Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Patients
2.2. Biochemical Characteristics of Patients
2.3. Clinical Markers of Peripheral Artery Disease Severity
2.4. Correlations of CD34+ Cells Number with Clinical and Biochemical Markers
3. Discussion
4. Materials and Methods
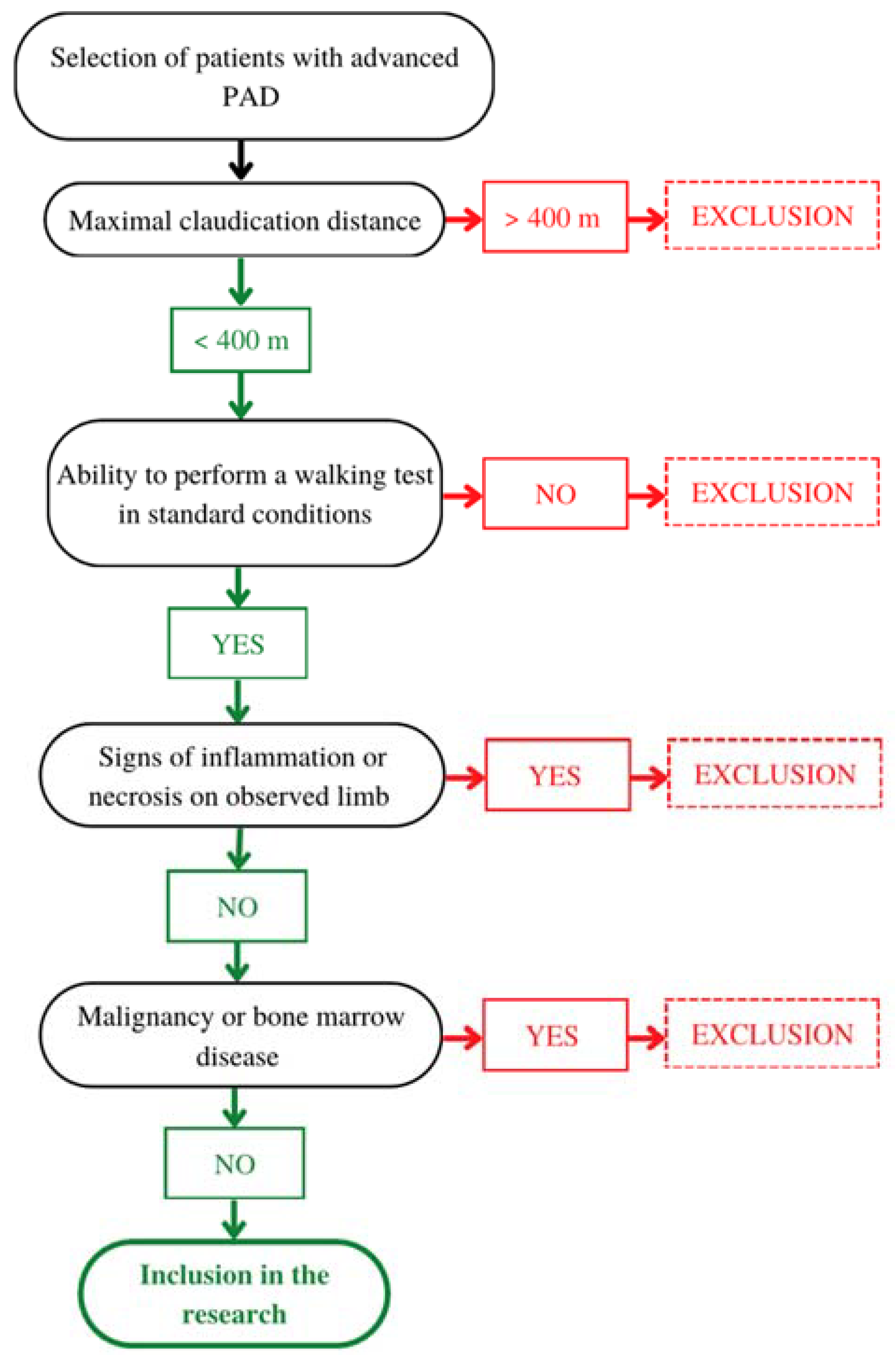
4.1. Patients
4.2. Clinical Examination
4.3. Laboratory Tests
4.4. Immunomagnetic Positive Selection of CD34+ Cells and Cell Counting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on Peripheral Arterial Disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.; Mafilios, M.S.; Bhounsule, P.; Hasegawa, J.T. The Burden of Critical Limb Ischemia: A Review of Recent Literature. Vasc. Health Risk Manag. 2019, 15, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Kibbe, M.R.; Mendelsohn, F.; Marston, W.; Driver, V.R.; Sharafuddin, M.; Teodorescu, V.; Wiechmann, B.N.; Thompson, C.; Kraiss, L.; et al. A Randomized, Controlled Pilot Study of Autologous CD34+ Cell Therapy for Critical Limb Ischemia. Circ. Cardiovasc. Interv. 2012, 5, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, B.; Fu, W.; Wang, Y.; Guo, D.; Wei, Z.; Xu, X.; Mendelsohn, F.O. Transplantation of Purified CD34+ Cells in the Treatment of Critical Limb Ischemia. J. Vasc. Surg. 2013, 58, 404–411.e3. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic Angiogenesis for Patients with Limb Ischaemia by Autologous Transplantation of Bone-Marrow Cells: A Pilot Study and a Randomised Controlled Trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Osipova, O.; Saaya, S.; Karpenko, A.; Zakian, S.; Aboian, E. Cell Therapy of Critical Limb Ischemia-Problems and Prospects. Vasa Eur. J. Vasc. Med. 2019, 48, 461–471. [Google Scholar] [CrossRef]
