Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line
Abstract
1. Introduction
2. Results
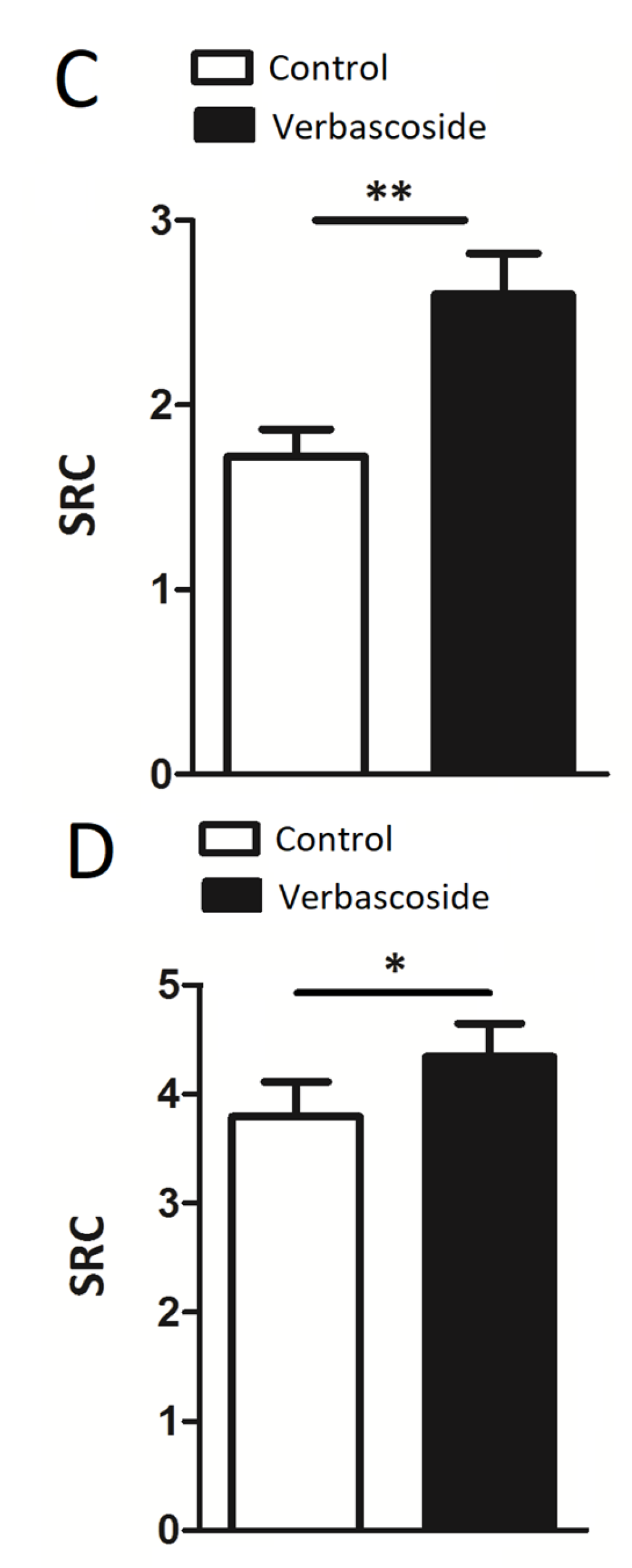
