Effects of Prokineticins on Cerebral Cell Function and Blood–Brain Barrier Permeability
Abstract
1. Introduction
2. Results
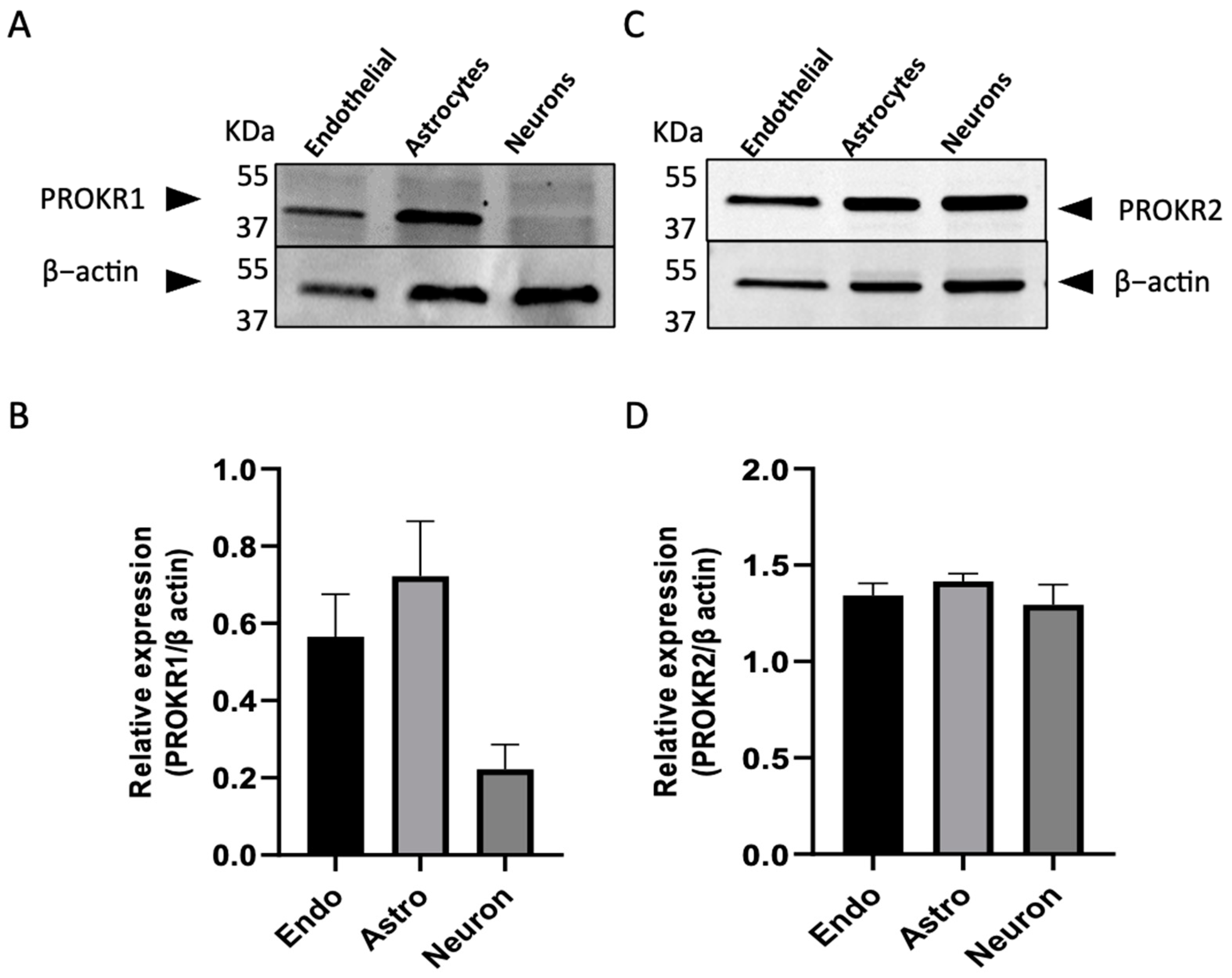
2.1. Expression of Prokineticin Receptors in Cerebral Cells
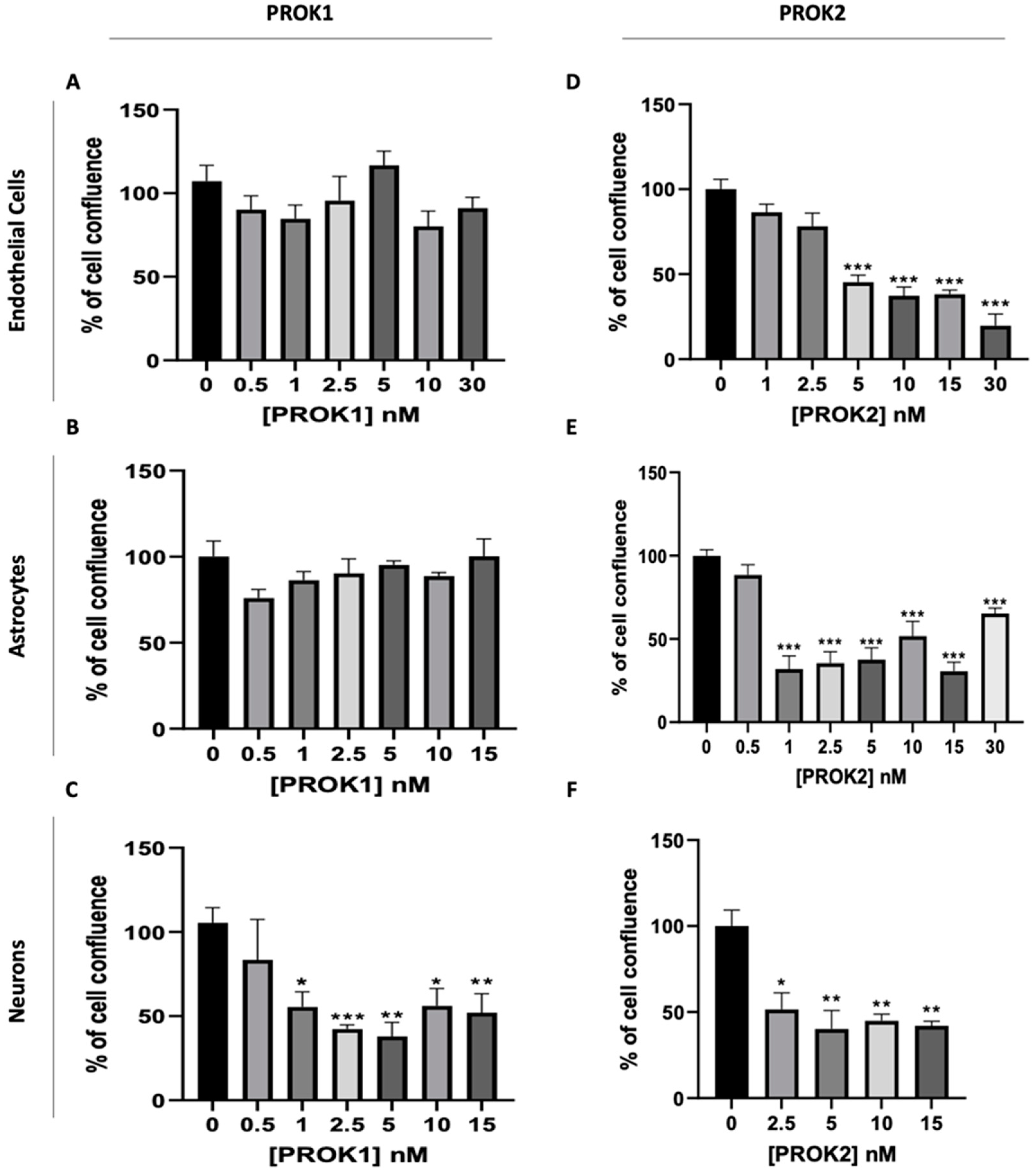
2.2. Effect of PROKs on the Proliferation of Cerebral Cells
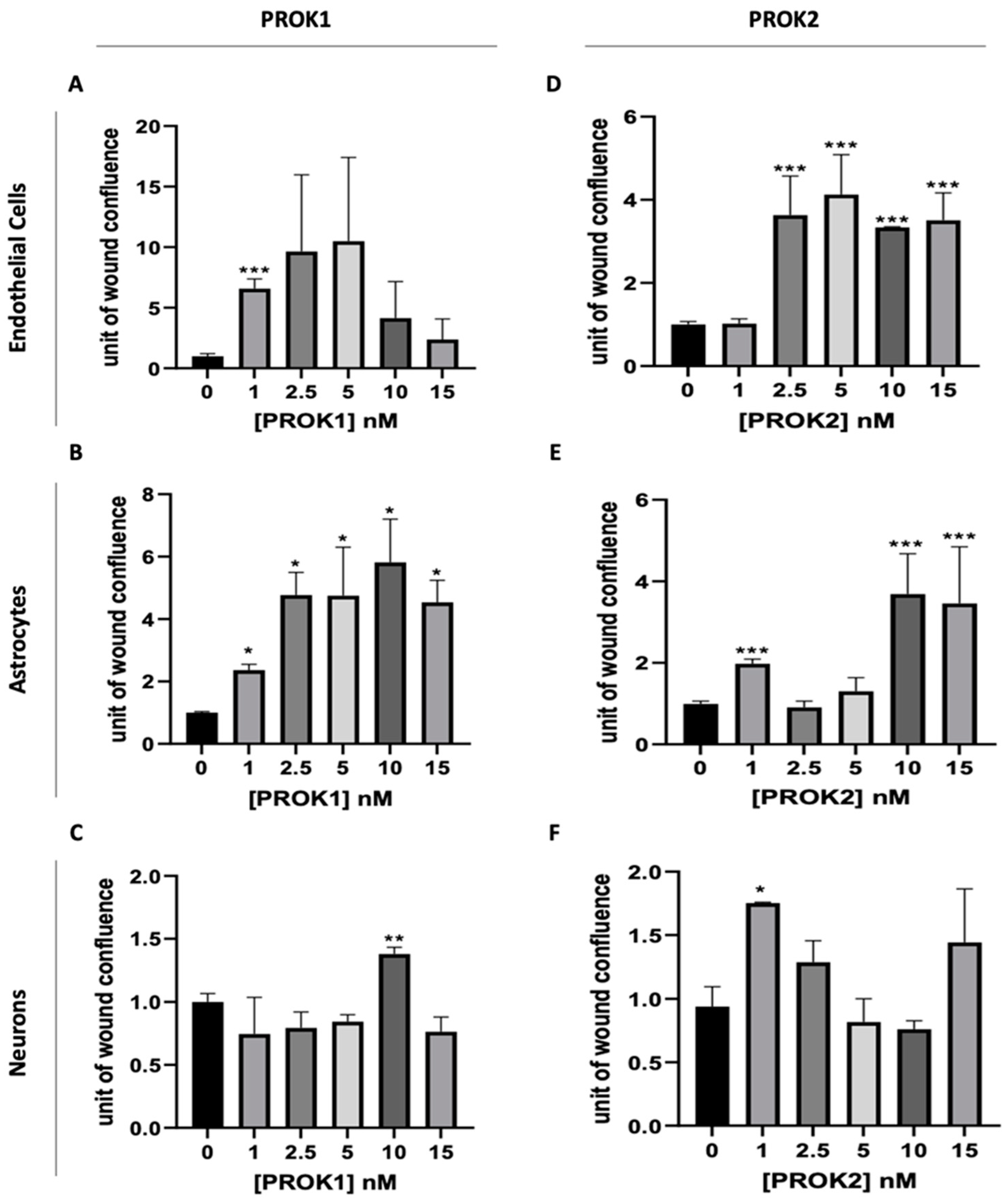
2.3. Effect of PROKs on the Migration of Cerebral Cells
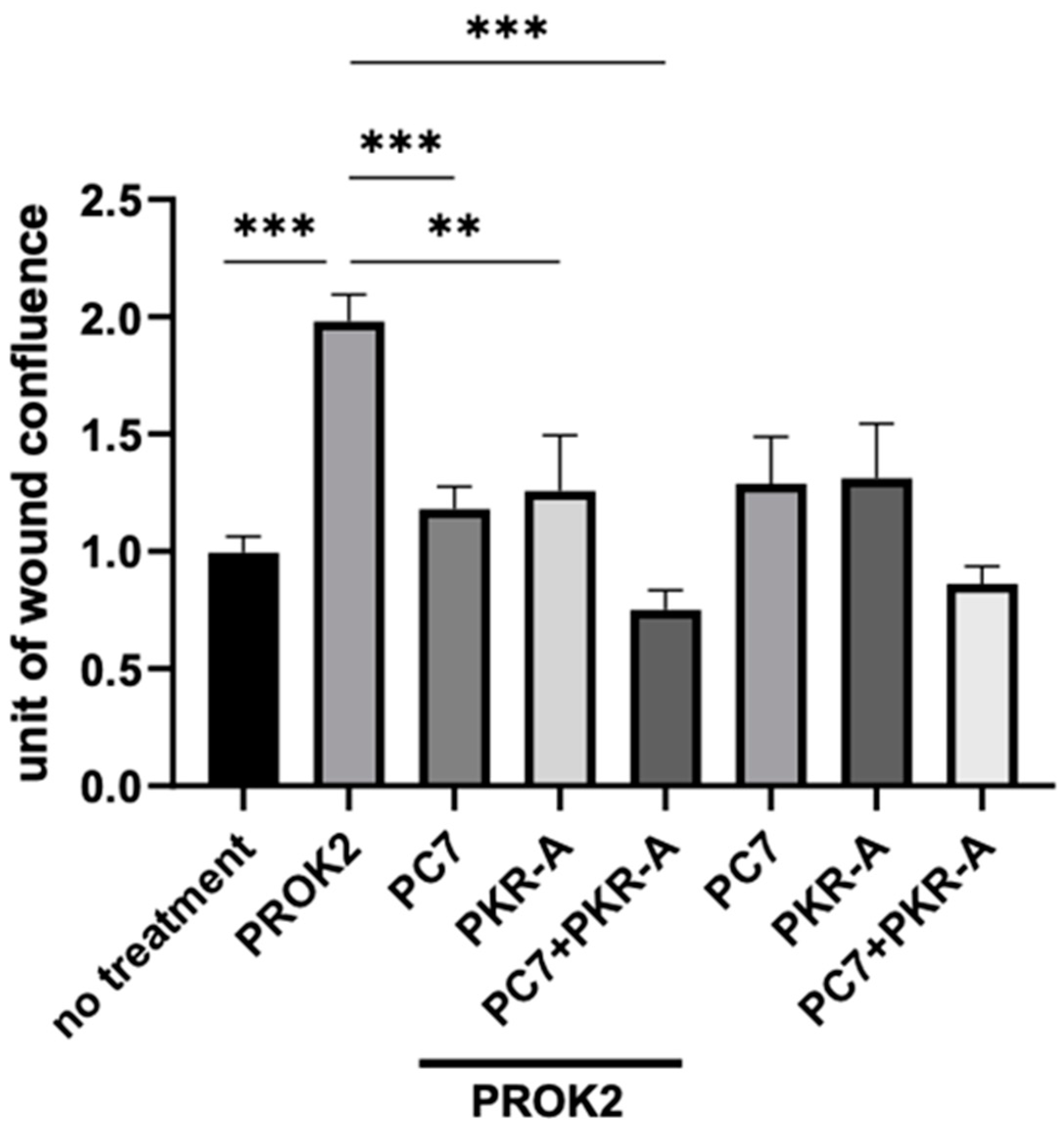
2.4. Effect of PROKRs’ Antagonists on the Migration of Cerebral Cells
2.5. Effect of PROKs and PROKRs’ Antagonist on BBB Co-Culture Model
2.5.1. Assessment of the TEER and Permeability of the Established In Vitro BBB Model
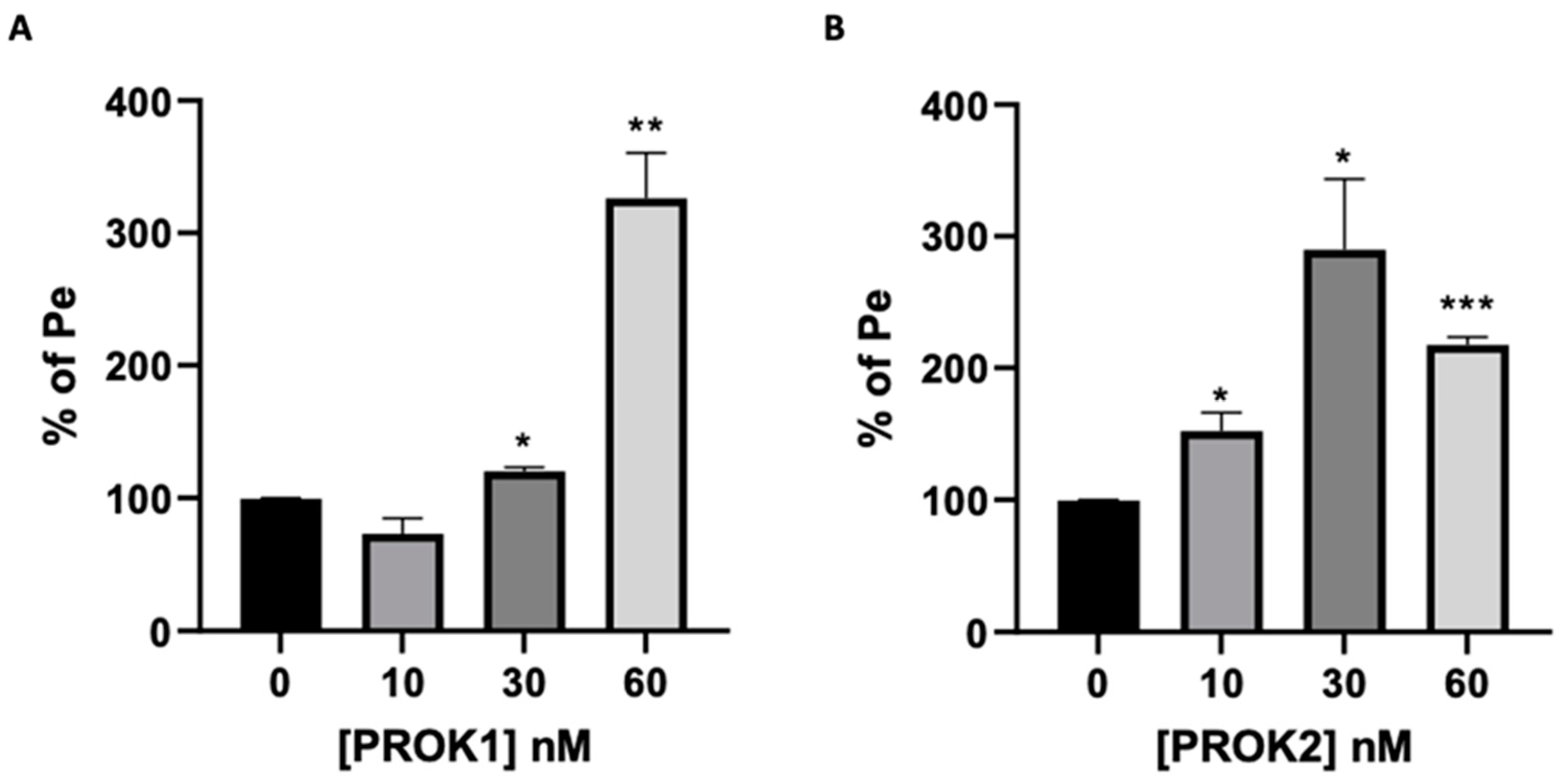
2.5.2. Prokineticins’ Effect on the Integrity of the BBB
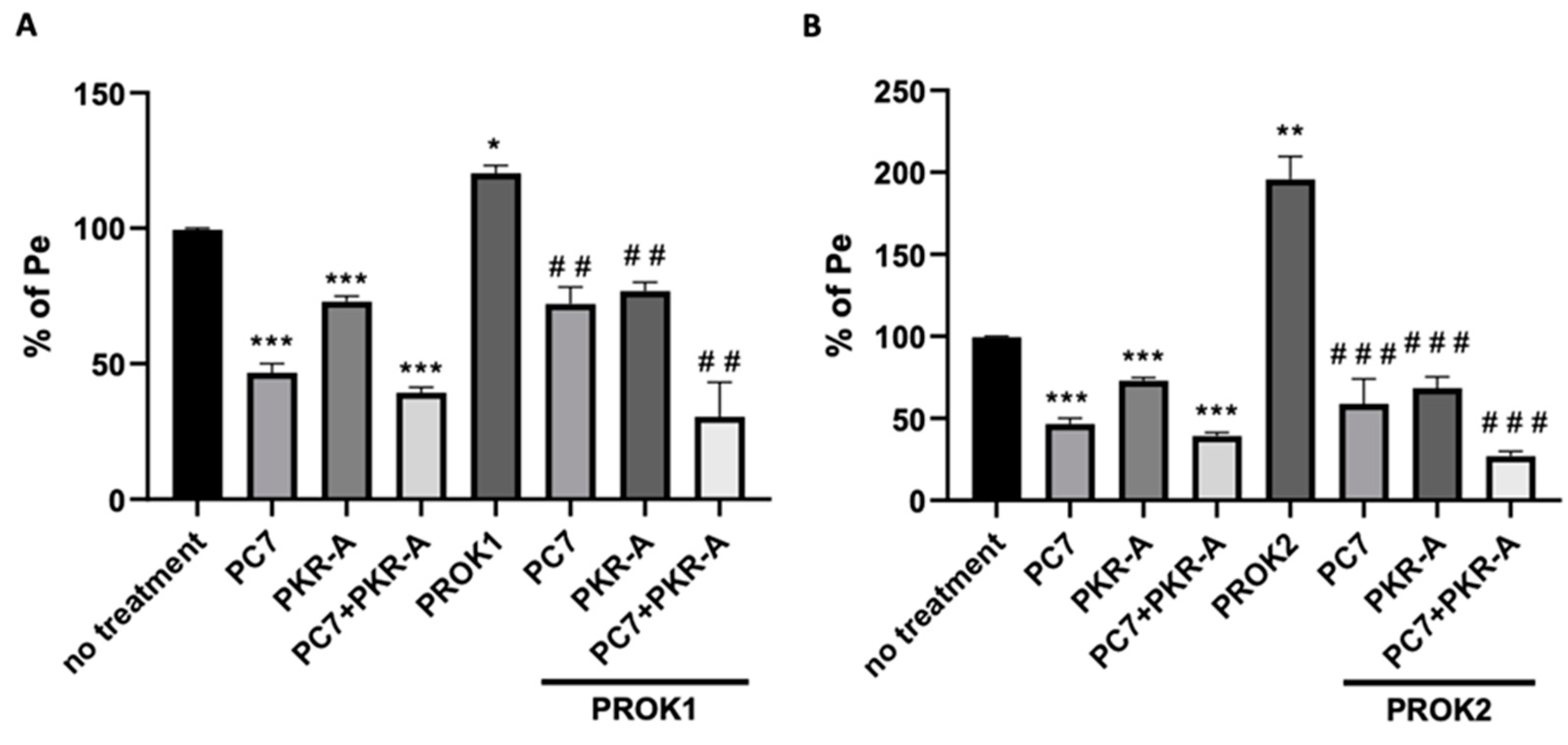
2.5.3. Effect of PROKRs’ Antagonists on the Permeability of the BBB Co-Culture Model
3. Discussion
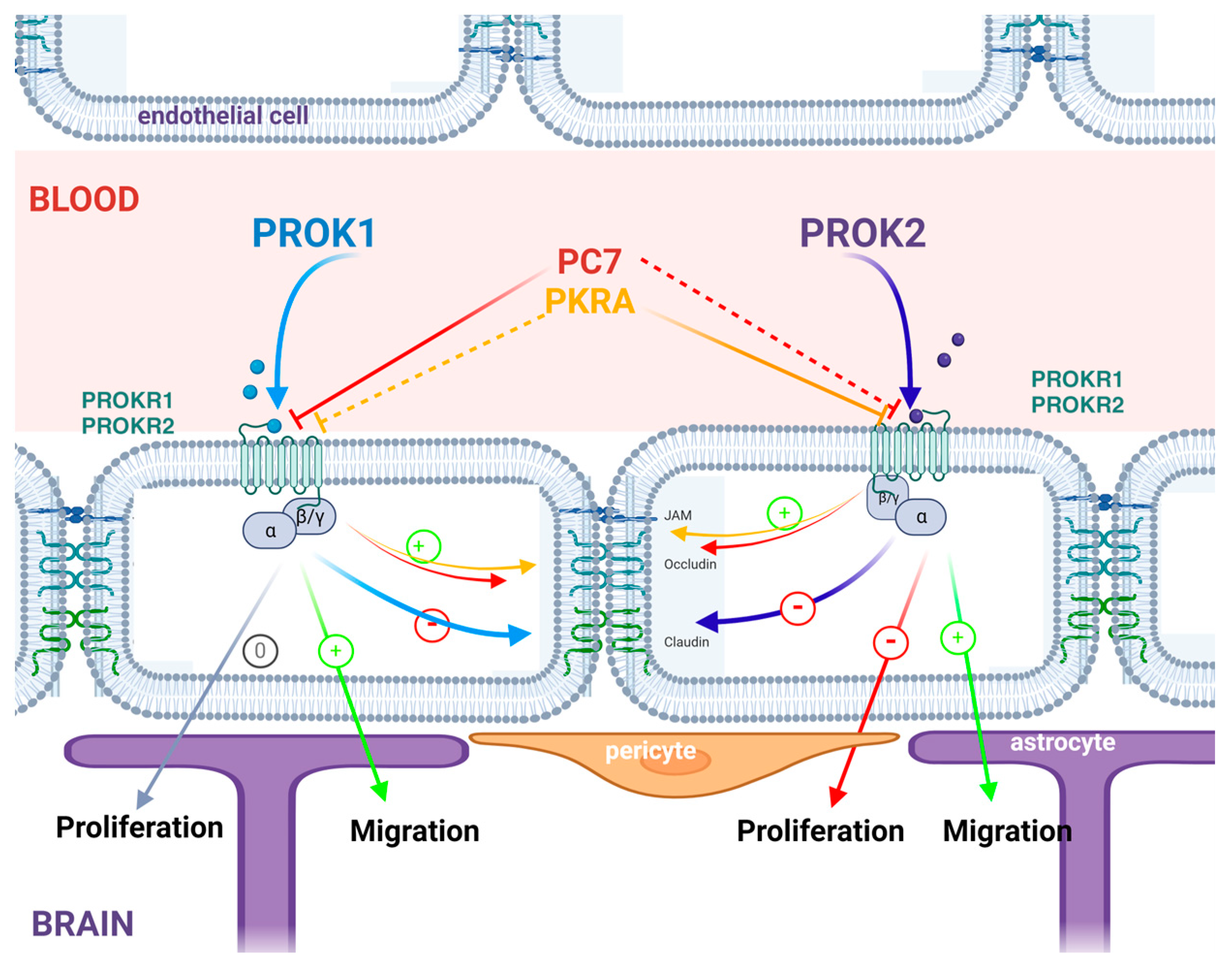
3.1. PROK1 and PROK2 Act on Cerebral Cells’ Proliferation and Migration
3.2. Effect of PROKs and PROKRs’ Antagonists on BBB
4. Materials and Methods
4.1. Compounds
4.2. Murine Brain Cell Culture
4.3. Western Blot
4.4. Proliferation Assay
4.5. Wound-Healing Migration Assay
4.6. Co-Culture of Endothelial Cells and Astrocytes
4.7. TEER Measurement
4.8. Permeability of FITC-Dextran
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joubert, F.J.; Strydom, D.J. Snake Venom. The Amino Acid Sequence of Protein A from Dendroaspis Polylepis Polylepis (Black Mamba) Venom. Hoppe Seylers Z Physiol. Chem. 1980, 361, 1787–1794. [Google Scholar] [CrossRef]
- Schweitz, H.; Pacaud, P.; Diochot, S.; Moinier, D.; Lazdunski, M. MIT(1), a Black Mamba Toxin with a New and Highly Potent Activity on Intestinal Contraction. FEBS Lett. 1999, 461, 183–188. [Google Scholar] [CrossRef]
- Mollay, C.; Wechselberger, C.; Mignogna, G.; Negri, L.; Melchiorri, P.; Barra, D.; Kreil, G. Bv8, a Small Protein from Frog Skin and Its Homologue from Snake Venom Induce Hyperalgesia in Rats. Eur. J. Pharmacol. 1999, 374, 189–196. [Google Scholar] [CrossRef]
