Hepatoprotective and Antioxidant Effects of Nanopiperine against Cypermethrin via Mitigation of Oxidative Stress, Inflammations and Gene Expression Using qRT-PCR
Abstract
:1. Introduction
2. Results
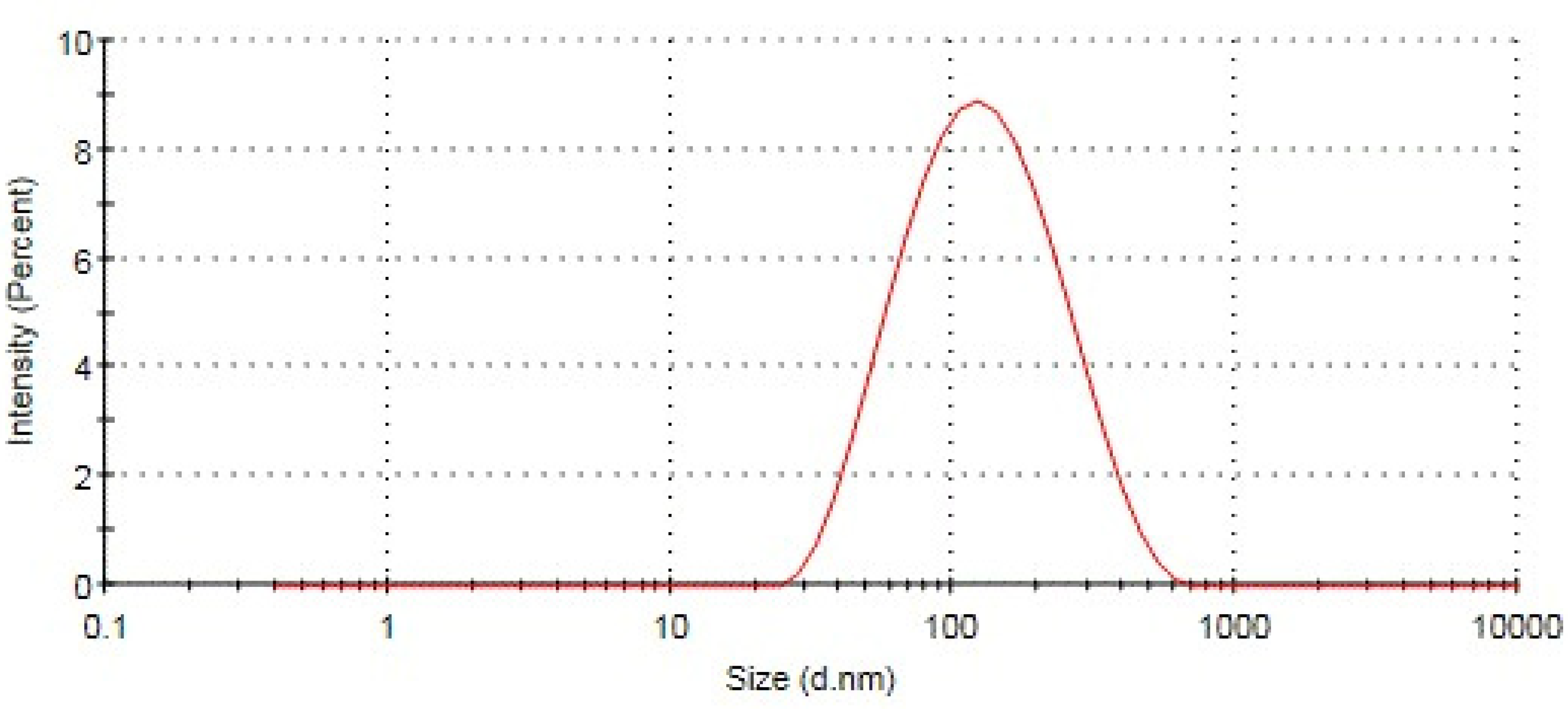
2.1. Particle Size, Polydispersity Index, and Zeta Potential
2.2. Blood Serum Assay
2.3. Effects on LPO and GSH
2.4. Effects on SOD and Catalase
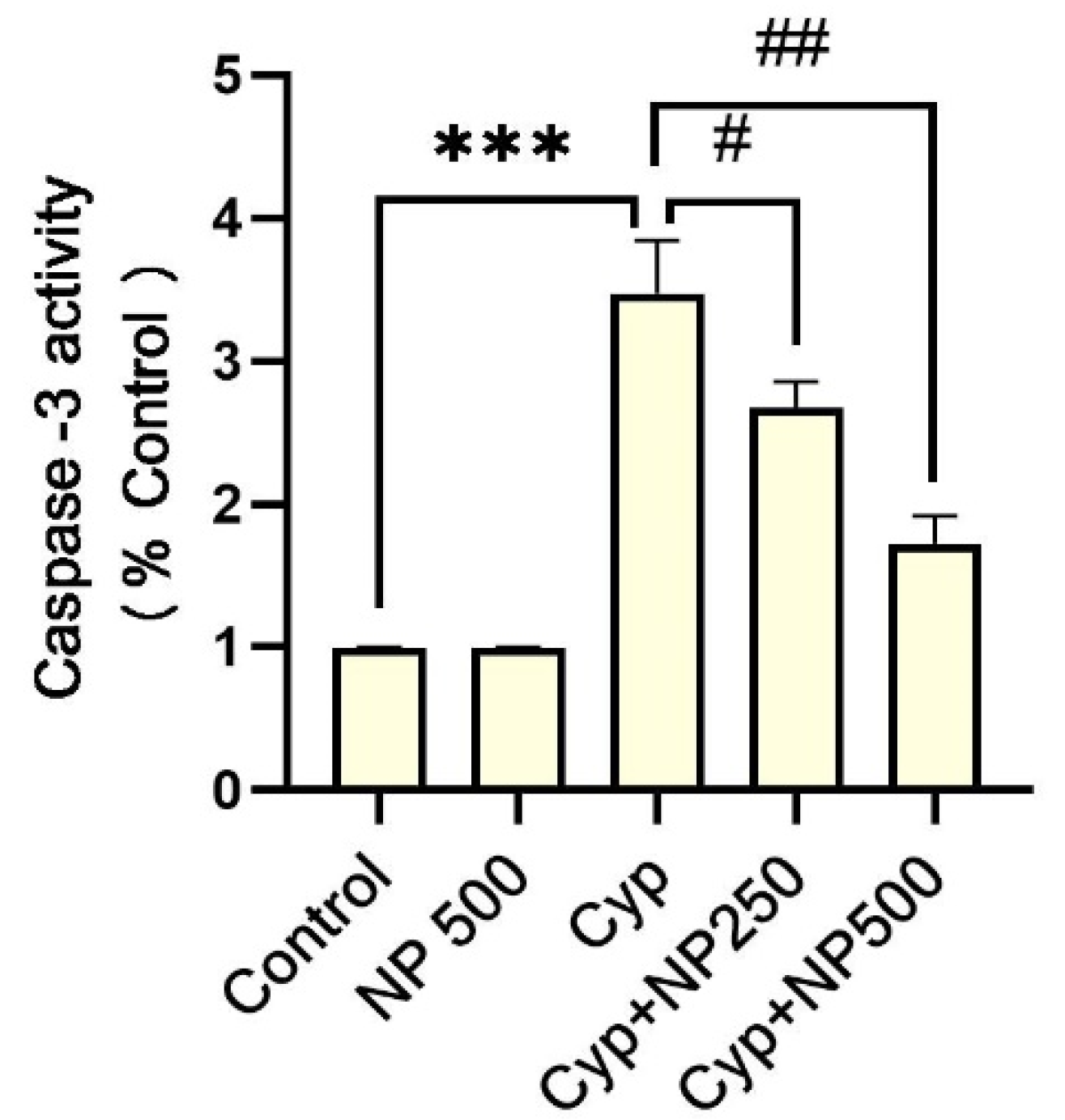
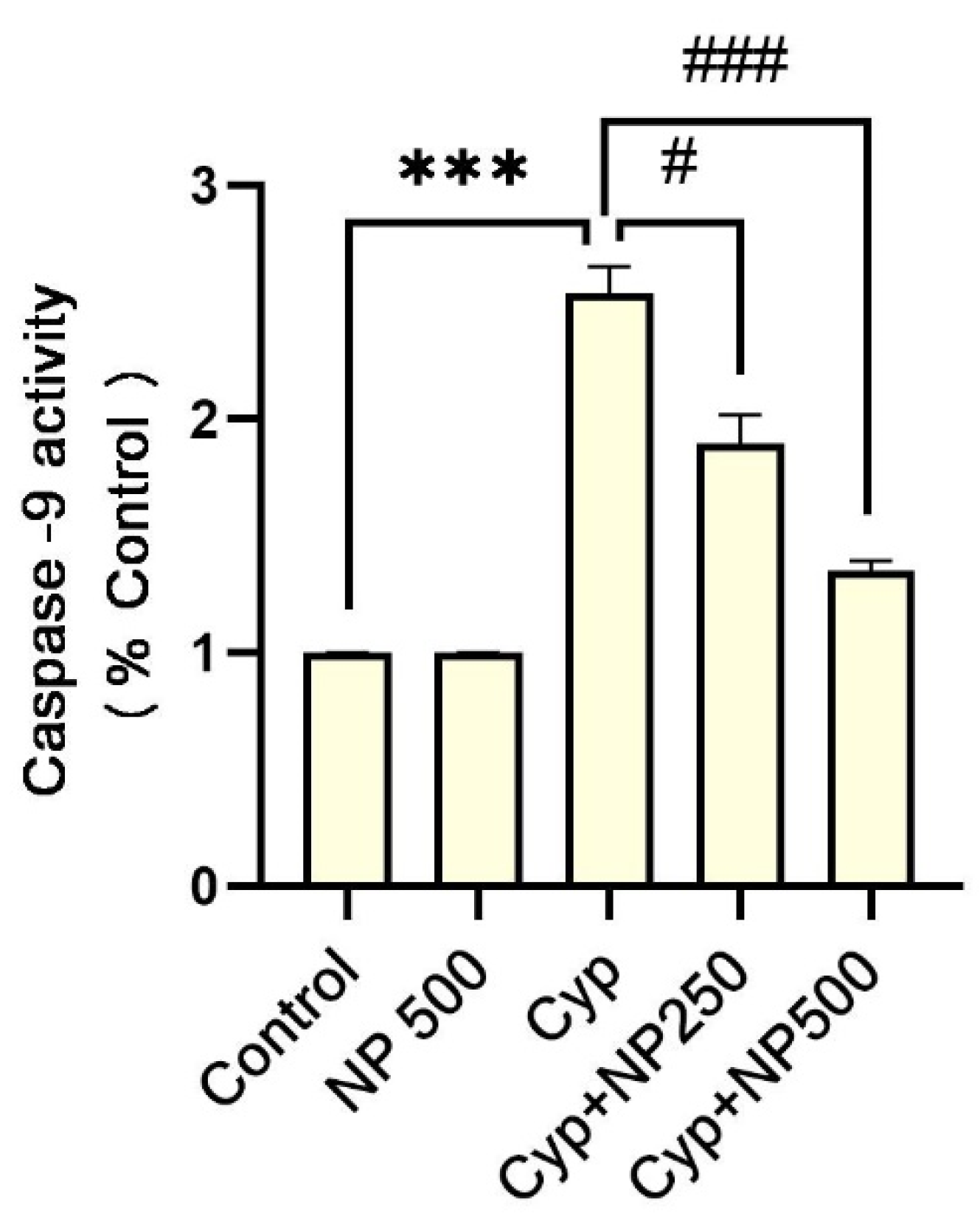
2.5. Caspases Assay
2.6. Inflammatory Markers
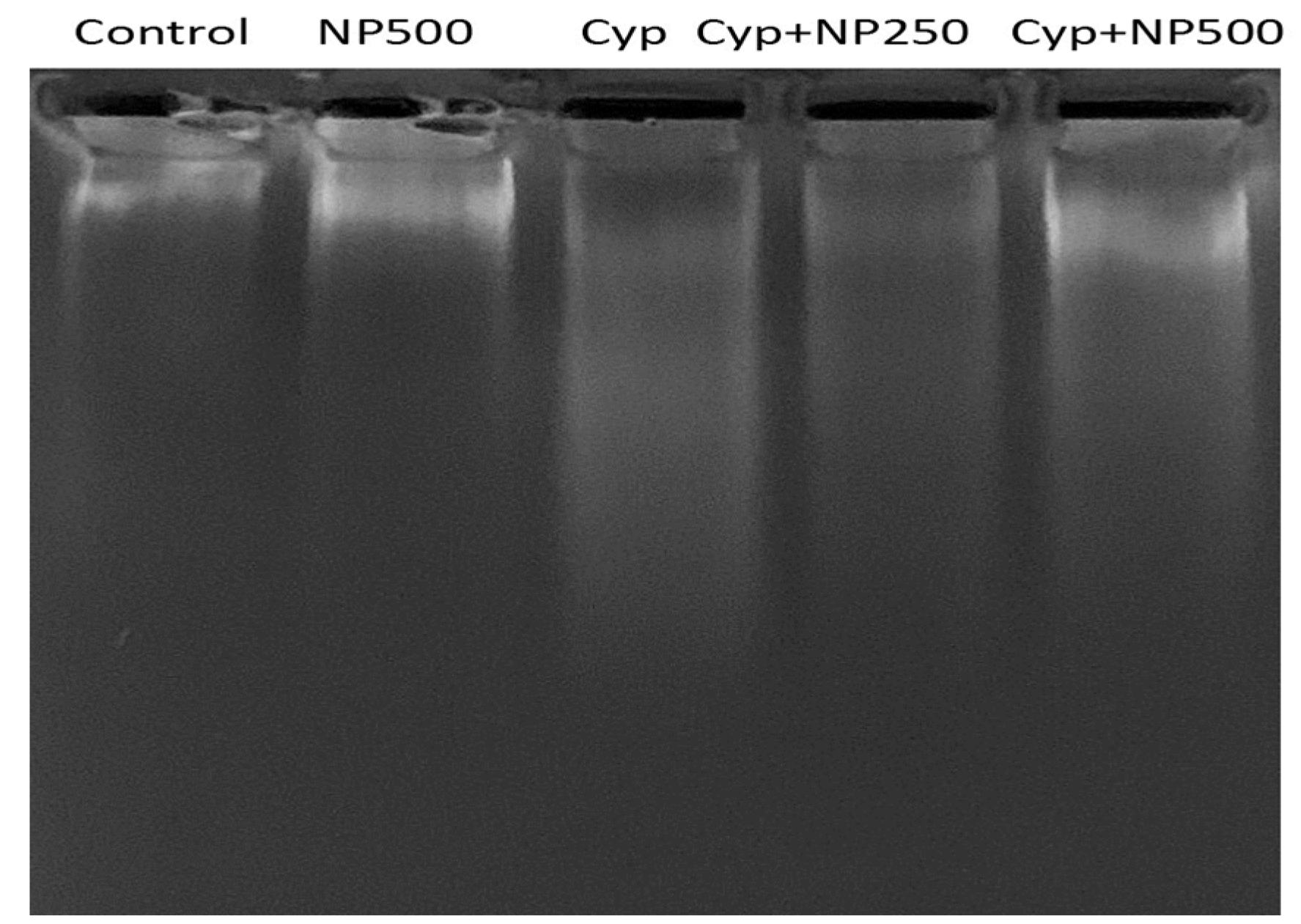
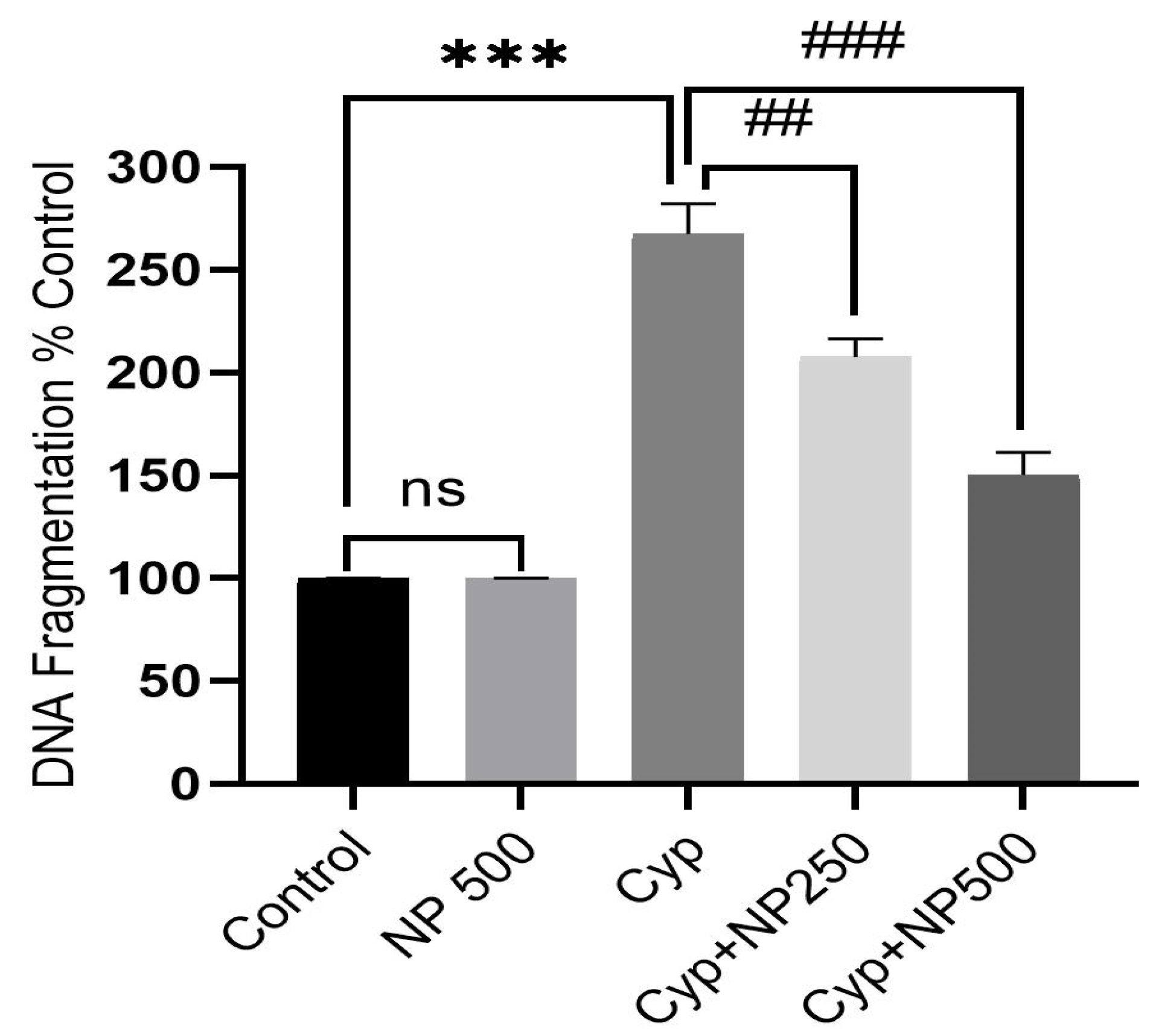
2.7. DNA Fragmentation Produced by Cyp
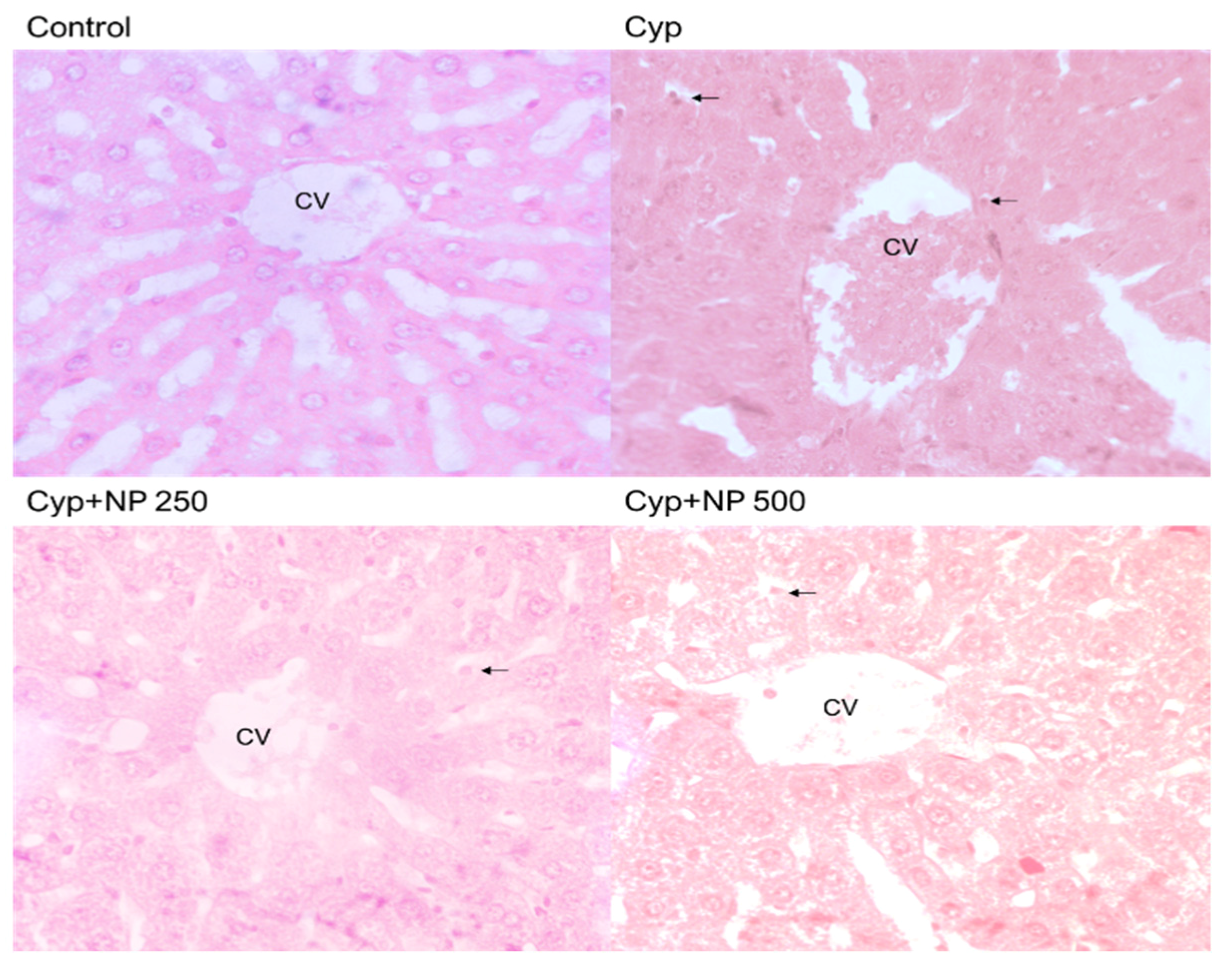
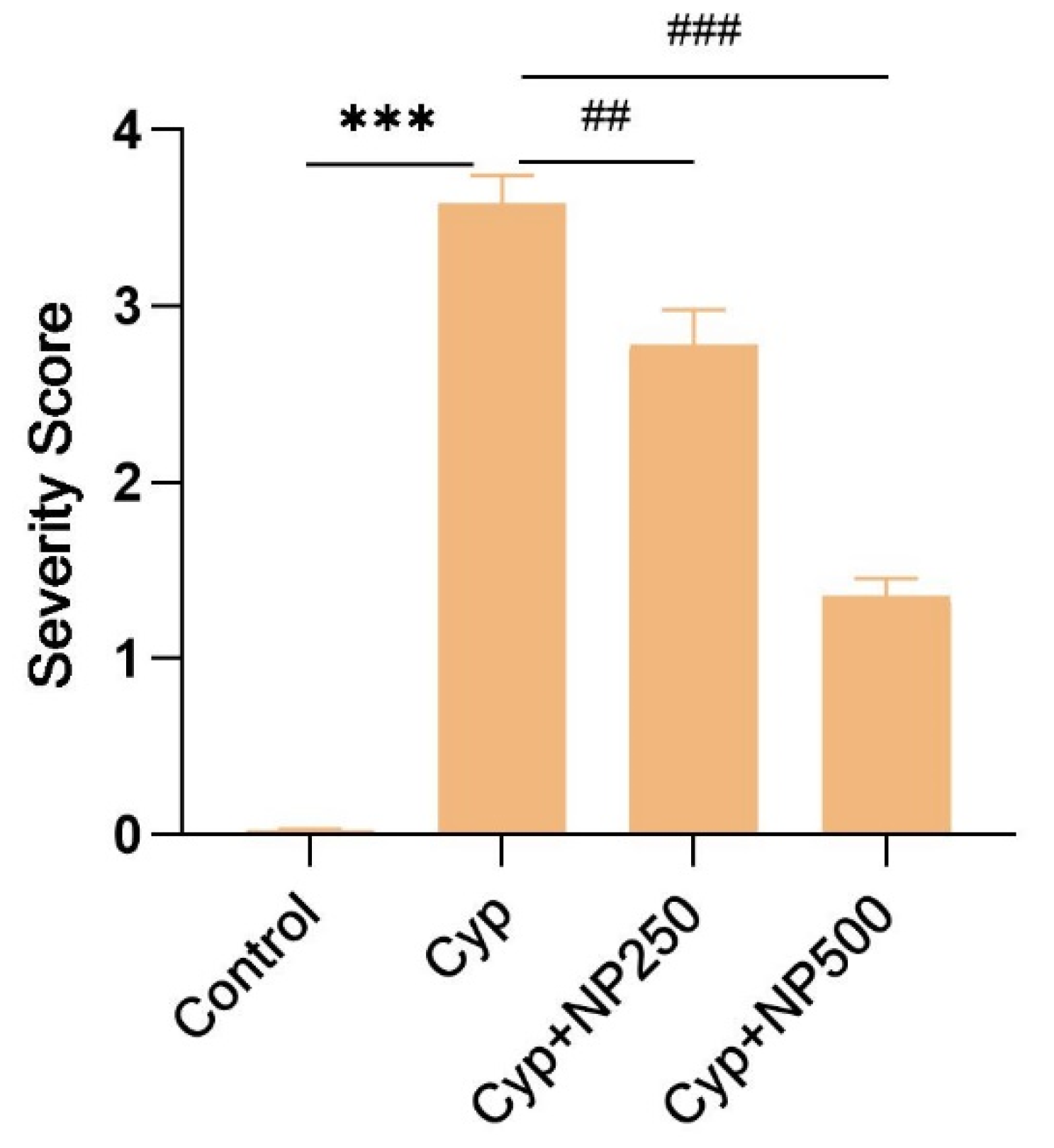
2.8. Histopathological Studies
2.9. Effect of NP on RNA Expression
3. Discussion
4. Materials and Method
4.1. Chemicals
4.2. Animals
4.3. Nano Formulation of Piperine (NP)
4.4. Dose Selection
4.5. Experimental Design
4.6. Sample Preparation
4.7. Serum Biochemical Assay
4.8. Estimation of LPO and GSH
4.9. Estimation of Activity of SOD
4.10. Estimation of Catalase Activity
4.11. Estimation of Interleukin Cytokines (IL-6 and IL-1β) and Apoptotic Markers (Caspase-3 and -9)
4.12. DNA Damage and Apoptosis Assay
4.13. Quantitative Real Time Polymerase Chain Reaction
4.14. Liver Histopathological Studies
4.15. Estimation of Protein
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Manfo, F.P.T.; Mboe, S.A.; Nantia, E.A.; Ngoula, F.; Telefo, P.B.; Moundipa, P.F.; Cho-Ngwa, F. Evaluation of the effects of agro pesticides use on liver and kidney function in farmers from Buea, Cameroon. J. Toxicol. 2020, 2020, 2305764. [Google Scholar] [CrossRef]
