Inflammatory Mediators of Axon Regeneration in the Central and Peripheral Nervous Systems
Abstract
1. Historical Background
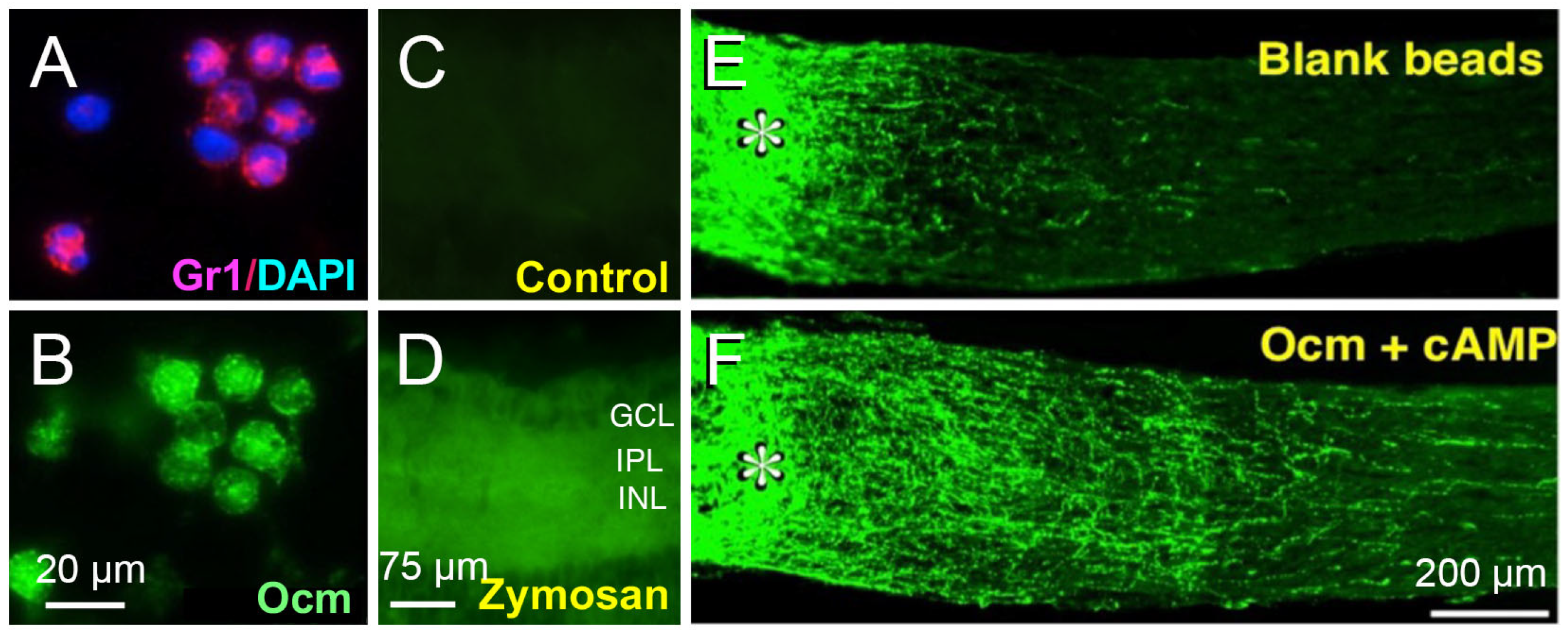
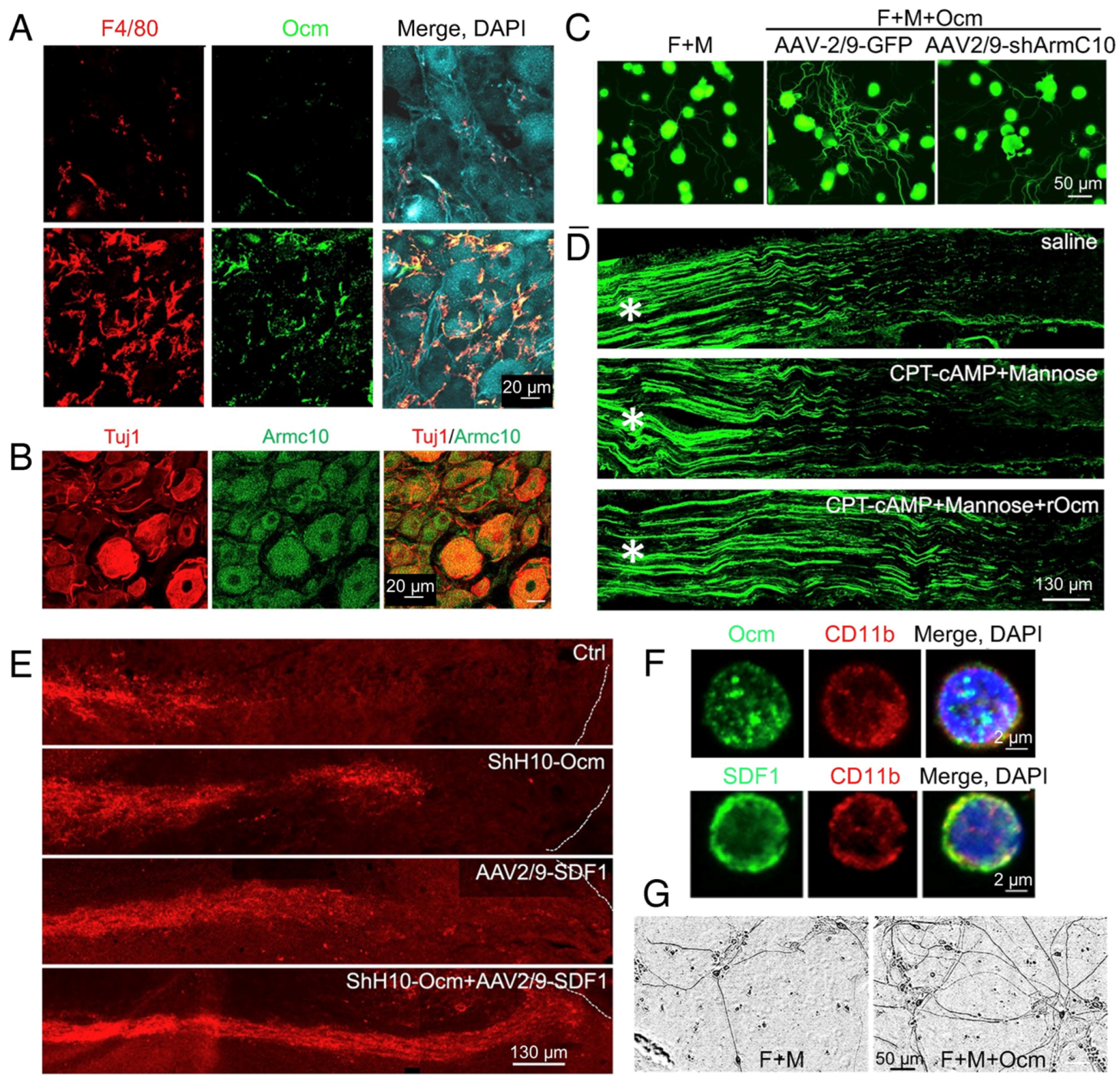
2. Intraocular Inflammation Enables Optic Nerve Regeneration: Role of Oncomodulin
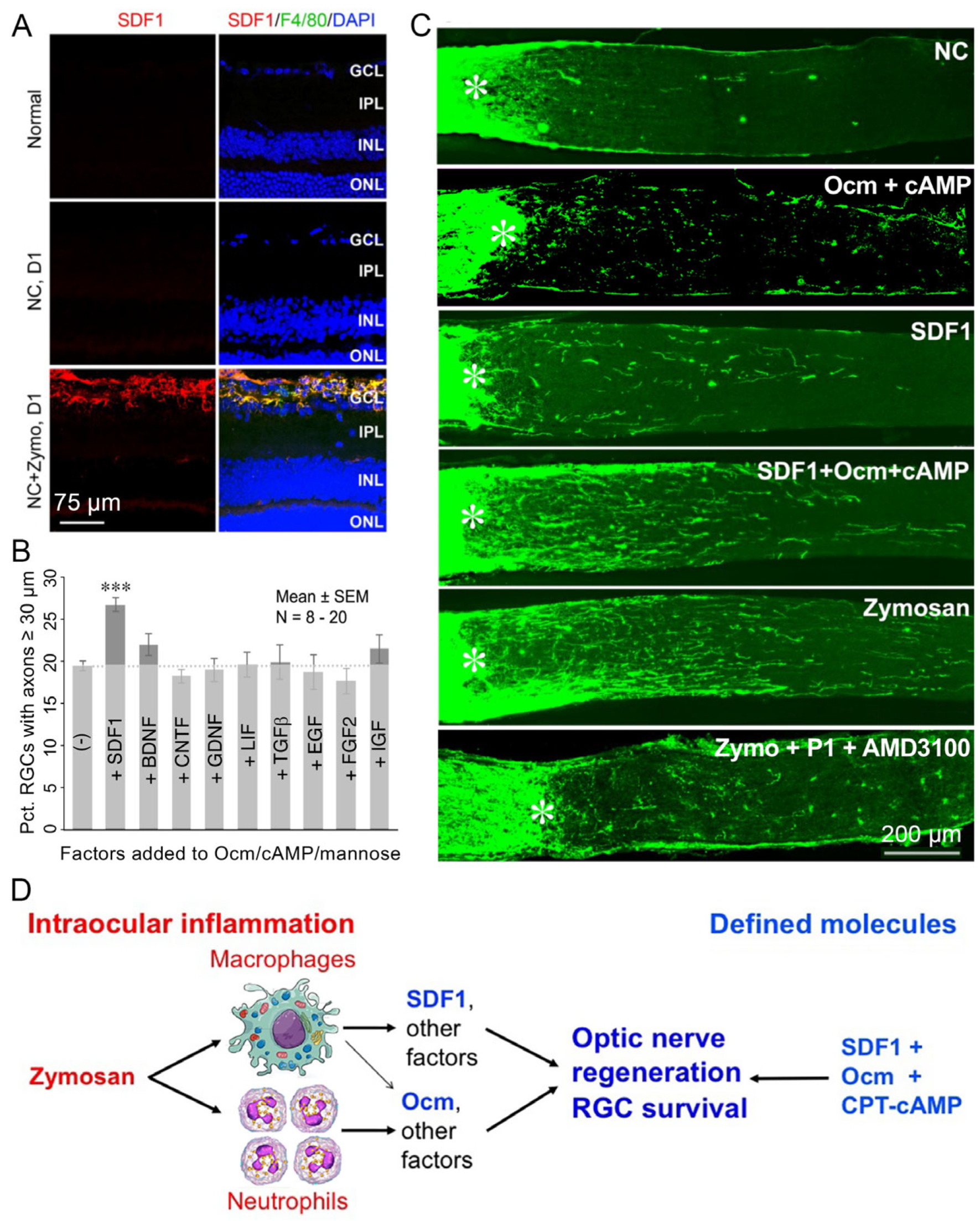
3. SDF1 Complements the Pro-Regenerative Effects of Ocm
4. CNTF Gene Therapy Induces Optic Nerve Regeneration Indirectly via CCL5
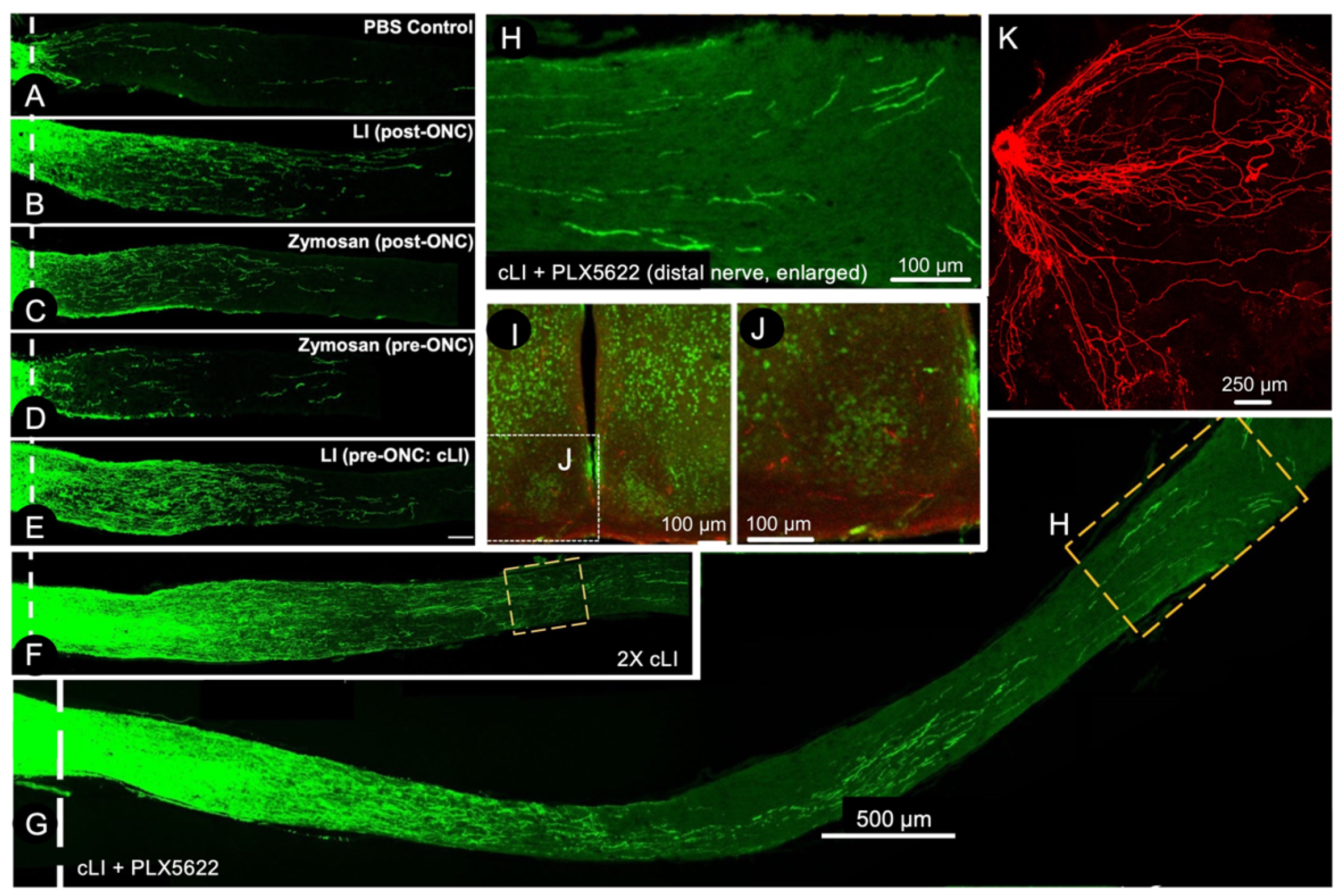
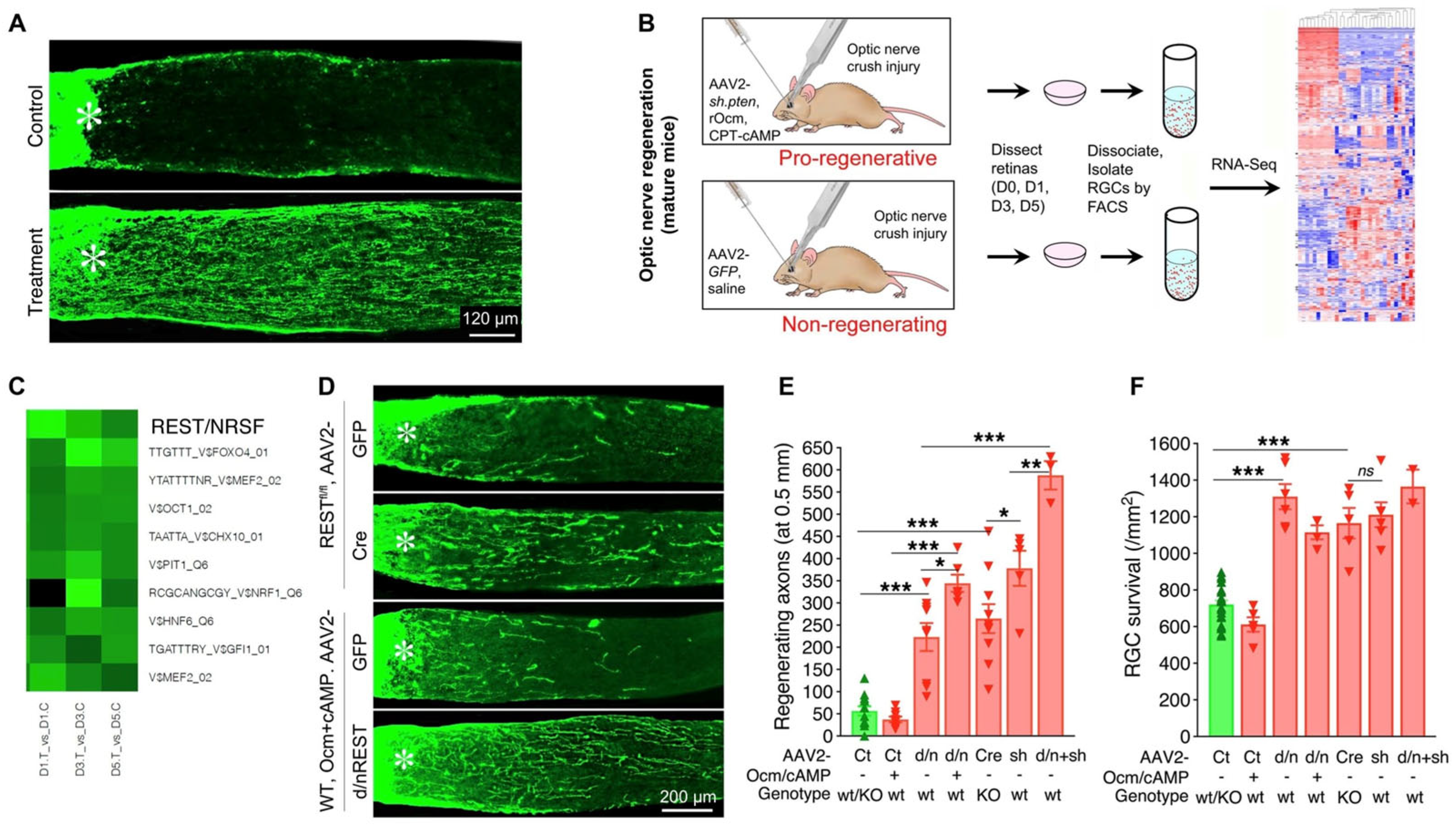
5. A Conditioning Effect in Optic Nerve Regeneration
6. Combinatorial Approaches
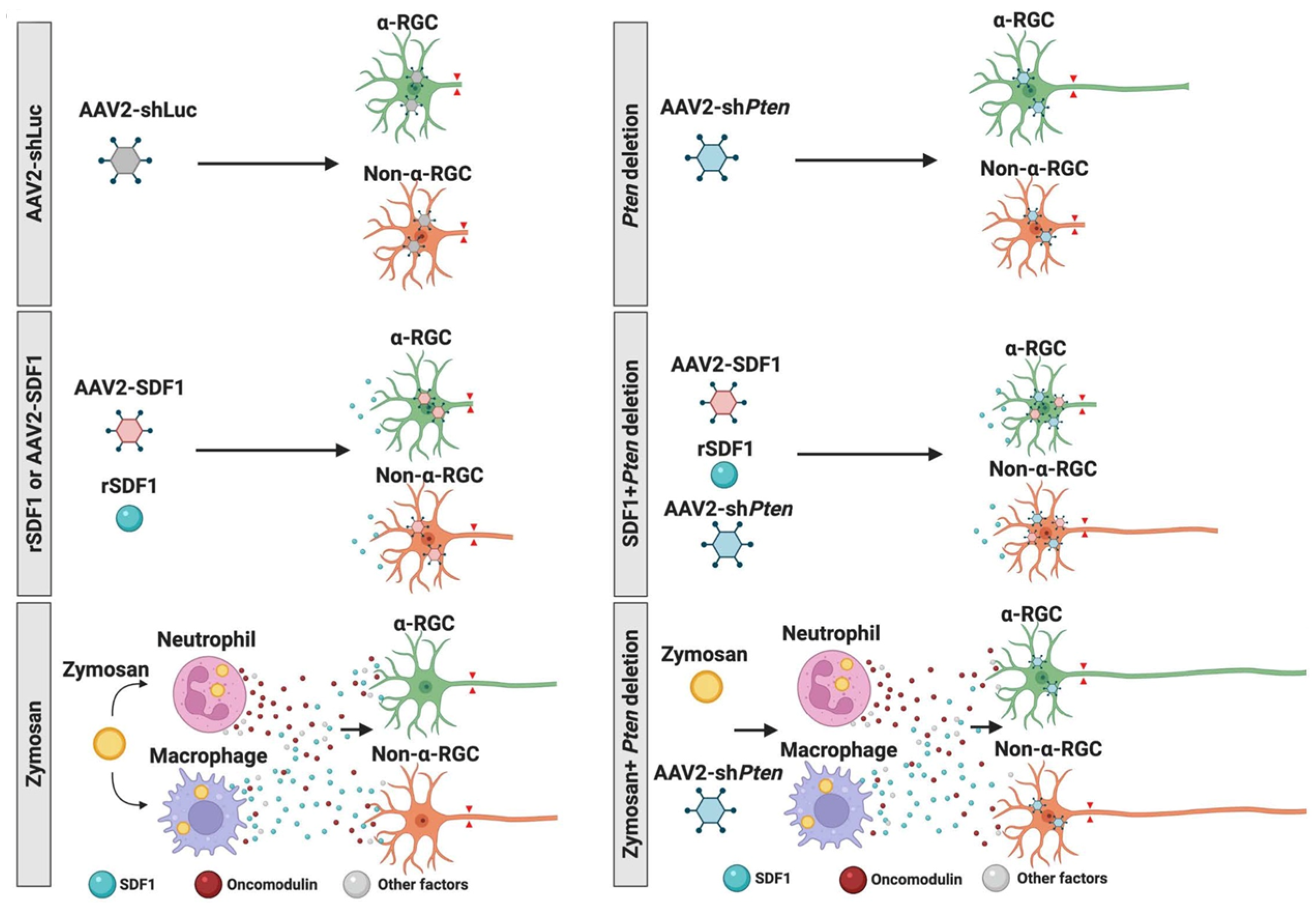
7. Differential Responses of RGC Subtypes
8. ArmC10, the Ocm Receptor, Is Critical for Inflammation-Induced Optic Nerve Regeneration and the Conditioning Lesion Effect in the Peripheral Nervous System
9. SDF1 Complements the Effects of Ocm on Peripheral Sensory Neurons
10. Transcriptional Regulation of the Regenerative Program
11. Role of Other Retinal Cell Types in Optic Nerve Regeneration
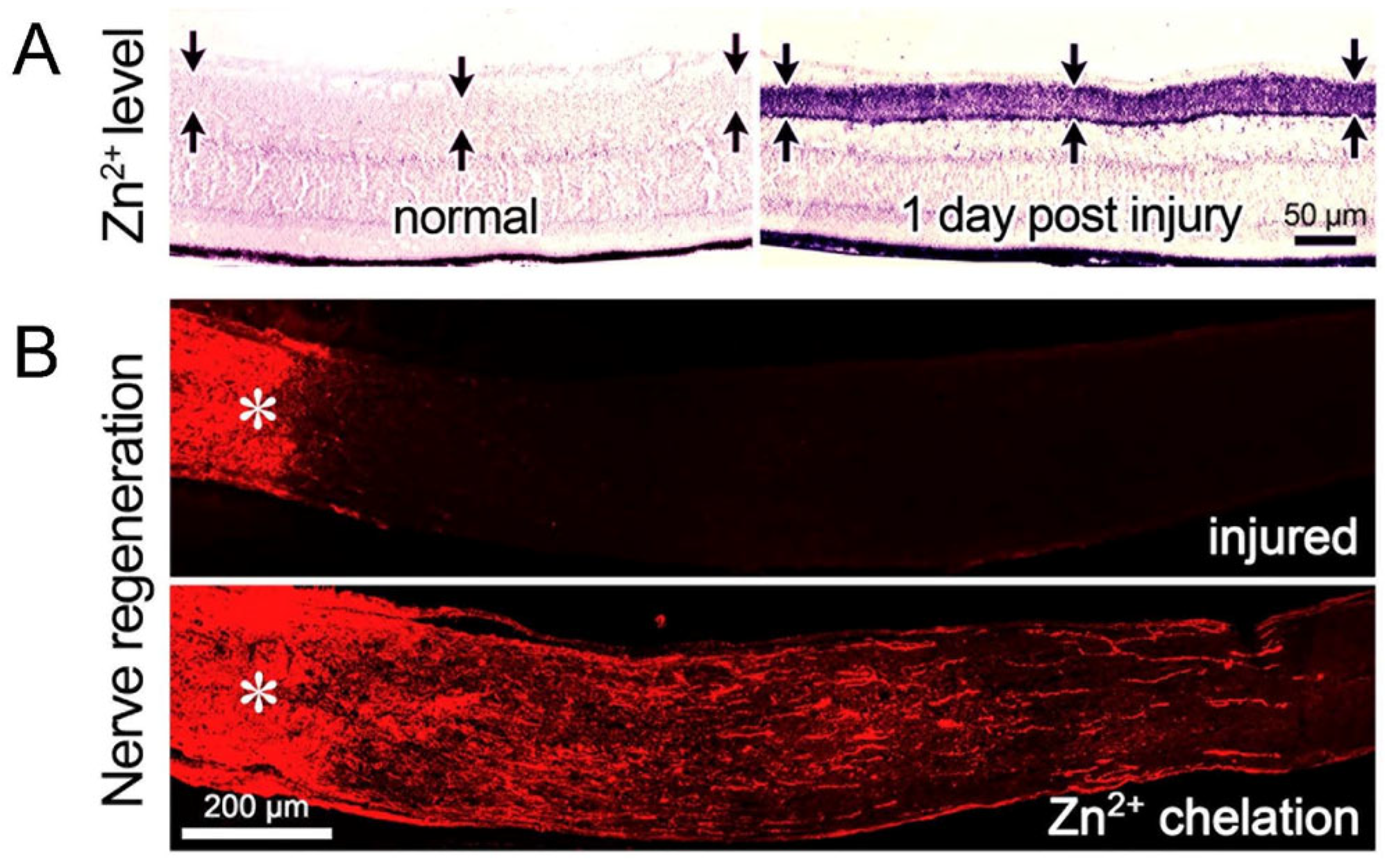
12. Regeneration and RGC Survival
13. The Other Side of Inflammation
14. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramon y Cajal, S. Degeneration and Regeneration of the Nervous System; Oxford University Press: New York, NY, USA, 1991; Volume 5. [Google Scholar]
- So, K.F.; Aguayo, A.J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985, 328, 349–354. [Google Scholar] [CrossRef]
- Aguayo, A.J.; Bray, G.M.; Carter, D.A.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Rasminsky, M. Regrowth and connectivity of injured central nervous system axons in adult rodents. Acta Neurobiol. Exp. 1990, 50, 381–389. [Google Scholar]
- Caroni, P.; Schwab, M.E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 1988, 106, 1281–1288. [Google Scholar] [CrossRef]
- Caroni, P.; Schwab, M.E. Oligodendrocyte- and myelin-associated inhibitors of neurite growth in the adult nervous system. Adv. Neurol. 1993, 61, 175–179. [Google Scholar]
- Shen, Y.J.; DeBellard, M.E.; Salzer, J.L.; Roder, J.; Filbin, M.T. Myelin-associated glycoprotein in myelin and expressed by Schwann cells inhibits axonal regeneration and branching. Mol. Cell Neurosci. 1998, 12, 79–91. [Google Scholar] [CrossRef]
- McKerracher, L.; David, S.; Jackson, D.L.; Kottis, V.; Dunn, R.J.; Braun, P.E. Identification of myelin-associated glycoprotein as a major myelin- derived inhibitor of neurite growth. Neuron 1994, 13, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Carlile, J.; Hunter, A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J. Neurocytol. 1996, 25, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.; Yin, Y.; Nguyen, J.; Irwin, N.; Benowitz, L.I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 2000, 20, 4615–4626. [Google Scholar] [CrossRef]
- Fischer, D.; Pavlidis, M.; Thanos, S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3943–3954. [Google Scholar]
- Yin, Y.; Cui, Q.; Li, Y.; Irwin, N.; Fischer, D.; Harvey, A.R.; Benowitz, L.I. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003, 23, 2284–2293. [Google Scholar] [CrossRef]
- Lazarov-Spiegler, O.; Solomon, A.S.; Schwartz, M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia 1998, 24, 329–337. [Google Scholar] [CrossRef]