- Li, M.; Bullock, C.M.; Knauer, D.J.; Ehlert, F.J.; Zhou, Q.Y. Identification of Two Prokineticin cDNAs: Recombinant Proteins Potently Contract Gastrointestinal Smooth Muscle. Mol. Pharmacol. 2001, 59, 692–698. [Google Scholar] [CrossRef]
- LeCouter, J.; Kowalski, J.; Foster, J.; Hass, P.; Zhang, Z.; Dillard-Telm, L.; Frantz, G.; Rangell, L.; DeGuzman, L.; Keller, G.A.; et al. Identification of an Angiogenic Mitogen Selective for Endocrine Gland Endothelium. Nature 2001, 412, 877–884. [Google Scholar] [CrossRef]
- LeCouter, J.; Lin, R.; Ferrara, N. Endocrine Gland-Derived VEGF and the Emerging Hypothesis of Organ-Specific Regulation of Angiogenesis. Nat. Med. 2002, 8, 913–917. [Google Scholar] [CrossRef]
- Negri, L.; Ferrara, N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent Angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef]
- Kisliouk, T.; Podlovni, H.; Spanel-Borowski, K.; Ovadia, O.; Zhou, Q.-Y.; Meidan, R. Prokineticins (Endocrine Gland-Derived Vascular Endothelial Growth Factor and BV8) in the Bovine Ovary: Expression and Role as Mitogens and Survival Factors for Corpus Luteum-Derived Endothelial Cells. Endocrinology 2005, 146, 3950–3958. [Google Scholar] [CrossRef]
- Ngan, E.S.W.; Lee, K.Y.; Sit, F.Y.L.; Poon, H.C.; Chan, J.K.Y.; Sham, M.H.; Lui, V.C.H.; Tam, P.K.H. Prokineticin-1 Modulates Proliferation and Differentiation of Enteric Neural Crest Cells. Biochim. Biophys. Acta 2007, 1773, 536–545. [Google Scholar] [CrossRef]
- Urayama, K.; Guilini, C.; Messaddeq, N.; Hu, K.; Steenman, M.; Kurose, H.; Ert, G.; Nebigil, C.G. The Prokineticin Receptor-1 (GPR73) Promotes Cardiomyocyte Survival and Angiogenesis. FASEB J. 2007, 21, 2980–2993. [Google Scholar] [CrossRef]
- Keramidas, M.; Faudot, C.; Cibiel, A.; Feige, J.-J.; Thomas, M. Mitogenic Functions of Endocrine Gland-Derived Vascular Endothelial Growth Factor and Bombina Variegata 8 on Steroidogenic Adrenocortical Cells. J. Endocrinol. 