; Peng, J. Tau modification by the norepinephrine metabolite DOPEGAL stimulates its pathology and propagation. Nat. Struct. Mol. Biol. 2022, 29, 292–305. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Trushina, N.I.; Bakota, L.; Mulkidjanian, A.Y.; Brandt, R. The Evolution of Tau Phosphorylation and Interactions. Front. Aging Neurosci. 2019, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Ercan, E.; Eid, S.; Weber, C.; Kowalski, A.; Bichmann, M.; Behrendt, A.; Matthes, F.; Krauss, S.; Reinhardt, P.; Fulle, S.; et al. A validated antibody panel for the characterization of tau post-translational modifications. Mol. Neurodegener. 2017, 12, 87. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Embaye, K.S.; Huang, F.; Li, L.; Zhu, F.; Wang, J.Z.; Liu, R.; Feng, J.; Wang, X. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 2022, 74, 101544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, J.; Zhu, Z.; Su, C.F.; Sreenivasmurthy, S.G.; Iyaswamy, A.; Lu, J.H.; Chen, G.; Song, J.X.; Li, M. Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: A mechanism review. Biomed. Pharmacother. 2021, 133, 110968. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wang, Y.B.; Song, J.X.; Deng, W.K.; Lu, J.H.; Ma, L.L.; Yang, C.B.; Li, M.; Xue, Y. Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy. Autophagy 2017, 13, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Jeong, Y.; Jeon, S.G.; Kim, S.; Lee, S.K.; Choi, H.S.; Im, C.S.; Kim, S.H.; Kim, S.H.; Park, J.H.; et al. Uncaria rhynchophylla ameliorates amyloid beta deposition and amyloid beta-mediated pathology in 5XFAD mice. Neurochem. Int. 2018, 121, 114–124. [Google Scholar] [CrossRef]
- Jiao, Y.N.; Zhang, J.S.; Qiao, W.J.; Tian, S.Y.; Wang, Y.B.; Wang, C.Y.; Zhang, Y.H.; Zhang, Q.; Li, W.; Min, D.Y.; et al. Kai-Xin-San Inhibits Tau Pathology and Neuronal Apoptosis in Aged SAMP8 Mice. Mol. Neurobiol. 2022, 59, 3294–3309. [Google Scholar] [CrossRef]
- Serpente, M.; Fenoglio, C.; Cioffi, S.M.G.; Oldoni, E.; Arcaro, M.; Arighi, A.; Fumagalli, G.G.; Ghezzi, L.; Scarpini, E.; Galimberti, D. Profiling of Specific Gene Expression Pathways in Peripheral Cells from Prodromal Alzheimer’s Disease Patients. J. Alzheimer’s Dis. JAD 2018, 61, 1289–1294. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, K.; Das, D.; Gowaikar, R.; Shaw, E.; Ramachandran, A.; Rupanagudi, K.V.; Kommaddi, R.P.; Bennett, D.A.; Ravindranath, V. Reactive Oxygen Species-Mediated Loss of Synaptic Akt1 Signaling Leads to Deficient Activity-Dependent Protein Translation Early in Alzheimer’s Disease. Antioxid. Redox Signal. 2017, 27, 1269–1280. [Google Scholar] [CrossRef]
- Singh, A.K.; Kashyap, M.P.; Tripathi, V.K.; Singh, S.; Garg, G.; Rizvi, S.I. Neuroprotection Through Rapamycin-Induced Activation of Autophagy and PI3K/Akt1/mTOR/CREB Signaling Against Amyloid-β-Induced Oxidative Stress, Synaptic/Neurotransmission Dysfunction, and Neurodegeneration in Adult Rats. Mol. Neurobiol. 2017, 54, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Bogár, F.; Paragi, G.; Gera, J.; Fülöp, L. Key Peptides and Proteins in Alzheimer’s Disease. Curr. Protein Pept. Sci. 2019, 20, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.S.; Crocker, S.J.; Nicholson, D.W.; Schulz, J.B. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000, 10, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Rahman, M.A.; Kabir, M.T.; Behl, T.; Mathew, B.; Perveen, A.; Barreto, G.E. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of Alzheimer’s disease. IUBMB Life 2020, 72, 1843–1855. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef]
- Ma, S.L.; Tang, N.L.; Zhang, Y.P.; Ji, L.D.; Tam, C.W.; Lui, V.W.; Chiu, H.F.; Lam, L.C. Association of prostaglandin-endoperoxide synthase 2 (PTGS2) polymorphisms and Alzheimer’s disease in Chinese. Neurobiol. Aging 2008, 29, 856–860. [Google Scholar] [CrossRef]
- Sakakibara, I.; Terabayashi, S.; Kubo, M.; Higuchi, M.; Komatsu, Y.; Okada, M.; Taki, K.; Kamei, J. Effect on locomotion of indole alkaloids from the hooks of uncaria plants. Phytomedicine 1999, 6, 163–168. [Google Scholar] [CrossRef]