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; Saito, Y.; Uemura, S.; Suzuki, H.; Fukumoto, S.; et al. Long-Term Clinical Outcome after Intramuscular Implantation of Bone Marrow Mononuclear Cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] Trial) in Patients with Chronic Limb Ischemia. Am. Heart J. 2008, 156, 1010–1018. [Google Scholar] [CrossRef]
- Kresnik, P.K.; Krasna, M.; Rozman, P.; Vrtovec, B.; Malicev, E. Collection and Immunoselection of CD34+ Cells: The Impact of Age, Sex, and Diabetes in Patients with Chronic Heart Failure. Transfusion 2016, 56, 1792–1800. [Google Scholar] [CrossRef]
- Muller-Ehmsen, J.; Braun, D.; Schneider, T.; Pfister, R.; Worm, N.; Wielckens, K.; Scheid, C.; Frommolt, P.; Flesch, M. Decreased Number of Circulating Progenitor Cells in Obesity: Beneficial Effects of Weight Reduction. Eur. Heart J. 2008, 29, 1560–1568. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Yannakoulia, M.; Chrysohoou, C.; Stefanadis, C. The Implication of Obesity and Central Fat on Markers of Chronic Inflammation: The ATTICA Study. Atherosclerosis 2005, 183, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.; Andre, C.; Pontvert-Delucq, S.; Drenou, B.; Baillou, C.; Guigon, M.; Najman, A.; Lemoine, F. Tumor Necrosis Factor α (TNFα) Downregulates c-Kit Proto-Oncogene Product Expression in Normal and Acute Myeloid Leukemia CD34+ Cells via P55 TNFα Receptors. Blood 1994, 84, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.; Fang, X.; Ding, M.; Wang, X.; Yuan, D.; Sui, X.; Liu, X.; Zhang, L.; Xu, H.; Li, Y.; et al. Smoking Is an Important Factor That Affects Peripheral Blood Progenitor Cells Yield in Healthy Male Donors. J. Clin. Apher. 2020, 35, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.S.; Cheng, S.; Larson, M.G.; Cupples, L.A.; McCabe, E.L.; Wang, Y.A.; Ngwa, J.S.; Martin, R.P.; Klein, R.J.; Hashmi, B.; et al. Circulating CD34(+) Progenitor Cell Frequency Is Associated with Clinical and Genetic Factors. Blood 2013, 121, e50–e56. [Google Scholar] [CrossRef]