2008, 196, 473–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngan, E.S.W.; Sit, F.Y.L.; Lee, K.; Miao, X.; Yuan, Z.; Wang, W.; Nicholls, J.M.; Wong, K.K.Y.; Garcia-Barcelo, M.; Lui, V.C.H.; et al. Implications of Endocrine Gland-Derived Vascular Endothelial Growth Factor/Prokineticin-1 Signaling in Human Neuroblastoma Progression. Clin. Cancer Res. 2007, 13, 868–875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guilini, C.; Urayama, K.; Turkeri, G.; Dedeoglu, D.B.; Kurose, H.; Messaddeq, N.; Nebigil, C.G. Divergent Roles of Prokineticin Receptors in the Endothelial Cells: Angiogenesis and Fenestration. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H844–H852. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Hoffmann, P.; Benharouga, M.; Salomon, A.; Schaal, J.-P.; Feige, J.-J.; Alfaidy, N. Molecular Characterization of EG-VEGF-Mediated Angiogenesis: Differential Effects on Microvascular and Macrovascular Endothelial Cells. Mol. Biol. Cell 2010, 21, 2832–2843. [Google Scholar] [CrossRef]
- Hoffmann, P.; Feige, J.-J.; Alfaidy, N. Expression and Oxygen Regulation of Endocrine Gland-Derived Vascular Endothelial Growth Factor/Prokineticin-1 and Its Receptors in Human Placenta during Early Pregnancy. Endocrinology 2006, 147, 1675–1684. [Google Scholar] [CrossRef]
- Hoffmann, P.; Saoudi, Y.; Benharouga, M.; Graham, C.H.; Schaal, J.-P.; Mazouni, C.; Feige, J.-J.; Alfaidy, N. Role of EG-VEGF in Human Placentation: Physiological and Pathological Implications. J. Cell Mol. Med. 2009, 13, 2224–2235. [Google Scholar] [CrossRef]
- Alfaidy, N.; Hoffmann, P.; Boufettal, H.; Samouh, N.; Aboussaouira, T.; Benharouga, M.; Feige, J.-J.; Brouillet, S. The Multiple Roles of EG-VEGF/PROK1 in Normal and Pathological Placental Angiogenesis. Biomed. Res. Int. 2014, 2014, 451906. [Google Scholar] [CrossRef]
- Nebigil, C.G. Prokineticin Receptors in Cardiovascular Function: Foe or Friend? Trends Cardiovasc. Med. 2009, 19, 55–60. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhou, Q.Y. Overexpression of Prokineticin 2 in Transgenic Mice Leads to Reduced Circadian Behavioral Rhythmicity and Altered Molecular Rhythms in the Suprachiasmatic Clock. J. Circadian Rhythm. 