- Shimada, Y.; Goto, H.; Itoh, T.; Sakakibara, I.; Kubo, M.; Sasaki, H.; Terasawa, K. Evaluation of the protective effects of alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J. Pharm. Pharmacol. 1999, 51, 715–722. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, C.F. Components of Goutengsan in Rat Plasma by Microdialysis Sampling and Its Protection on Aβ1–42-Induced PC12 Cells Injury. Evid.-Based Complement. Altern. Med. 2017, 2017, 7593027. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Ip, S.P.; Su, Z.R.; Lai, X.P. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, W.G.; Zhu, Z.R.; Wu, Y.L.; Zhang, Z.Y.; Qu, S.C. Rhynchophylline suppresses soluble Aβ1–42-induced impairment of spatial cognition function via inhibiting excessive activation of extrasynaptic NR2B-containing NMDA receptors. Neuropharmacology 2018, 135, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Turab Naqvi, A.A.; Hasan, G.M.; Hassan, M.I. Targeting Tau Hyperphosphorylation via Kinase Inhibition: Strategy to Address Alzheimer’s Disease. Curr. Top. Med. Chem. 2020, 20, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhou, F.; Sun, J.; Li, Y. NPD1 Enhances Autophagy and Reduces Hyperphosphorylated Tau and Amyloid-β42 by Inhibiting GSK3β Activation in N2a/APP695swe Cells. J. Alzheimer’s Dis. JAD 2021, 84, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Nesi, G.; Sestito, S.; Digiacomo, M.; Rapposelli, S. Oxidative Stress, Mitochondrial Abnormalities and Proteins Deposition: Multitarget Approaches in Alzheimer’s Disease. Curr. Top. Med. Chem. 2017, 17, 3062–3079. [Google Scholar] [CrossRef]
- You, J.S.; Li, C.Y.; Chen, W.; Wu, X.L.; Huang, L.J.; Li, R.K.; Gao, F.; Zhang, M.Y.; Liu, H.L.; Qu, W.L. A network pharmacology-based study on Alzheimer disease prevention and treatment of Qiong Yu Gao. BioData Min. 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, X.; Wang, P.; Zhao, C.; Qin, Y.; Liu, F.; Yu, L.; Xu, H. An Integrative Pharmacology-Based Approach for Evaluating the Potential Effects of Purslane Seed in Diabetes Mellitus Treatment Using UHPLC-LTQ-Orbitrap and TCMIP V2.0. Front. Pharmacol. 2020, 11, 593693. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, T.; Shi, C.; Wang, Z.; Fu, X. Network pharmacology-based approach to understand the effect and mechanism of Danshen against anemia. J. Ethnopharmacol. 2022, 282, 114615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; He, K.; Guan, Y.; Yang, X.; Chen, Y.; Sun, M.; Qiu, X.; Yan, F.; Huang, H.; Yao, L.; et al. Network pharmacology-based strategy to investigate pharmacological mechanisms of Tinospora sinensis for treatment of Alzheimer’s disease. J. Ethnopharmacol. 2020, 259, 112940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dan, W. Exploring the Biological Mechanism of Huang Yam in Treating Tumors and Preventing Antitumor Drug-Induced Cardiotoxicity Using Network Pharmacology and Molecular Docking Technology. Evid.-Based Complement. Altern. Med. 2021, 2021, 9988650. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Ma, X.H.; Duan, W.J.; Mo, Y.S.; Chen, J.L.; Li, S.; Zhao, W.; Yang, L.; Mi, S.Q.; Mao, X.L.; Wang, H.; et al. Neuroprotective effect of paeoniflorin on okadaic acid-induced tau hyperphosphorylation via calpain/Akt/GSK-3β pathway in SH-SY5Y cells. Brain Res. 2018, 1690, 1–11. [Google Scholar] [CrossRef]


| No. | Name | Uniprot ID | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|---|
| 1 | INS | P01308 | 95 | 0.120473547 | 0.694690265 |
| 2 | AKT1 | P31749 | 94 | 0.108167587 | 0.707207207 |
| 3 | CASP3 | P42574 | 74 | 0.024572104 | 0.633064516 |
| 4 | EGFR | P00533 | 72 | 0.029317310 | 0.620553360 |
| 5 | STAT3 | P40763 | 63 | 0.021227013 | 0.601532567 |
| 6 | APP | P05067 | 62 | 0.035526890 | 0.608527132 |
| 7 | MTOR | P42345 | 58 | 0.018850365 | 0.596958175 |
| 8 | MMP9 | P14780 | 58 | 0.013916864 | 0.579335793 |
| 9 | ESR1 | P03372 | 57 | 0.020596430 | 0.594696970 |