- Blair, A.; Ritz, B.; Wesseling, C.; Freeman, L.B. Pesticides and human health. Occup. Environ. Med. 2015, 72, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.O. Pesticides and cancer. Cancer Causes Control 1997, 8, 420–443. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Rull, R.P. Assessment of environmental exposures from agricultural pesticides in childhood leukemia studies: Challenges and opportunities. Radiat. Prot. Dosim. 2008, 132, 148–155. [Google Scholar] [CrossRef]
- Gunier, R.B.; Ward, M.H.; Airola, M.; Bell, E.M.; Colt, J.; Nishioka, M.; Nuckols, J.R. Determinants of agricultural pesticide concentrations in carpet dust. Environ. Health Perspect. 2011, 119, 970–976. [Google Scholar] [CrossRef]
- Gunier, R.B.; Harnly, M.E.; Reynolds, P.; Hertz, A.; Von, B.J. Agricultural pesticide use in California: Pesticide prioritization, use densities, and population distributions for a childhood cancer study. Environ. Health Perspect. 2001, 109, 1071–1078. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Khan, S.A.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef]
- Jin, X.; Chen, M.; Song, L.; Li, H.; Li, Z. The evaluation of p,p′-DDT exposure on cell adhesion of hepatocellular carcinoma. Toxicology 2014, 322, 99–108. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, W.; Liu, J. Environmentally relevant exposure to cypermethrin aggravates diet-induced diabetic symptoms in mice: The interaction between environmental chemicals and diet. Environ. Int. 2023, 178, 108090. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Bhattacharyya, D.; Mandal, T.K.; Das, S. Repeated dose toxicity of alfa-cypermethrin in rats. J. Vet. Sci. 2004, 5, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Abdou, R.H.; Sayed, N. Antioxidant and Anti-Inflammatory Effects of Nano-Selenium against Cypermethrin-Induced Liver Toxicity. Cell Bio. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Kasuba, V.; Tariba Lovakovic, B.; Lucic Vrdoljak, A.; Katic, A.; Kopjar, N.; Micek, V.; Milic, M.; Pizent, A.; Zeljezic, D.; Zunec, S. Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats. Toxics 2022, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.F.H.; El-Sayed, N.M.; Khodeer, D.M.; Ahmed, A.A.M.; Hanna, P.A.; Moustafa, Y.M.A. Nano selenium ameliorates oxidative stress and inflammatory response associated with cypermethrin-induced neurotoxicity in rats. Ecotoxicol. Environ. Saf. 2020, 195, 110479. [Google Scholar] [CrossRef]
- Sankar, D.; Sambandam, G.; Ramakrishna Rao, M.; Pugalendi, K.V. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin. Chim. Acta 2005, 355, 97–104. [Google Scholar] [CrossRef]
- Giray, B.; Gürbay, A.; Hincal, F. Cypermethrin-Induced Oxidative Stress in Rat Brain and Liver Is Prevented by Vitamin E or Allopurinol. Toxicol. Lett. 2001, 118, 139–146. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hussien, H.M.; Yousef, M.I. Deleterious effects of cypermethrin on rat liver and kidney: Protective role of sesame oil. J. Environ. Sci. Health B 2012, 47, 306–314. [Google Scholar] [CrossRef]
- Ileriturk, M.; Kandemir, O.; Kandemir, F.M. Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ. Toxicol. 2022, 37, 2639–2650. [Google Scholar] [CrossRef]
- Aziz, F.M.; Maulood, I.M.; Chawsheen, M.A. Effects of melatonin, vitamin C and E alone or in combination on lead-induced injury in liver and kidney organs of rats. Pak. J. Zool. 2014, 46, 1425–1431. [Google Scholar] [CrossRef]
- Qin, B.; Yang, K.; Cao, R. Synthesis and antioxidative activity of piperine derivatives containing phenolic hydroxyl. J. Chem. 2020, 2020, 1–99. [Google Scholar] [CrossRef]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Protective effects of amide constituents from the fruit of Piper chaba on D-galactosamine/ TNF-α-induced cell death in mouse