2018, 16, 13. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Bullock, C.M.; Li, C.; Lee, A.G.; Bermak, J.C.; Belluzzi, J.; Weaver, D.R.; Leslie, F.M.; Zhou, Q.-Y. Prokineticin 2 Transmits the Behavioural Circadian Rhythm of the Suprachiasmatic Nucleus. Nature 2002, 417, 405–410. [Google Scholar] [CrossRef]
- Melchiorri, D.; Bruno, V.; Besong, G.; Ngomba, R.T.; Cuomo, L.; De Blasi, A.; Copani, A.; Moschella, C.; Storto, M.; Nicoletti, F.; et al. The Mammalian Homologue of the Novel Peptide Bv8 Is Expressed in the Central Nervous System and Supports Neuronal Survival by Activating the MAP Kinase/PI-3-Kinase Pathways. Eur. J. Neurosci. 2001, 13, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Lattanzi, R.; Giannini, E.; Melchiorri, P. Bv8/Prokineticin Proteins and Their Receptors. Life Sci. 2007, 81, 1103–1116. [Google Scholar] [CrossRef]
- Ng, K.L.; Li, J.-D.; Cheng, M.Y.; Leslie, F.M.; Lee, A.G.; Zhou, Q.-Y. Dependence of Olfactory Bulb Neurogenesis on Prokineticin 2 Signaling. Science 2005, 308, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.-I.; Yamazaki, C.; Masumoto, K.-H.; Nagano, M.; Naito, M.; Soga, T.; Hiyama, H.; Matsumoto, M.; Takasaki, J.; Kamohara, M.; et al. Abnormal Development of the Olfactory Bulb and Reproductive System in Mice Lacking Prokineticin Receptor PKR2. Proc. Natl. Acad. Sci. USA 2006, 103, 4140–4145. [Google Scholar] [CrossRef]
- Pitteloud, N.; Quinton, R.; Pearce, S.; Raivio, T.; Acierno, J.; Dwyer, A.; Plummer, L.; Hughes, V.; Seminara, S.; Cheng, Y.-Z.; et al. Digenic Mutations Account for Variable Phenotypes in Idiopathic Hypogonadotropic Hypogonadism. J. Clin. Investig. 2007, 117, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dodé, C.; Rondard, P. PROK2/PROKR2 Signaling and Kallmann Syndrome. Front. Endocrinol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Lee, A.G.; Culbertson, C.; Sun, G.; Talati, R.K.; Manley, N.C.; Li, X.; Zhao, H.; Lyons, D.M.; Zhou, Q.-Y.; et al. Prokineticin 2 Is an Endangering Mediator of Cerebral Ischemic Injury. Proc. Natl. Acad. Sci. USA 2012, 109, 5475–5480. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Lattanzi, R.; Gerace, E.; Scartabelli, T.; Balboni, G.; Negri, L.; Pellegrini-Giampietro, D.E. Prokineticins Are Neuroprotective in Models of Cerebral Ischemia and Ischemic Tolerance in Vitro. Neuropharmacology 2016, 108, 39–48. [Google Scholar] [CrossRef]
- Mundim, M.V.; Zamproni, L.N.; Pinto, A.A.S.; Galindo, L.T.; Xavier, A.M.; Glezer, I.; Porcionatto, M. A New Function for Prokineticin 2: Recruitment of SVZ-Derived Neuroblasts to the Injured Cortex in a Mouse Model of Traumatic Brain Injury. Mol. Cell Neurosci. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, Y.; Chen, B.; Miao, Z.; Tu, Y.; Li, C.; Chao, H.; Ye, Y.; Xu, X.; Sun, G.; et al. Prokineticin-2 Prevents Neuronal Cell Deaths in a Model of Traumatic Brain Injury. Nat. Commun. 2021, 12, 4220. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a Is Critical for the Formation and Function of the Blood-Brain Barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T Cell Responses in the Central Nervous System. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of Microglial and Macrophage Responses after Spinal Cord Injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Pansieri, J.; Hadley, G.; Lockhart, A.; Pisa, M.; DeLuca, G.C. Regional Contribution of Vascular Dysfunction in White Matter Dementia: Clinical and Neuropathological Insights. Front. Neurol. 2023, 14, 1199491. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Miele, R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 2021, 9, 1648. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte–Endothelial Interactions and Blood–Brain Barrier Permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Severini, C.; Lattanzi, R.; Maftei, D.; Marconi, V.; Ciotti, M.T.; Petrocchi Passeri, P.; Florenzano, F.; Del Duca, E.; Caioli, S.; Zona, C.; et al. Bv8/Prokineticin 2 Is Involved in Aβ-Induced Neurotoxicity. Sci. Rep. 2015, 5, 15301. [Google Scholar] [CrossRef] [PubMed]