- Michaud, S.É.; Dussault, S.; Haddad, P.; Groleau, J.; Rivard, A. Circulating Endothelial Progenitor Cells from Healthy Smokers Exhibit Impaired Functional Activities. Atherosclerosis 2006, 187, 423–432. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and Migratory Activity of Circulating Endothelial Progenitor Cells Inversely Correlate with Risk Factors for Coronary Artery Disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Asada, A.; Kasahara, E.; Sato, E.F.; Shindo, M.; Inoue, M. Smoking a Single Cigarette Rapidly Reduces Combined Concentrations of Nitrate and Nitrite and Concentrations of Antioxidants in Plasma. Circulation 2002, 105, 1155–1157. [Google Scholar] [CrossRef]
- Thum, T.; Fraccarollo, D.; Schultheiss, M.; Froese, S.; Galuppo, P.; Widder, J.D.; Tsikas, D.; Ertl, G.; Bauersachs, J. Endothelial Nitric Oxide Synthase Uncoupling Impairs Endothelial Progenitor Cell Mobilization and Function in Diabetes. Diabetes 2007, 56, 666–674. [Google Scholar] [CrossRef]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human Endothelial Progenitor Cells from Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation into Vascular Structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef]
- Vigorelli, V.; Resta, J.; Bianchessi, V.; Lauri, A.; Bassetti, B.; Agrifoglio, M.; Pesce, M.; Polvani, G.; Bonalumi, G.; Cavallotti, L.; et al. Abnormal DNA Methylation Induced by Hyperglycemia Reduces CXCR4 Gene Expression in CD34+ Stem Cells. J. Am. Heart Assoc. 2019, 8, e010012. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, Regional, and National Prevalence and Risk Factors for Peripheral Artery Disease in 2015: An Updated Systematic Review and Analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of Classification Systems in Peripheral Artery Disease. Semin. Intervent. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Massa, M.; Rosti, V.; Ferrario, M.; Campanelli, R.; Ramajoli, I.; Rosso, R.; De Ferrari, G.M.; Ferlini, M.; Goffredo, L.; Bertoletti, A.; et al. Increased Circulating Hematopoietic and Endothelial Progenitor Cells in the Early Phase of Acute Myocardial Infarction. Blood 2005, 105, 199–206. [Google Scholar] [CrossRef]