hepatocytes. Bioorganic Med. Chem. Lett. 2008, 18, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Siddiqui, R.; Alam, M.I.; Mohammed, M.; Almoshari, Y.; Alqahtani, S.S. Cardioprotective Effects of Nano-Piperine Against Cypermethrin Toxicity Through Oxidative Stress, Histopathological and Immunohistochemical Studies in Male Wistar Rats. Nat. Prod. Commun. 2023, 18, 1934578X231154029. [Google Scholar] [CrossRef]
- Preetz, C.; Rübe, A.; Reiche, I.; Hause, G.; Mäder, K. Preparation and characterization of biocompatible oil-loaded polyelectrolyte nanocapsules. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B.; Ur Rehman, S.; Saleemi, M.K. Nephroprotective effects of curcumin loaded chitosan nanoparticles in cypermethrin induced renal toxicity in rabbits. Environ. Sci. Pollut. Res. Int. 2020, 27, 14771–14779. [Google Scholar] [CrossRef]
- Ashafaq, M.; Hussain, S.; Alshahrani, S.; Siddiqui, R.; Alam, M.I.; Elhassan Taha, M.M.; Almoshari, Y.; Alqahtani, S.S.; Jali, A.M.; Aljohani, H.M. Neuroprotective Effects of Nano-Curcumin against Cypermethrin Associated Oxidative Stress and Up-Regulation of Apoptotic and Inflammatory Gene Expression in Rat Brains. Antioxidants 2023, 12, 644. [Google Scholar] [CrossRef]
- Yousef, M.I.; Omar, S.M.A.M.; El-Guendi, M.I.; Abdelmegid, L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010, 48, 3246–3261. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M.; Kamel, K.I.; Al-Salhen, K.S. Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicology 2003, 189, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Rivarola, V.A.; Balegno, H.F. Effect of 2,4-dichlorophenxyacetic acid on polyamine synthesis in Chinese hamster ovary cells. Toxicol. Lett. 1991, 56, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Al-Omair, M.A.; Sedky, A.; Al-Otaibi, L. Protective effect of α-lipoic acid against α-cypermethrin-induced changes in rat cerebellum. J. Chem. Neuroanat. 