- Caioli, S.; Severini, C.; Ciotti, T.; Florenzano, F.; Pimpinella, D.; Petrocchi Passeri, P.; Balboni, G.; Polisca, P.; Lattanzi, R.; Nisticò, R.; et al. Prokineticin System Modulation as a New Target to Counteract the Amyloid Beta Toxicity Induced by Glutamatergic Alterations in an in Vitro Model of Alzheimer’s Disease. Neuropharmacology 2017, 116, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.; Luo, J.; Harischandra, D.S.; Gordon, R.; Sarkar, S.; Jin, H.; Anantharam, V.; Désaubry, L.; Kanthasamy, A.; Kanthasamy, A. Prokineticin-2 Promotes Chemotaxis and Alternative A2 Reactivity of Astrocytes. Glia 2018, 66, 2137–2157. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Kiyo-oka, M.; Osakada, M.; Horiguchi, N.; Shintani, N.; Ago, Y.; Kakuda, M.; Baba, A.; Matsuda, T. Expression of Prokineticin Receptors in Mouse Cultured Astrocytes and Involvement in Cell Proliferation. Brain Res. 2006, 1112, 65–69. [Google Scholar] [CrossRef]
- Slessareva, J.E.; Ma, H.; Depree, K.M.; Flood, L.A.; Bae, H.; Cabrera-Vera, T.M.; Hamm, H.E.; Graber, S.G. Closely Related G-Protein-Coupled Receptors Use Multiple and Distinct Domains on G-Protein Alpha-Subunits for Selective Coupling. J. Biol. Chem. 2003, 278, 50530–50536. [Google Scholar] [CrossRef]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight Junction Protein Expression and Barrier Properties of Immortalized Mouse Brain Microvessel Endothelial Cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef]
- Idris, F.; Muharram, S.H.; Zaini, Z.; Alonso, S.; Diah, S. Invasion of a Murine in Vitro Blood-Brain Barrier Co-Culture Model by Dengue Virus Serotypes 1 to 4. Arch. Virol. 2019, 164, 1069–1083. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Rubin, L.L.; Staddon, J.M. The Cell Biology of the Blood-Brain Barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in 601 Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef]
- Traboulsi, W.; Sergent, F.; Boufettal, H.; Brouillet, S.; Slim, R.; Hoffmann, P.; Benlahfid, M.; Zhou, Q.Y.; Balboni, G.; Onnis, V.; et al. Antagonism of EG-VEGF Receptors as Targeted Therapy for Choriocarcinoma Progression In Vitro and In Vivo. Clin. Cancer. Res. 2017, 23, 7130–7140. [Google Scholar] [CrossRef]