- Fischer, D.; Petkova, V.; Thanos, S.; Benowitz, L.I. Switching mature retinal ganglion cells to a robust growth state in vivo: Gene expression and synergy with RhoA inactivation. J. Neurosci. 2004, 24, 8726–8740. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Carbajal, K.S.; Segal, B.M.; Giger, R.J. Neuroinflammation triggered by beta-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Hauk, T.G.; Leibinger, M.; Muller, A.; Andreadaki, N.; Knippschild, U.; Fischer, D. Intravitreal application of the Toll-like receptor 2 agonist Pam3Cys stimulates axon regeneration in the mature optic nerve. Investig. Ophthalmol. Vis. Sci. 2009, 51, 459–464. [Google Scholar] [CrossRef]
- Sas, A.R.; Carbajal, K.S.; Jerome, A.D.; Menon, R.; Yoon, C.; Kalinski, A.L.; Giger, R.J.; Segal, B.M. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020, 21, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yin, Y.; Zhang, A.; Bernstein, A.M.; Kawaguchi, R.; Gao, K.; Potter, K.; Gilbert, H.Y.; Ao, Y.; Ou, J.; et al. Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice. Nat. Commun. 2022, 13, 4418. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef]
- MacManus, J.P.; Brewer, L.M. Isolation, localization, and properties of the oncodevelopmental calcium-binding protein oncomodulin. Methods Enzymol. 1987, 139, 156–168. [Google Scholar] [PubMed]
- Henzl, M.T.; Shibasaki, O.; Comegys, T.H.; Thalmann, I.; Thalmann, R. Oncomodulin is abundant in the organ of Corti. Hear. Res. 1997, 106, 105–111. [Google Scholar] [CrossRef]
- Simmons, D.D.; Tong, B.; Schrader, A.D.; Hornak, A.J. Oncomodulin identifies different hair cell types in the mammalian inner ear. J. Comp. Neurol. 2010, 518, 3785–3802. [Google Scholar] [CrossRef]
- Kurimoto, T.; Yin, Y.; Habboub, G.; Gilbert, H.Y.; Li, Y.; Nakao, S.; Hafezi-Moghadam, A.; Benowitz, L.I. Neutrophils express oncomodulin and promote optic nerve regeneration. J. Neurosci. 2013, 33, 14816–14824. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cui, Q.; Gilbert, H.Y.; Yang, Y.; Yang, Z.; Berlinicke, C.; Li, Z.; Zaverucha-do-Valle, C.; He, H.; Petkova, V.; et al. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 19587–19592. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cen, L.P.; Li, Y.; Gilbert, H.Y.; Strelko, O.; Berlinicke, C.; Stavarache, M.A.; Ma, M.; Wang, Y.; Cui, Q.; et al. Monocyte-derived SDF1 supports optic nerve regeneration and alters retinal ganglion cells’ response to Pten deletion. Proc. Natl. Acad. Sci. USA 2022, 119, e2113751119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Liang, J.J.; Ng, T.K.; Hu, Z.; Xu, C.; Chen, S.; Chen, S.L.; Xu, Y.; Zhuang, X.; Huang, S.; et al. CXCL5/CXCR2 modulates inflammation-mediated neural repair after optic nerve injury. Exp. Neurol. 2021, 341, 113711. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Tran, N.M.; Chenette, D.M.; DeLuca, K.; Foli, Y.; Kannan, R.; Sekine, Y.; Wang, X.; Wollan, M.; Kim, I.J.; et al. Optic nerve regeneration screen identifies multiple genes restricting adult neural repair. Cell Rep. 2021, 34, 108777. [Google Scholar] [CrossRef]
- Bray, E.R.; Yungher, B.J.; Levay, K.; Ribeiro, M.; Dvoryanchikov, G.; Ayupe, A.C.; Thakor, K.; Marks, V.; Randolph, M.; Danzi, M.C.; et al. Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron 2019, 103, 642–657.e7. [Google Scholar]
- Leaver, S.G.; Cui, Q.; Bernard, O.; Harvey, A.R. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur. J. Neurosci. 2006, 24, 3323–3332. [Google Scholar] [CrossRef]
- Pernet, V.; Joly, S.; Dalkara, D.; Jordi, N.; Schwarz, O.; Christ, F.; Schaffer, D.V.; Flannery, J.G.; Schwab, M.E. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis. 2013, 51, 202–213. [Google Scholar] [CrossRef]
- Yungher, B.J.; Ribeiro, M.; Park, K.K. Regenerative Responses and Axon Pathfinding of Retinal Ganglion Cells in Chronically Injured Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1743–1750. [Google Scholar] [CrossRef]