2017, 86, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.A. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2004, 37, 1499–1507. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney, and heart of high-fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Nachiappan, V. Cassia auriculata flower extract attenuates hyperlipidemia in male Wistar rats by regulating the hepatic cholesterol metabolism. Biomed Pharm. 2017, 95, 394–401. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants, and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef]
- Mendes, A.F.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Hydrogen peroxide mediates interleukin-1beta induced AP-1 activation in articular chondrocytes: Implications for the regulation of iNOS expression. Cell Biol. Toxicol. 2003, 19, 203–214. [Google Scholar] [CrossRef]
- Abdul-Hamida, M.; Moustafa, N.; Abd Alla Asranb, A.M.A.; Mowafyb, L. Cypermethrin-induced histopathological, ultrastructural and biochemical changes in the liver of albino rats: The protective role of propolis and curcumin. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 160–173. [Google Scholar] [CrossRef]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Bokar, I.A.; Siddiqui, R.; Alam, M.I.; Taha, M.M.E.; Almoshari, Y.; Alqahtani, S.S.; Ahmed, R.A.; et al. Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats. Molecules 2023, 28, 881. [Google Scholar] [CrossRef] [PubMed]
- Sabina, E.P.; Souriyan, A.D.H.; Jackline, D.; Rasool, M.K. Piperine, an active ingredient of black pepper attenuates acetaminophen-induced hepatotoxicity in mice. Asian Pac. J. Trop. Med. 2010, 3, 971–976. [Google Scholar] [CrossRef]