- LeCouter, J.; Zlot, C.; Tejada, M.; Peale, F.; Ferrara, N. Bv8 and Endocrine Gland-Derived Vascular Endothelial Growth Factor Stimulate Hematopoiesis and Hematopoietic Cell Mobilization. Proc. Natl. Acad. Sci. USA 2004, 101, 16813–16818. [Google Scholar] [CrossRef]
- Dorsch, M.; Qiu, Y.; Soler, D.; Frank, N.; Duong, T.; Goodearl, A.; O’Neil, S.; Lora, J.; Fraser, C.C. PK1/EG-VEGF Induces Monocyte Differentiation and Activation. J. Leukoc. Biol. 2005, 78, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Kindo, M.; Arora, H.; Boulberdaa, M.; Steenman, M.; Nebigil, C.G. Prokineticin Receptor-1-Dependent Paracrine and Autocrine Pathways Control Cardiac Tcf21+ Fibroblast Progenitor Cell Transformation into Adipocytes and Vascular Cells. Sci. Rep. 2017, 7, 12804. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G. Updates on Endothelial Functions of Proangiogenic Prokineticin. Hypertension 2016, 68, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Miele, C. Non-Peptide Agonists and Antagonists of the Prokineticin Receptors. Curr. Issues Mol. Biol. 2022, 44, 6323–6332. [Google Scholar] [CrossRef]
- LeCouter, J.; Lin, R.; Tejada, M.; Frantz, G.; Peale, F.; Hillan, K.J.; Ferrara, N. The Endocrine-Gland-Derived VEGF Homologue Bv8 Promotes Angiogenesis in the Testis: Localization of Bv8 Receptors to Endothelial Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Dormishian, M.; Turkeri, G.; Urayama, K.; Nguyen, T.L.; Boulberdaa, M.; Messaddeq, N.; Renault, G.; Henrion, D.; Nebigil, C.G. Prokineticin Receptor-1 Is a New Regulator of Endothelial Insulin Uptake and Capillary Formation to Control Insulin Sensitivity and Cardiovascular and Kidney Functions. J. Am. Heart Assoc. 2013, 2, e000411. [Google Scholar] [CrossRef]