- Yungher, B.J.; Luo, X.; Salgueiro, Y.; Blackmore, M.G.; Park, K.K. Viral vector-based improvement of optic nerve regeneration: Characterization of individual axons’ growth patterns and synaptogenesis in a visual target. Gene Ther. 2015, 22, 811–821. [Google Scholar] [CrossRef]
- Cen, L.P.; Liang, J.J.; Chen, J.H.; Harvey, A.R.; Ng, T.K.; Zhang, M.; Pang, C.P.; Cui, Q.; Fan, Y.M. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 2017, 343, 472–482. [Google Scholar] [PubMed]
- Bei, F.; Lee, H.H.; Liu, X.; Gunner, G.; Jin, H.; Ma, L.; Wang, C.; Hou, L.; Hensch, T.K.; Frank, E.; et al. Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell 2016, 164, 219–232. [Google Scholar] [PubMed]
- Hellstrom, M.; Pollett, M.A.; Harvey, A.R. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J. Neurotrauma 2011, 28, 2475–2483. [Google Scholar]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Kuwako, K.; Martinez-Carrasco, I.; Connolly, L.; He, Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 2009, 64, 617–623. [Google Scholar]
- Pernet, V.; Di Polo, A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 2006, 129, 1014–1026. [Google Scholar]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [PubMed]
- Kobayashi, H.; Mizisin, A.P. CNTFR alpha alone or in combination with CNTF promotes macrophage chemotaxis in vitro. Neuropeptides 2000, 34, 338–347. [Google Scholar]
- Cen, L.P.; Luo, J.M.; Zhang, C.W.; Fan, Y.M.; Song, Y.; So, K.F.; van Rooijen, N.; Pang, C.P.; Lam, D.S.; Cui, Q. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4257–4266. [Google Scholar] [CrossRef]
- Xue, T.; Do, M.T.; Riccio, A.; Jiang, Z.; Hsieh, J.; Wang, H.C.; Merbs, S.L.; Welsbie, D.S.; Yoshioka, T.; Weissgerber, P.; et al. Melanopsin signalling in mammalian iris and retina. Nature 2011, 479, 67–73. [Google Scholar]
- Xie, L.; Yin, Y.; Benowitz, L. Chemokine CCL5 promotes robust optic nerve regeneration and mediates many of the effects of CNTF gene therapy. Proc. Natl. Acad. Sci. USA 2021, 118, e2017282118. [Google Scholar] [PubMed]
- Lu, X.; Richardson, P.M. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci. 1991, 11, 972–978. [Google Scholar] [PubMed]
- Richardson, P.M.; Verge, V.M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987, 411, 406–408. [Google Scholar] [PubMed]
- Neumann, S.; Woolf, C.J. Regeneration of Dorsal Column Fibers into and beyond the Lesion Site following Adult Spinal Cord Injury. Neuron 1999, 23, 83–91. [Google Scholar]
- Schreyer, D.J.; Skene, J.H. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: Delayed accumulation and correlation with regenerative potential. J. Neurosci. 1991, 11, 3738–3751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, M.J.; Kim, J.; Shin, H.; Jeong, S.R.; Kang, Y.M.; Choi, J.Y.; Hwang, D.H.; Kim, B.G. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J. Neurosci. 2013, 33, 15095–15108. [Google Scholar]
- Niemi, J.P.; DeFrancesco-Lisowitz, A.; Roldan-Hernandez, L.; Lindborg, J.A.; Mandell, D.; Zigmond, R.E. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci. 2013, 33, 16236–16248. [Google Scholar]
- Kwon, M.J.; Seo, Y.; Cho, H.; Kim, H.S.; Oh, Y.J.; Kim, M.; Park, H.H.; Joe, E.-H.; Kwon, M.-H.; Kang, H.C.; et al. Oncomodulin derived from regeneration-associated macrophages in dorsal root ganglia after preconditioning injury promotes sensory axon regeneration in the spinal cord. Theranostics. 2022, 12, 5856–5876. [Google Scholar] [CrossRef]
- Xie, L.; Yin, Y.; Jayakar, S.; Kawaguchi, R.; Wang, Q.; Peterson, S.; Shi, C.; Turnes, B.L.; Zhang, Z.; Oses-Prieto, J.; et al. The oncomodulin receptor ArmC10 enables axon regeneration in mice after nerve injury and neurite outgrowth in human iPSC-derived sensory neurons. Sci. Transl. Med. 2023, 15, eadg6241. [Google Scholar] [CrossRef]