2.1. Verbascoside Improved Mitochondrial Spare Respiratory Capacity
2.2. Verbascoside Partially Restored Mitochondrial Function under Oxidative Conditions
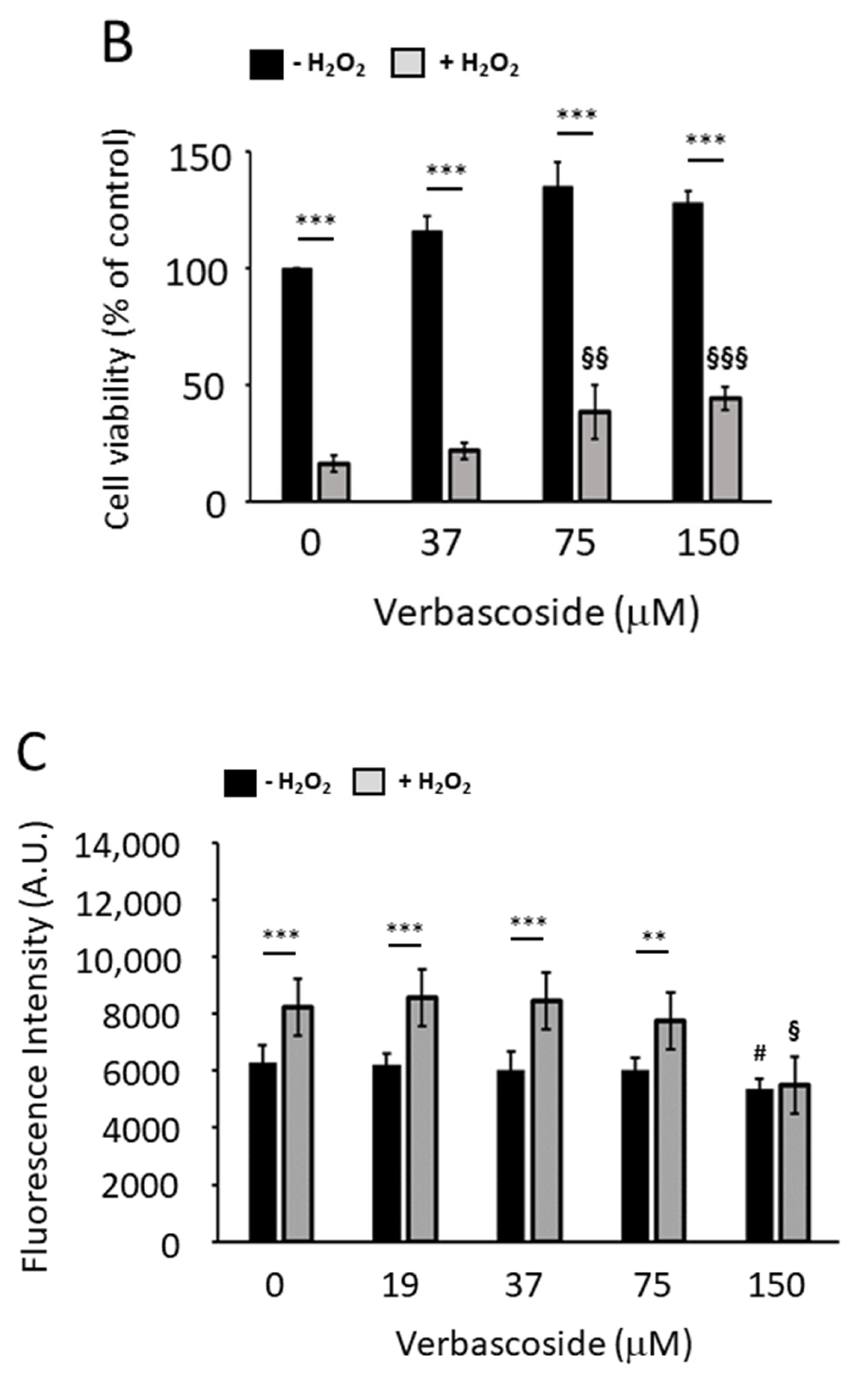
2.3. Verbascoside Protected C2C12 Myoblasts and Myotubes from H2O2-Induced Reactive Oxygen Species