- Mackie, A.R.; Losordo, D.W. CD34-Positive Stem Cells in the Treatment of Heart and Vascular Disease in Human Beings. Tex. Heart Inst. J. 2011, 38, 474. [Google Scholar] [PubMed]
- Hamburg, N.M.; Creager, M.A. Pathophysiology of Intermittent Claudication in Peripheral Artery Disease. Circ. J. 2017, 81, 281–289. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Jacobsen, F.W.; Fahlman, C.; Rusten, L.S. TNF-Alpha, the Great Imitator: Role of P55 and P75 TNF Receptors in Hematopoiesis. Stem Cells 1994, 12 (Suppl. S1), 111–126; discussion 126–128. [Google Scholar] [PubMed]
- Ugovšek, S.; Rehberger Likozar, A.; Finderle, S.; Poglajen, G.; Okrajšek, R.; Vrtovec, B.; Šebeštjen, M. TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity ArteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S1), S5–S67. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | |
|---|---|
| Male sex | 23 (76.7) |
| Age (years) | 68 ± 9 |
| Body mass (kg) | 79.7 ± 16.5 |
| BMI (kg/m2) | 26.7 ± 4.7 |
| >30 kg/m2 | 9 (30) |
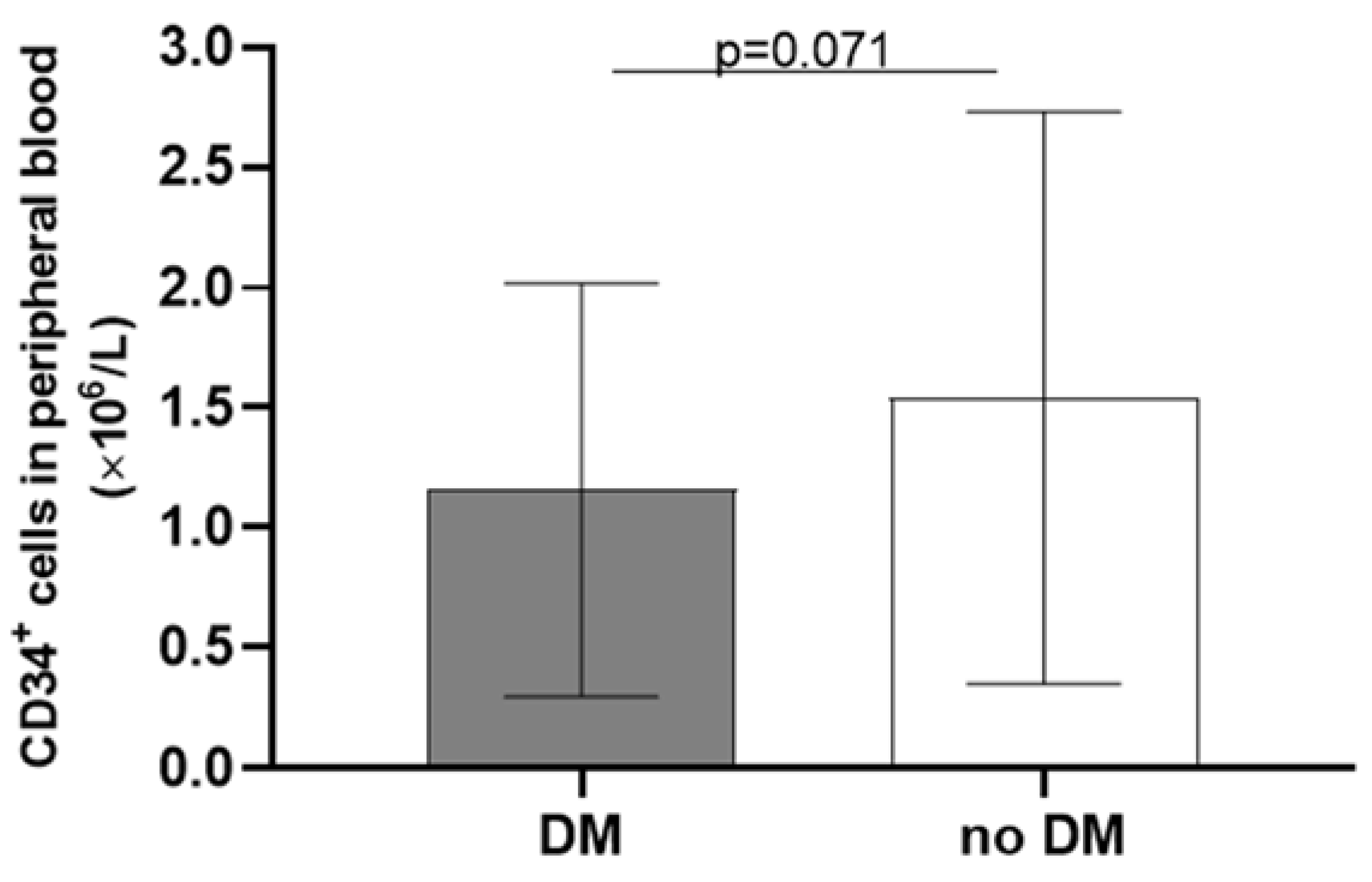
| Diabetes type II | 12 (40.0) |
| Chronic kidney disease (eGFR < 60 mL/min/1.73 m2) | 6 (20.0) |
| Dyslipidaemia | 19 (63.3) |
| Arterial hypertension | 26 (86.7) |
| Smokers; active/past | 12 (40.0)/16 (53.3) |
| Chronic limb-threatening ischaemia | 2 (6.6) |
| Previous revascularisation | 11 (36.7) |
| Laboratory Values | |
|---|---|
| Haemoglobin (mg/L) | 99 ± 85 |
| Leukocytes (×109/L) | 9.6 ± 2.6 |
| Thrombocytes (×109/L) | 241 ± 75 |
| CD34+ cells (×106/L) | 0.42 ± 0.72 |
| Glucose (mmol/L) | 7.26 ± 2.21 |
| HbA1c (%) | 6.10 ± 0.72 |
| Urea (mmol/L) | 9.13 ± 3.02 |
| Creatinine (μmol/L) | 98.03 ± 33.91 |
| eGFR (mL/min/1.73 m2) | 67.0 ± 21.7 |
| Cholesterol (mmol/L) | 4.37 ± 0.46 |