- Feng, Q.; Wong, K.A.; Benowitz, L.I. Full-length optic nerve regeneration in the absence of genetic manipulations. JCI Insight 2023, 8, e164579. [Google Scholar]
- Peterson, S.L.; Li, Y.; Sun, C.J.; Wong, K.A.; Leung, K.S.; de Lima, S.; Hanovice, N.J.; Yuki, K.; Stevens, B.; Benowitz, L.I. Retinal Ganglion Cell Axon Regeneration Requires Complement and Myeloid Cell Activity within the Optic Nerve. J. Neurosci. 2021, 41, 8508–8531. [Google Scholar]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- GrandPre, T.; Nakamura, F.; Vartanian, T.; Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000, 403, 439–444. [Google Scholar] [CrossRef]
- Mukhopadhyay, G.; Doherty, P.; Walsh, F.S.; Crocker, P.R.; Filbin, M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 1994, 13, 757–767. [Google Scholar] [CrossRef]
- Liu, B.P.; Fournier, A.; GrandPre, T.; Strittmatter, S.M. Myelin-Associated Glycoprotein as a Functional Ligand for the Nogo-66 Receptor. Science 2002, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef] [PubMed]
- Atwal, J.K.; Pinkston-Gosse, J.; Syken, J.; Stawicki, S.; Wu, Y.; Shatz, C.; Tessier-Lavigne, M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 2008, 322, 967–970. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Bazan, J.F.; McDermott, G.; Park, J.B.; Wang, K.; Tessier-Lavigne, M.; He, Z.; Garcia, K.C. Structure of the Nogo receptor ectodomain: A recognition module implicated in myelin inhibition. Neuron 2003, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar]
- McKeon, R.J.; Hoke, A.; Silver, J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 1995, 136, 32–43. [Google Scholar] [CrossRef]
- Pearson, C.S.; Mencio, C.P.; Barber, A.C.; Martin, K.R.; Geller, H.M. Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife 2018, 7, e37139. [Google Scholar]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [PubMed]
- Fawcett, J.W.; Schwab, M.E.; Montani, L.; Brazda, N.; Muller, H.W. Defeating inhibition of regeneration by scar and myelin components. Handb. Clin. Neurol. 2012, 109, 503–522. [Google Scholar] [PubMed]
- Brown, J.M.; Xia, J.; Zhuang, B.; Cho, K.S.; Rogers, C.J.; Gama, C.I.; Rawat, M.; Tully, S.E.; Uetani, N.; Mason, D.E.; et al. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4768–4773. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Xing, B.; Dill, J.; Li, H.; Hoang, H.H.; Zhao, Z.; Yang, X.L.; Bachoo, R.; Cannon, S.; Longo, F.M.; et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 2011, 31, 14051–14066. [Google Scholar]
- Fawcett, J. Molecular control of brain plasticity and repair. Prog. Brain Res. 2009, 175, 501–509. [Google Scholar]
- Lingor, P.; Teusch, N.; Schwarz, K.; Mueller, R.; Mack, H.; Bahr, M.; Mueller, B.K. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J. Neurochem. 2007, 103, 181–189. [Google Scholar]
- Steinmetz, M.P.; Horn, K.P.; Tom, V.J.; Miller, J.H.; Busch, S.A.; Nair, D.; Silver, D.J.; Silver, J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J. Neurosci. 2005, 25, 8066–8076. [Google Scholar]
- Monnier, P.P.; Sierra, A.; Schwab, J.M.; Henke-Fahle, S.; Mueller, B.K. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell Neurosci. 2003, 22, 319–330. [Google Scholar]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar]
- Asher, R.A.; Morgenstern, D.A.; Moon, L.D.; Fawcett, J.W. Chondroitin sulphate proteoglycans: Inhibitory components of the glial scar. Prog. Brain Res. 2001, 132, 611–619. [Google Scholar]