- Katragadda, V.; Adem, M.; Mohammad, R.A.; Sri Bhasyam, S.; Battini, K. Testosterone recuperates deteriorated male fertility in cypermethrin-intoxicated rats. Toxicol. Res. 2021, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.K.; Kaur, K.P.; Khera, K.S.; Singh, B. Toxicological effects of cypermethrin on female albino rats. Toxicol. Int. 2011, 18, 5–8. [Google Scholar] [CrossRef]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Siddiqui, R.; Ahmed, R.A.; Khuwaja, G.; Islam, F. Cinnamon oil against acetaminophen-induced acute liver toxicity by attenuating inflammation, oxidative stress and apoptosis. Toxicol. Rep. 2020, 7, 1296–1304. [Google Scholar] [CrossRef]
- Hussain, S.; Alshahrani, S.; Siddiqui, R.; Khan, A.; Elhassan Taha, M.M.; Ahmed, R.A.; Jali, A.M.; Qadri, M.; Khairat, K.H.M.; Ashafaq, M. Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation. Plants 2023, 12, 2290. [Google Scholar] [CrossRef]
- Alshahrani, S.; Ashafaq, M.; Hussain, S.; Mohammed, M.; Sultan, M.; Jali, A.M.; Siddiqui, R.; Islam, F. Renoprotective effects of cinnamon oil against APAP-Induced nephrotoxicity by ameliorating oxidative stress, apoptosis and inflammation in rats. Saudi Pharm. J. 2021, 29, 194–200. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ashafaq, M.; Tabassum, H.; Parvez, S. Modulation of Behavioral Deficits and Neurodegeneration by Tannic Acid in Experimental Stroke Challenged Wistar Rats. Mol. Neurobiol. 2016, 54, 5941–5951. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]


| Groups | ALT | AST | ALP | Protein | Albumin |
|---|---|---|---|---|---|
| Control | 40.52 ± 5.63 | 37.11 ± 2.92 | 92.883 ± 9.675 | 8.882 ± 0.067 | 4.36 ± 1.15 |
| NP500 | 44.378 ± 6.52 | 31.073 ± 0.983 | 85.614 ± 3.018 | 7.31 ± 0.1.28 | 4.07 ± 0.88 |
| Cyp | 179.46 ± 12.29 *** | 81.051 ± 7.35 *** | 181.831 ± 1.527 *** | 4.01 ± 0.052 ** | 2.19 ± 1.86 ** |