| HDL (mmol/L) | 1.21 ± 0.45 |
| LDL (mmol/L) | 2.35 ± 0.20 |
| Triglycerides (mmol/L) | 1.84 (1.15–2.37) |
| Apolipoprotein A1 (g/L) | 1.50 ± 0.43 |
| Apolipoprotein B (g/L) | 0.88 ± 0.06 |
| Lipoprotein (a) (mg/L) | 105.93 ± 319.22 |
| Angiopoietin 2 (ng/L) | 2.03 ± 1.07 |
| VEGF (ng/L) | 0.15 ± 0.09 |
| VCAM-1 (ng/mL) | 721.10 ± 242.66 |
| TNFα (ng/L) | 37.92 ± 52.04 |
| IL-6 (ng/L) | 1.26 ± 1.26 |
| IL-8 (ng/L) | 59.35 ± 37.26 |
| Haemodynamic Tests | |
|---|---|
| Ankle–Brachial Index | 0.51 ± 0.16 |
| <0.4 | 7 (23.3) |
| 0.41–0.9 | 21 (70.0) |
| 0.91–1.4 | 2 (6.7) |
| Toe pressure (mm Hg) | 64 ± 29 |
| <30 | 3 (10) |
| 30–70 | 15 (50) |
| >70 | 12 (40) |
| Clinical Characteristics | Correlation |
|---|---|
| Age | r = 0.002; p = 0.993 |
| BMI | r = 0.112; p = 0.557 |
| Smoking (pack-years) | r = −0.300; p = 0.107 |
| Parameter | Correlation |
|---|---|
| ABI | r = 0.165; p = 0.392 |
| Toe pressure | r = −0.102; p = 0.598 |
| Claudication distance | r = −0.403; p = 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sernek, B.; Kamnikar, R.; Sebestjen, M.; Boc, A.; Boc, V. Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease. Int. J. Mol. Sci. 2023, 24, 15346. https://doi.org/10.3390/ijms242015346
Sernek B, Kamnikar R, Sebestjen M, Boc A, Boc V. Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease. International Journal of Molecular Sciences. 2023; 24(20):15346. https://doi.org/10.3390/ijms242015346
Chicago/Turabian StyleSernek, Barbara, Rok Kamnikar, Miran Sebestjen, Anja Boc, and Vinko Boc. 2023. "Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease" International Journal of Molecular Sciences 24, no. 20: 15346. https://doi.org/10.3390/ijms242015346
APA StyleSernek, B., Kamnikar, R., Sebestjen, M., Boc, A., & Boc, V. (2023). Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease. International Journal of Molecular Sciences, 24(20), 15346. https://doi.org/10.3390/ijms242015346