- Hoke, A.; Silver, J. Proteoglycans and other repulsive molecules in glial boundaries during development and regeneration of the nervous system. Prog. Brain Res. 1996, 108, 149–163. [Google Scholar] [PubMed]
- Lehmann, M.; Fournier, A.; Selles-Navarro, I.; Dergham, P.; Sebok, A.; Leclerc, N.; Tigyi, G.; McKerracher, L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999, 19, 7537–7547. [Google Scholar]
- Zheng, B.; Ho, C.; Li, S.; Keirstead, H.; Steward, O.; Tessier-Lavigne, M. Lack of enhanced spinal regeneration in nogo-deficient mice. Neuron 2003, 38, 213–224. [Google Scholar] [PubMed]
- Fischer, D.; He, Z.; Benowitz, L.I. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 2004, 24, 1646–1651. [Google Scholar] [PubMed]
- Dickendesher, T.L.; Baldwin, K.T.; Mironova, Y.A.; Koriyama, Y.; Raiker, S.J.; Askew, K.L.; Wood, A.; Geoffroy, C.G.; Zheng, B.; Liepmann, C.D.; et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 2012, 15, 703–712. [Google Scholar]
- Wang, X.; Hasan, O.; Arzeno, A.; Benowitz, L.I.; Cafferty, W.B.; Strittmatter, S.M. Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways. Exp. Neurol. 2012, 237, 55–69. [Google Scholar]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar]
- De Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.Y.; Fagiolini, M.; Martinez, A.M.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar]
- Kurimoto, T.; Yin, Y.; Omura, K.; Gilbert, H.Y.; Kim, D.; Cen, L.P.; Moko, L.; Kugler, S.; Benowitz, L.I. Long-distance axon regeneration in the mature optic nerve: Contributions of oncomodulin, cAMP, and pten gene deletion. J. Neurosci. 2010, 30, 15654–15663. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Zhang, K.; Yeung, C.; Feng, G.; Yankner, B.A.; et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 2012, 480, 372–375. [Google Scholar]
- Luo, X.; Salgueiro, Y.; Beckerman, S.R.; Lemmon, V.P.; Tsoulfas, P.; Park, K.K. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol. 2013, 247, 653–662. [Google Scholar] [PubMed]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar]
- Kay, J.N.; De la Huerta, I.; Kim, I.J.; Zhang, Y.; Yamagata, M.; Chu, M.W.; Meister, M.; Sanes, J.R. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J. Neurosci. 2011, 31, 7753–7762. [Google Scholar] [CrossRef]
- Huberman, A.D.; Wei, W.; Elstrott, J.; Stafford, B.K.; Feller, M.B.; Barres, B.A. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 2009, 62, 327–334. [Google Scholar]
- Dhande, O.S.; Huberman, A.D. Visual circuits: Mouse retina no longer a level playing field. Curr. Biol. 2014, 24, R155–R156. [Google Scholar] [CrossRef][Green Version]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar]
- Peng, Y.R.; Shekhar, K.; Yan, W.; Herrmann, D.; Sappington, A.; Bryman, G.S.; van Zyl, T.; Do, M.T.H.; Regev, A.; Sanes, J.R. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell 2019, 176, 1222–1237.e22. [Google Scholar]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.J.; He, Z.; Sanes, J.R. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, A.; Tran, N.M.; Yan, W.; Benhar, I.; Tian, F.; Schaffer, R.; He, Z.; Sanes, J.R. Overlapping transcriptional programs promote survival and axonal regeneration of injured retinal ganglion cells. Neuron 2022, 110, 2625–2645.e7. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.Z.; Atkatsh, K.; Tavarez, U.; Erdos, M.R.; Gruenbaum, Y.; Collins, F.S. Biotinylation by antibody recognition-a method for proximity labeling. Nat. Methods 2018, 15, 127–133. [Google Scholar] [CrossRef]
- Niemi, J.P.; DeFrancesco-Oranburg, T.; Cox, A.; Lindborg, J.A.; Echevarria, F.D.; McCluskey, J.; Simmons, D.D.; Zigmond, R.E. The Conditioning Lesion Response in Dorsal Root Ganglion Neurons Is Inhibited in Oncomodulin Knock-Out Mice. eNeuro 2022, 9, ENEURO.0477-21. [Google Scholar] [CrossRef]