- Urayama, K.; Dedeoglu, D.B.; Guilini, C.; Frantz, S.; Ertl, G.; Messaddeq, N.; Nebigil, C.G. Transgenic Myocardial Overexpression of Prokineticin Receptor-2 (GPR73b) Induces Hypertrophy and Capillary Vessel Leakage. Cardiovasc. Res. 2009, 81, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Pathophysiological Consequences of VEGF-Induced Vascular Permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Crouch, E.E.; Joseph, T.; Marsan, E.; Huang, E.J. Disentangling Brain Vasculature in Neurogenesis and Neurodegeneration Using Single-Cell Transcriptomics. Trends Neurosci. 2023, 46, 551–565. [Google Scholar] [CrossRef]
- Vincenzi, M.; Kremic, A.; Jouve, A.; Lattanzi, R.; Miele, R.; Benharouga, M.; Alfaidy-Benharouga, N.; Migrenne-Li, S.; Kanthasamy, A.G.; Porcionatto, M.; et al. Therapeutic Potential of Targeting Prokineticin Receptors in Diseases. Pharmacol. Rev. 2023, 75, 1167–1199. [Google Scholar] [CrossRef] [PubMed]
- Balboni, G.; Lazzari, I.; Trapella, C.; Negri, L.; Lattanzi, R.; Giannini, E.; Nicotra, A.; Melchiorri, P.; Visentin, S.; Nuccio, C.D.; et al. Triazine Compounds as Antagonists at Bv8-Prokineticin Receptors. J. Med. Chem. 2008, 51, 7635–7639. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younes, H.; Kyritsi, I.; Mahrougui, Z.; Benharouga, M.; Alfaidy, N.; Marquette, C. Effects of Prokineticins on Cerebral Cell Function and Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2023, 24, 15428. https://doi.org/10.3390/ijms242015428
Younes H, Kyritsi I, Mahrougui Z, Benharouga M, Alfaidy N, Marquette C. Effects of Prokineticins on Cerebral Cell Function and Blood–Brain Barrier Permeability. International Journal of Molecular Sciences. 2023; 24(20):15428. https://doi.org/10.3390/ijms242015428
Chicago/Turabian StyleYounes, Hadi, Ioanna Kyritsi, Zineb Mahrougui, Mohamed Benharouga, Nadia Alfaidy, and Christel Marquette. 2023. "Effects of Prokineticins on Cerebral Cell Function and Blood–Brain Barrier Permeability" International Journal of Molecular Sciences 24, no. 20: 15428. https://doi.org/10.3390/ijms242015428
APA StyleYounes, H., Kyritsi, I., Mahrougui, Z., Benharouga, M., Alfaidy, N., & Marquette, C. (2023). Effects of Prokineticins on Cerebral Cell Function and Blood–Brain Barrier Permeability. International Journal of Molecular Sciences, 24(20), 15428. https://doi.org/10.3390/ijms242015428