| Cyp + NP 250 | 158.51 ± 9.84 ## | 51.66 ± 4.42 ## | 149.62 ±11.04 ### | 6.37 ± 0.48 # | 3.01 ± 1.05 ## |
| Cyp + NP 500 | 95.66 ± 8.42 ### | 44.56 ± 5.37 ### | 131.79 ±11.53 ### | 7.85 ± 1.03 ## | 3.25 ± 2.13 ### |
| Groups | MDA (nmol/g Tissue) | GSH (DTNB Conjugate Formed/mg Protein) | SOD (nmol Epinephrine Protected from Oxidation/min/mg Protein) | CAT (nmol of H2O2 Consumed/min/mg Protein) |
|---|---|---|---|---|
| Control | 1.148 ± 0.059 | 28.117 ± 1.056 | 25.883 ± 2.675 | 5.882 ± 0.067 |
| NP500 | 1.198 ± 0.068 | 29.073 ± 0.983 | 26.614 ± 3.018 | 6.001 ± 0.083 |
| Cyp | 2.081 ± 0.161 *** | 17.051 ± 1.681 *** | 14.831 ± 1.527 *** | 3.181 ± 0.052 ** |
| Cyp + NP250 | 1.781 ± 0.089 # | 21.129 ± 1.671 # | 19.390 ± 2.118 # | 4.173 ± 0.064 # |
| Cyp + NP500 | 1.354 ± 0.087 ### | 24.280 ± 1.143 ## | 23.137 ± 2.482 ## | 4.951 ± 0.48 ## |
| Groups | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|
| Control | 224.67 ± 20.56 | 281.40 ± 21.63 | 236.75 ± 17.96 |
| NP500 | 223.98 ± 21.35 | 282.67 ± 19.43 | 235.88 ± 23.48 |
| Cyp | 845.19 ± 32.72 *** | 910.27 ± 36.79 *** | 518.07 ± 33.79 *** |
| Cyp + NP250 | 571.34 ± 26.37 ## | 710.91 ± 31.58 # | 362.43 ± 28.56 ## |
| Cyp + NP500 | 411.95 ± 18.26 ### | 522.64 ± 27.11 ### | 259.21 ± 19.74 ### |
| Oligo | Primer Sequences | Tm (c) |
|---|---|---|
| IL-1beta | 5′-ATGGCAACCGTACCTGAACCCA-3′ F 5′-GCTCGAAATGTCCCAGGAA-3′ R | 64.0 58.4 |
| IL-6 | 5′-AGTTGCTTCTTGGGACTGATGT-3′ F 5′-TGCTCTGAATGACTCTGGCTTTG-3′ R | 62.9 62.9 |
| NF-kB | 5′-CATGAAGAGAAGACACTGACC-3′ F 5′-TGGATAGAGGCTAAGTGTAGA-3′ R | 59.4 57.4 |
| Bax | 5′-GCCTCCTTTCCTACTTCGGG-3′ F 5′-CTTTCCCCGTTCCCCATTCA-3′ R | 62.5 60.5 |
| Caspase-9 | 5′-TGTCCTACTCTACTTTCCCAGGT-3′ F 5′- GTGAGCCCACTGCTCAAAGAT -3′ R | 62.5 60.5 |
| Caspase-3 | 5′-GCTACGATCCACCAGCATTT-3′ F 5′-ATGCCACCTCTCCTTTCCTT-3′ R | 58.4 58.4 |