- Kwon, M.J.; Seo, Y.; Cho, H.; Kim, H.S.; Oh, Y.J.; Geniscan, S.; Kim, M.; Park, H.H.; Joe, E.H.; Kwon, M.H.; et al. Nanogel-mediated delivery of oncomodulin secreted from regeneration-associated macrophages promotes sensory axon regeneration in the spinal cord. Theranostics 2022, 12, 5856–5876. [Google Scholar] [CrossRef]
- Noro, T.; Shah, S.H.; Yin, Y.; Kawaguchi, R.; Yokota, S.; Chang, K.C.; Madaan, A.; Sun, C.; Coppola, G.; Geschwind, D.; et al. Elk-1 regulates retinal ganglion cell axon regeneration after injury. Sci. Rep. 2022, 12, 17446. [Google Scholar] [CrossRef]
- Raivich, G.; Bohatschek, M.; Da Costa, C.; Iwata, O.; Galiano, M.; Hristova, M.; Nateri, A.S.; Makwana, M.; Riera-Sans, L.; Wolfer, D.P.; et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 2004, 43, 57–67. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Walzer, M.A.; Snider, W.D. Turning On the Machine; Genetic Control of Axon Regeneration by c-Jun. Neuron 2004, 43, 1–2. [Google Scholar] [CrossRef]
- Seijffers, R.; Allchorne, A.J.; Woolf, C.J. The transcription factor ATF-3 promotes neurite outgrowth. Mol. Cell Neurosci. 2006, 32, 143–154. [Google Scholar] [CrossRef]
- Veldman, M.B.; Bemben, M.A.; Thompson, R.C.; Goldman, D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev. Biol. 2007, 312, 596–612. [Google Scholar] [CrossRef]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF family members regulate intrinsic axon regeneration ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; Goldberg, J.L. Multiple transcription factor families regulate axon growth and regeneration. Dev. Neurobiol. 2011, 71, 1186–1211. [Google Scholar] [PubMed]
- Fernandes, K.A.; Harder, J.M.; Kim, J.; Libby, R.T. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp. Eye Res. 2013, 112, 106–117. [Google Scholar]
- Belin, S.; Nawabi, H.; Wang, C.; Tang, S.; Latremoliere, A.; Warren, P.; Schorle, H.; Uncu, C.; Woolf, C.J.; He, Z.; et al. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 2015, 86, 1000–1014. [Google Scholar]
- Apara, A.; Galvao, J.; Wang, Y.; Blackmore, M.; Trillo, A.; Iwao, K.; Brown, D.P., Jr.; Fernandes, K.A.; Huang, A.; Nguyen, T.; et al. KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. J. Neurosci. 2017, 37, 9632–9644. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.L.; Joseph, C.G.; Fierke, C.A. Activation and inhibition of histone deacetylase 8 by monovalent cations. J. Biol. Chem. 2010, 285, 6036–6043. [Google Scholar] [CrossRef]
- Gaub, P.; Tedeschi, A.; Puttagunta, R.; Nguyen, T.; Schmandke, A.; Di Giovanni, S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010, 17, 1392–1408. [Google Scholar]
- Gaub, P.; Joshi, Y.; Wuttke, A.; Naumann, U.; Schnichels, S.; Heiduschka, P.; Di Giovanni, S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 2011, 134, 2134–2148. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gong, Y.Y.; Shi, Y.H.; Zhang, W.; Qin, X.H.; Wu, X.W. Valproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience 2012, 224, 282–293. [Google Scholar]
- Schmitt, H.M.; Pelzel, H.R.; Schlamp, C.L.; Nickells, R.W. Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury. Mol. Neurodegener. 2014, 9, 39. [Google Scholar]
- Chindasub, P.; Lindsey, J.D.; Duong-Polk, K.; Leung, C.K.; Weinreb, R.N. Inhibition of histone deacetylases 1 and 3 protects injured retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.L.; Klassen, M.P.; Hua, Y.; Barres, B.A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 2002, 296, 1860–1864. [Google Scholar] [PubMed]