| 10 | PPARG | P37231 | 57 | 0.020462185 | 0.581481481 |
| 11 | MAPK1 | P28482 | 57 | 0.022965376 | 0.592452830 |
| 12 | NGF | P01138 | 57 | 0.018801898 | 0.594696970 |
| 13 | TLR4 | O00206 | 53 | 0.016064887 | 0.566787004 |
| 14 | PTGS2 | P35354 | 52 | 0.007247996 | 0.570909091 |
| 15 | ERBB2 | P04626 | 50 | 0.010049860 | 0.564748201 |
| 16 | LEP | P41159 | 50 | 0.014671748 | 0.572992701 |
| 17 | CAV1 | Q03135 | 49 | 0.012189853 | 0.568840580 |
| ID | Name | Status | Ion Mode |
|---|---|---|---|
| GT2 | Akuammigine | Confirmed | Positive |
| GT3 | Angustidine | Confirmed | Positive |
| GT4 | Angustoline | Confirmed | Positive |
| GT5 | Corynantheine | Confirmed | Positive |
| GT6 | Corynanthine | Confirmed | Positive |
| GT7 | Corynoxeine | Confirmed | Positive |
| GT8 | Corynoxine | Confirmed | Positive |
| GT9 | Corynoxine B | Confirmed | Positive |
| GT10 | Dihydrocorynantheine | Confirmed | Positive |
| GT11 | Geissoschizine methyl ether | Confirmed | Positive |
| GT13 | Harman | Confirmed | Positive |
| GT14 | Hirsuteine | Confirmed | Positive |
| GT15 | Hirsutine | Confirmed | Positive |
| GT16 | Isocorynoxeine | Confirmed | Positive |
| GT18 | Isopteropodine | Confirmed | Positive |
| GT19 | Isorhynchophyllic acid | Confirmed | Positive |
| GT20 | Uncarine C | Confirmed | Positive |
| GT21 | Rhynchophylline | Confirmed | Positive |
| GT22 | Tetrahydroalstonine | Confirmed | Positive |
| GT23 | Vallesiachotamine | Confirmed | Positive |
| GT24 | Isorhynchophylline | Confirmed | Positive |
| GT25 | Isomitraphylline | Confirmed | Positive |
| GT26 | Mitraphylline | Confirmed | Positive |
| GT27 | Speciophylline/uncarine D | Confirmed | Positive |
| GT28 | Uncarine F | Confirmed | Positive |
| GT29 | Geissoschizinc acid | Confirmed | Positive |
| GT30 | Rhynchophylline A | Confirmed | Positive |
| GT31 | Methyl-(E)-2-[(2S,3Z,12bS)-3-ethylidene-2,4,6,7,12,12b-hexahydro-1H-indolo [3,2-h]quinolizin-2-yl]-3-methoxyprop-2-enoate | Confirmed | Positive |
| GT33 | Mitraphyllic acid | Confirmed | Positive |
| GT35 | (1′R,3S,4a’S,5a’S,10a’R)-1′-Methyl-2-oxo-1′,4a’,5′,5a’,7′,8′,10′,10a’-octahydrospiro [indoline-3,6′-pyrano [3,4-f]indolizine]-4′-carboxylic acid | Confirmed | Positive |
| GT36 | (2S,12bR)-Methyl-2-((E)-1-oxobut-2-en-2-yl)-1,2,6,7,12,12b-hexahydroindolo [2,3-a]quinolizine-3-carboxylate | Confirmed | Positive |
| GT37 | Vincoside lactam_qt | Confirmed | Positive |
| GT40 | Yohimbin | Confirmed | Positive |
| GT41 | Isocorynantheic acid | Confirmed | Positive |
| GT42 | β-Yohimbin | Confirmed | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Borjigin, G.; Sun, J.; Li, Q.; Wang, Q.; Mu, Y.; Shi, X.; Li, Q.; Wang, X.; Song, X.; et al. Identification of Uncaria rhynchophylla in the Potential Treatment of Alzheimer’s Disease by Integrating Virtual Screening and In Vitro Validation. Int. J. Mol. Sci. 2023, 24, 15457. https://doi.org/10.3390/ijms242015457
Jiang S, Borjigin G, Sun J, Li Q, Wang Q, Mu Y, Shi X, Li Q, Wang X, Song X, et al. Identification of Uncaria rhynchophylla in the Potential Treatment of Alzheimer’s Disease by Integrating Virtual Screening and In Vitro Validation. International Journal of Molecular Sciences. 2023; 24(20):15457. https://doi.org/10.3390/ijms242015457
Chicago/Turabian StyleJiang, Shuang, Gilwa Borjigin, Jiahui Sun, Qi Li, Qianbo Wang, Yuanqiu Mu, Xuepeng Shi, Qian Li, Xiaotong Wang, Xiaodan Song, and et al. 2023. "Identification of Uncaria rhynchophylla in the Potential Treatment of Alzheimer’s Disease by Integrating Virtual Screening and In Vitro Validation" International Journal of Molecular Sciences 24, no. 20: 15457. https://doi.org/10.3390/ijms242015457
APA StyleJiang, S., Borjigin, G., Sun, J., Li, Q., Wang, Q., Mu, Y., Shi, X., Li, Q., Wang, X., Song, X., Wang, Z., & Yang, C. (2023). Identification of Uncaria rhynchophylla in the Potential Treatment of Alzheimer’s Disease by Integrating Virtual Screening and In Vitro Validation. International Journal of Molecular Sciences, 24(20), 15457. https://doi.org/10.3390/ijms242015457