| Beta-actin | 5′-CAACCTTCTTGCAGCTCCTC-3′ F 5′-TTCTGACCCATACCCACCAT-3′ R | 60.5 58.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Jali, A.M.; Alshahrani, S.; Khairat, K.H.M.; Siddiqui, R.; Alam, M.I.; Ali, R.; Mohammed, M.; Khan, A.; Al Shahi, H.; et al. Hepatoprotective and Antioxidant Effects of Nanopiperine against Cypermethrin via Mitigation of Oxidative Stress, Inflammations and Gene Expression Using qRT-PCR. Int. J. Mol. Sci. 2023, 24, 15361. https://doi.org/10.3390/ijms242015361
Hussain S, Jali AM, Alshahrani S, Khairat KHM, Siddiqui R, Alam MI, Ali R, Mohammed M, Khan A, Al Shahi H, et al. Hepatoprotective and Antioxidant Effects of Nanopiperine against Cypermethrin via Mitigation of Oxidative Stress, Inflammations and Gene Expression Using qRT-PCR. International Journal of Molecular Sciences. 2023; 24(20):15361. https://doi.org/10.3390/ijms242015361
Chicago/Turabian StyleHussain, Sohail, Abdulmajeed M. Jali, Saeed Alshahrani, Khairat H. M. Khairat, Rahimullah Siddiqui, Mohammad Intakhab Alam, Raisuddin Ali, Manal Mohammed, Andleeb Khan, Hamad Al Shahi, and et al. 2023. "Hepatoprotective and Antioxidant Effects of Nanopiperine against Cypermethrin via Mitigation of Oxidative Stress, Inflammations and Gene Expression Using qRT-PCR" International Journal of Molecular Sciences 24, no. 20: 15361. https://doi.org/10.3390/ijms242015361
APA StyleHussain, S., Jali, A. M., Alshahrani, S., Khairat, K. H. M., Siddiqui, R., Alam, M. I., Ali, R., Mohammed, M., Khan, A., Al Shahi, H., Hanbashi, A., Qadri, M., & Ashafaq, M. (2023). Hepatoprotective and Antioxidant Effects of Nanopiperine against Cypermethrin via Mitigation of Oxidative Stress, Inflammations and Gene Expression Using qRT-PCR. International Journal of Molecular Sciences, 24(20), 15361. https://doi.org/10.3390/ijms242015361