- Zhang, Y.; Williams, P.R.; Jacobi, A.; Wang, C.; Goel, A.; Hirano, A.A.; Brecha, N.C.; Kerschensteiner, D.; He, Z. Elevating Growth Factor Responsiveness and Axon Regeneration by Modulating Presynaptic Inputs. Neuron 2019, 103, 39–51.e5. [Google Scholar] [CrossRef]
- Nathan, F.M.; Ohtake, Y.; Wang, S.; Jiang, X.; Sami, A.; Guo, H.; Zhou, F.Q.; Li, S. Upregulating Lin28a Promotes Axon Regeneration in Adult Mice with Optic Nerve and Spinal Cord Injury. Mol. Ther. 2020, 28, 1902–1917. [Google Scholar]
- Li, Y.; Andereggen, L.; Yuki, K.; Omura, K.; Yin, Y.; Gilbert, H.Y.; Erdogan, B.; Asdourian, M.S.; Shrock, C.; de Lima, S.; et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E209–E218. [Google Scholar] [PubMed]
- Chierzi, S.; Strettoi, E.; Cenni, M.C.; Maffei, L. Optic nerve crush: Axonal responses in wild-type and bcl-2 transgenic mice. J. Neurosci. 1999, 19, 8367–8376. [Google Scholar] [CrossRef]
- Knoferle, J.; Koch, J.C.; Ostendorf, T.; Michel, U.; Planchamp, V.; Vutova, P.; Tonges, L.; Stadelmann, C.; Bruck, W.; Bahr, M.; et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 6064–6069. [Google Scholar]
- Welsbie, D.S.; Yang, Z.; Ge, Y.; Mitchell, K.L.; Zhou, X.; Martin, S.E.; Berlinicke, C.A.; Hackler, L., Jr.; Fuller, J.; Fu, J.; et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. USA 2013, 110, 4045–4050. [Google Scholar]
- Welsbie, D.S.; Mitchell, K.L.; Jaskula-Ranga, V.; Sluch, V.M.; Yang, Z.; Kim, J.; Buehler, E.; Patel, A.; Martin, S.E.; Zhang, P.W.; et al. Enhanced Functional Genomic Screening Identifies Novel Mediators of Dual Leucine Zipper Kinase-Dependent Injury Signaling in Neurons. Neuron 2017, 94, 1142–1154.e6. [Google Scholar]
- Watkins, T.A.; Wang, B.; Huntwork-Rodriguez, S.; Yang, J.; Jiang, Z.; Eastham-Anderson, J.; Modrusan, Z.; Kaminker, J.S.; Tessier-Lavigne, M.; Lewcock, J.W. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. USA 2013, 110, 4039–4044. [Google Scholar] [CrossRef]
- Patel, A.K.; Broyer, R.M.; Lee, C.D.; Lu, T.; Louie, M.J.; La Torre, A.; Al-Ali, H.; Vu, M.T.; Mitchell, K.L.; Wahlin, K.J.; et al. Inhibition of GCK-IV kinases dissociates cell death and axon regeneration in CNS neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 33597–33607. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, J.; Starr, C.; Mohns, E.J.; Li, Y.; Chen, E.P.; Yoon, Y.; Kellner, C.P.; Tanaka, K.; Wang, H.; et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell 2021, 184, 4299–4314.e12. [Google Scholar] [PubMed]
- Nakazawa, T.; Nakazawa, C.; Matsubara, A.; Noda, K.; Hisatomi, T.; She, H.; Michaud, N.; Hafezi-Moghadam, A.; Miller, J.W.; Benowitz, L.I. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006, 26, 12633–12641. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res. 2008, 173, 409–421. [Google Scholar] [PubMed]
- Wax, M.B.; Tezel, G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp. Eye Res. 2009, 88, 825–830. [Google Scholar] [CrossRef]
- Roh, M.; Zhang, Y.; Murakami, Y.; Thanos, A.; Lee, S.C.; Vavvas, D.G.; Benowitz, L.I.; Miller, J.W. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE 2012, 7, e40065. [Google Scholar]
- Tezel, G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 23–31. [Google Scholar]
- Krizaj, D.; Ryskamp, D.A.; Tian, N.; Tezel, G.; Mitchell, C.H.; Slepak, V.Z.; Shestopalov, V.I. From mechanosensitivity to inflammatory responses: New players in the pathology of glaucoma. Curr. Eye Res. 2014, 39, 105–119. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Ambati, B.K.; Vetter, M.L. In vivo dynamics of retinal microglial activation during neurodegeneration: Confocal ophthalmoscopic imaging and cell morphometry in mouse glaucoma. J. Vis. Exp. 2015, e52731. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar]
- Bosco, A.; Anderson, S.R.; Breen, K.T.; Romero, C.O.; Steele, M.R.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Tomlinson, S.; Vetter, M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018, 26, 2379–2396. [Google Scholar] [PubMed]
- Williams, P.A.; Marsh-Armstrong, N.; Howell, G.R.; Bosco, A.; Danias, J.; Simon, J.; Di Polo, A.; Kuehn, M.H.; Przedborski, S.; Raff, M.; et al. Neuroinflammation in glaucoma: A new opportunity. Exp. Eye Res. 2017, 157, 20–27. [Google Scholar] [PubMed]
- Saini, C.; Jiang, S.; Devlin, J.; Pan, L.; Tang, Y.; Tang, J.; Sun, J.A.; Lorenzo, M.M.; Wang, Q.; Pasquale, L.R.; et al. Association between HSP-Specific T-Cell Counts and Retinal Nerve Fiber Layer Thickness in Patients with Primary Open-Angle Glaucoma. Ophthalmol. Sci. 2023, 3, 100310. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflamm. 2019, 16, 184. [Google Scholar] [CrossRef]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Munch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Zhu, Y.; Harada, T.; Liu, L.; Lush, M.E.; Guignard, F.; Harada, C.; Burns, D.K.; Bajenaru, M.L.; Gutmann, D.H.; Parada, L.F. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development 2005, 132, 5577–5588. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Wood, D.L.; Collins, F.S. Identification of the neurofibromatosis type 1 gene product. Proc. Natl. Acad. Sci. USA 1991, 88, 9658–9662. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Kettenmann, H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 2019, 104, 442–449. [Google Scholar]
- Guo, X.; Pan, Y.; Xiong, M.; Sanapala, S.; Anastasaki, C.; Cobb, O.; Dahiya, S.; Gutmann, D.H. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat. Commun. 2020, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Jecrois, E.S.; Zheng, W.; Bornhorst, M.; Li, Y.; Treisman, D.M.; Muguyo, D.; Huynh, S.; Andrew, S.F.; Wang, Y.; Jiang, J.; et al. Treatment during a developmental window prevents NF1-associated optic pathway gliomas by targeting Erk-dependent migrating glial progenitors. Dev. Cell 2021, 56, 2871–2885.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, W.; Jecrois, E.S.; Pierce, B.R.; Treisman, D.M. A therapeutic window for preventive therapy in NF1-associated optic pathway glioma. Mol. Cell Oncol. 2021, 8, 1989262. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, C.; Mo, J.; Chen, J.K.; Chatterjee, J.; Pan, Y.; Scheaffer, S.M.; Cobb, O.; Monje, M.; Le, L.Q.; Gutmann, D.H. Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat. Commun. 2022, 13, 2785. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benowitz, L.I.; Xie, L.; Yin, Y. Inflammatory Mediators of Axon Regeneration in the Central and Peripheral Nervous Systems. Int. J. Mol. Sci. 2023, 24, 15359. https://doi.org/10.3390/ijms242015359
Benowitz LI, Xie L, Yin Y. Inflammatory Mediators of Axon Regeneration in the Central and Peripheral Nervous Systems. International Journal of Molecular Sciences. 2023; 24(20):15359. https://doi.org/10.3390/ijms242015359
Chicago/Turabian StyleBenowitz, Larry I., Lili Xie, and Yuqin Yin. 2023. "Inflammatory Mediators of Axon Regeneration in the Central and Peripheral Nervous Systems" International Journal of Molecular Sciences 24, no. 20: 15359. https://doi.org/10.3390/ijms242015359
APA StyleBenowitz, L. I., Xie, L., & Yin, Y. (2023). Inflammatory Mediators of Axon Regeneration in the Central and Peripheral Nervous Systems. International Journal of Molecular Sciences, 24(20), 15359. https://doi.org/10.3390/ijms242015359