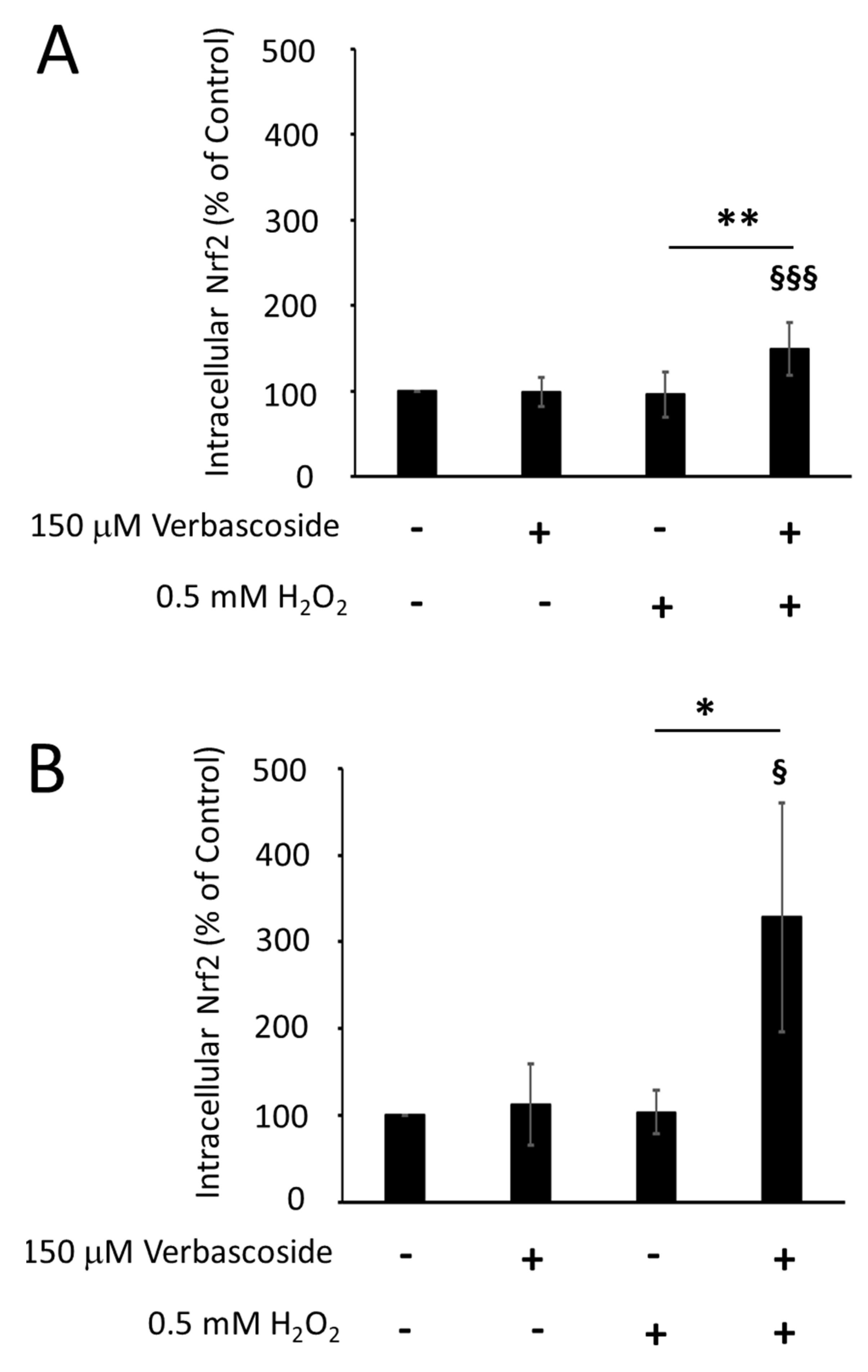
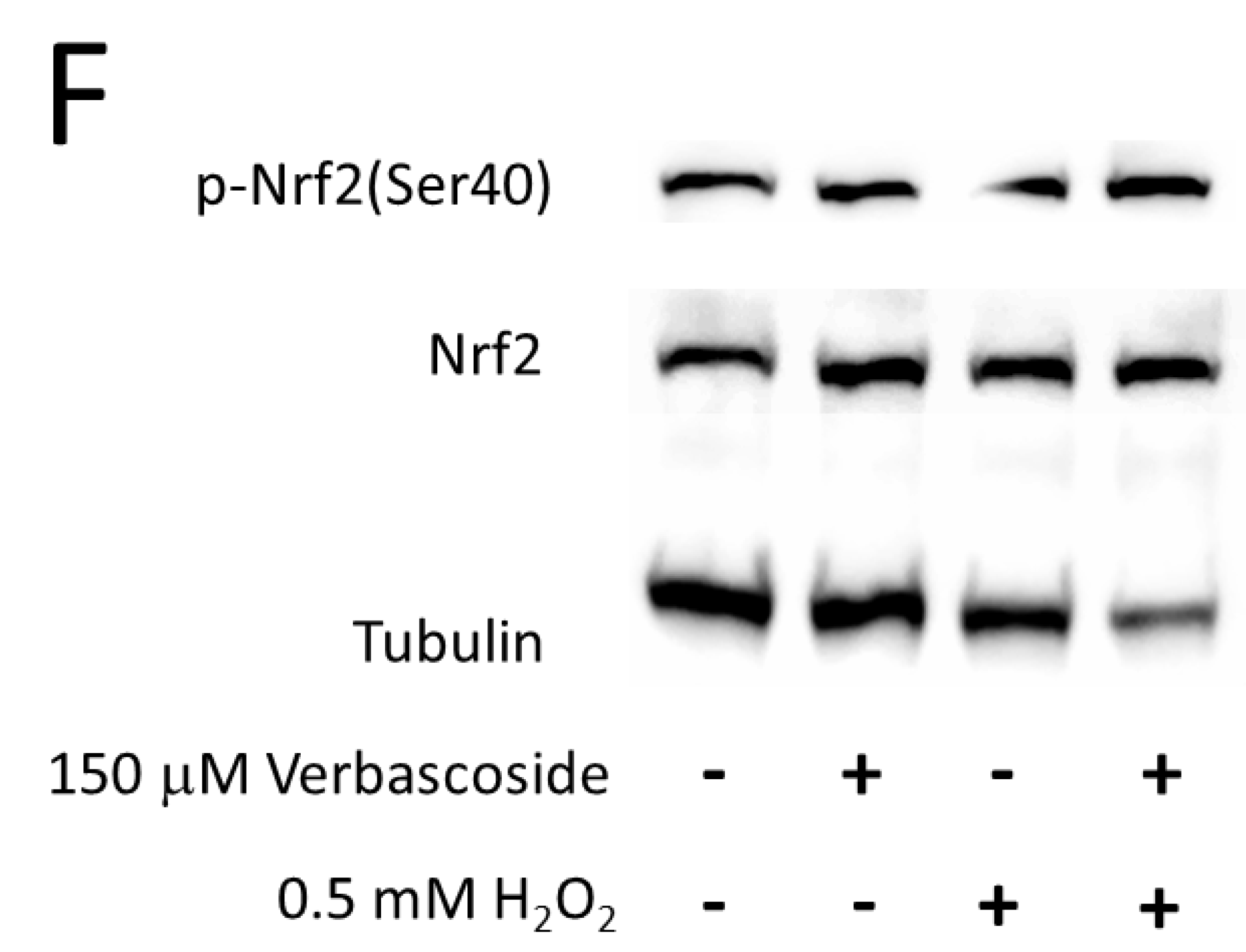
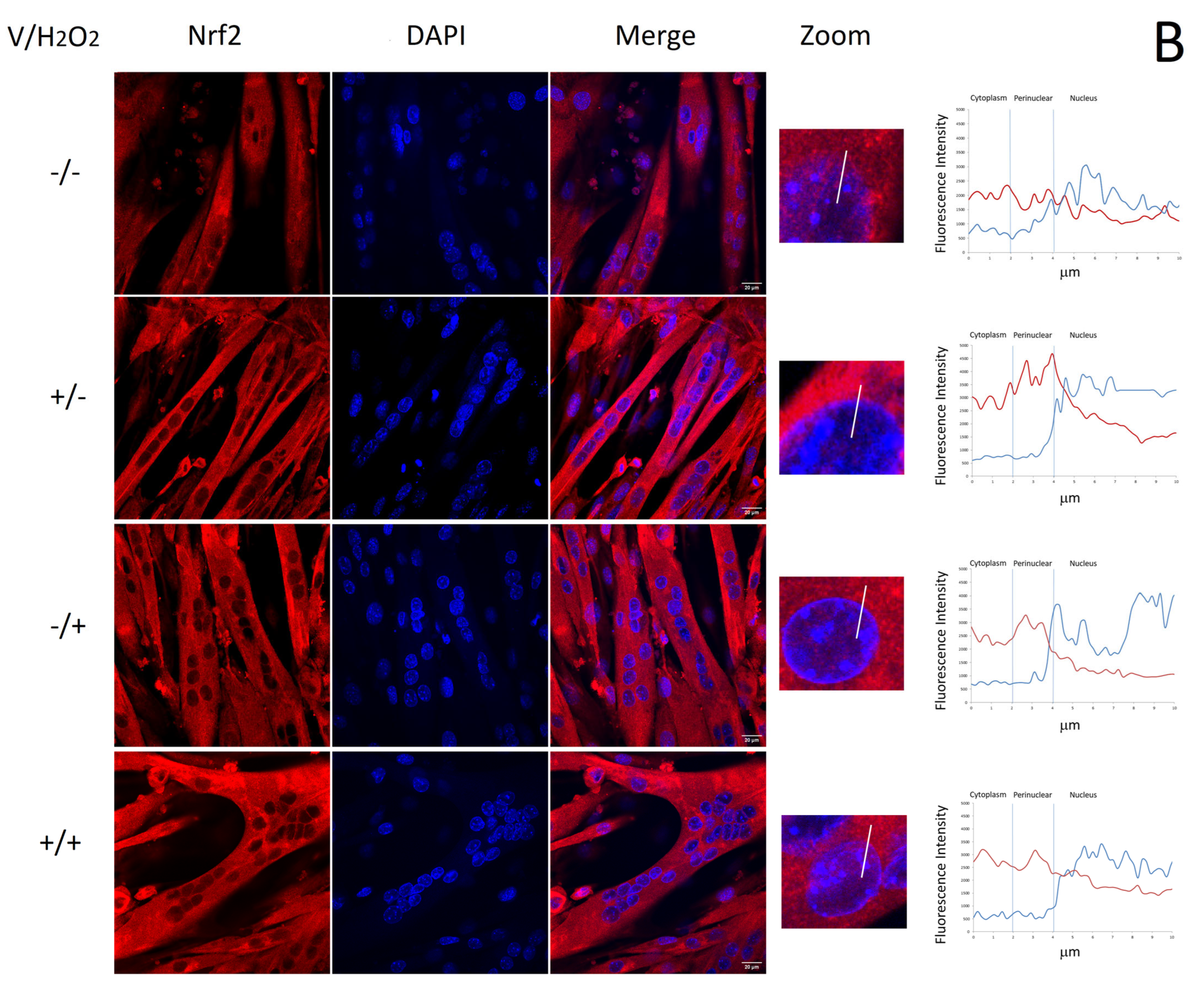
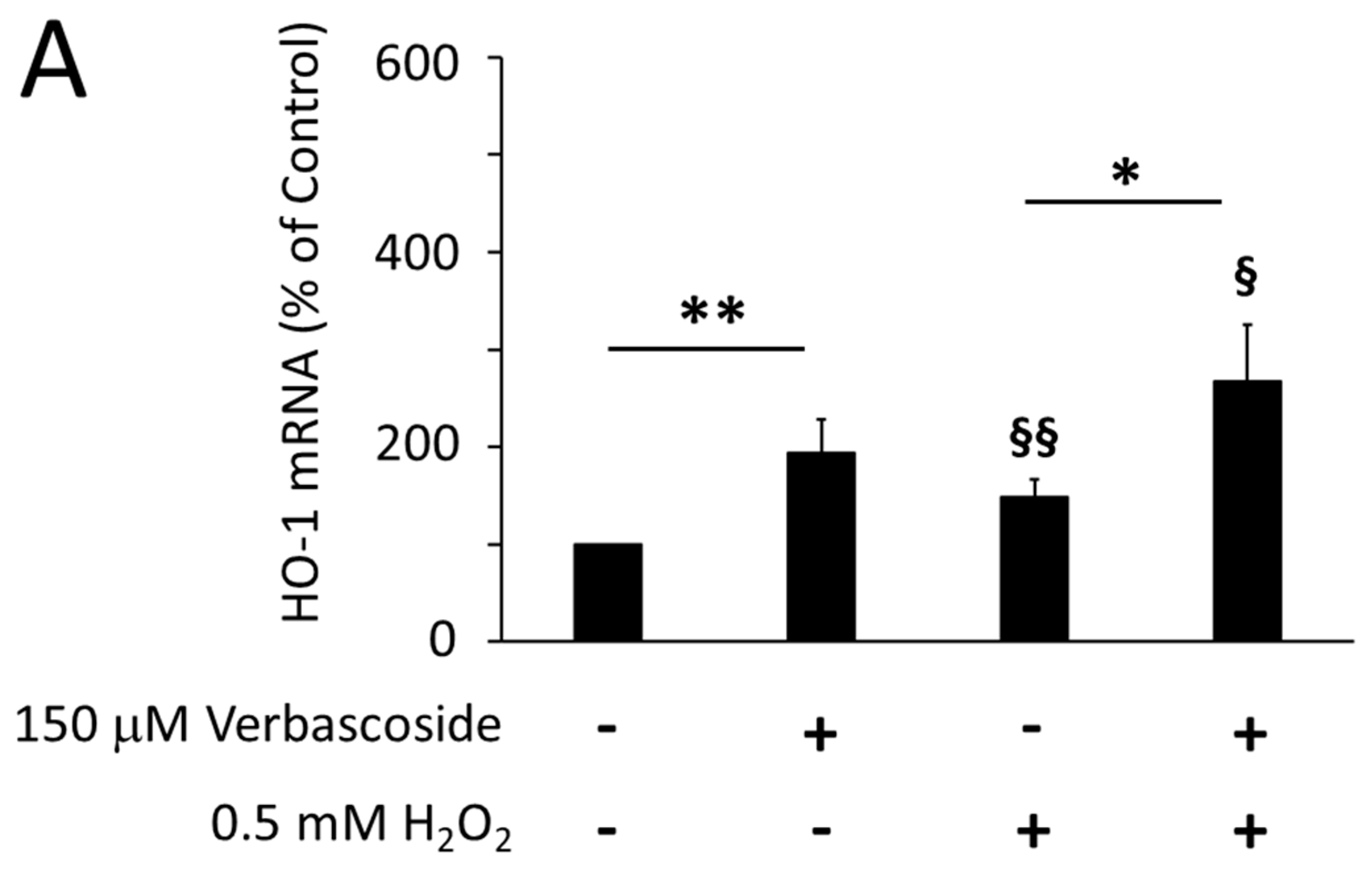
2.4. Verbascoside Reduced Oxidative Damage by Activating the Nrf2/HO-1 Axis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
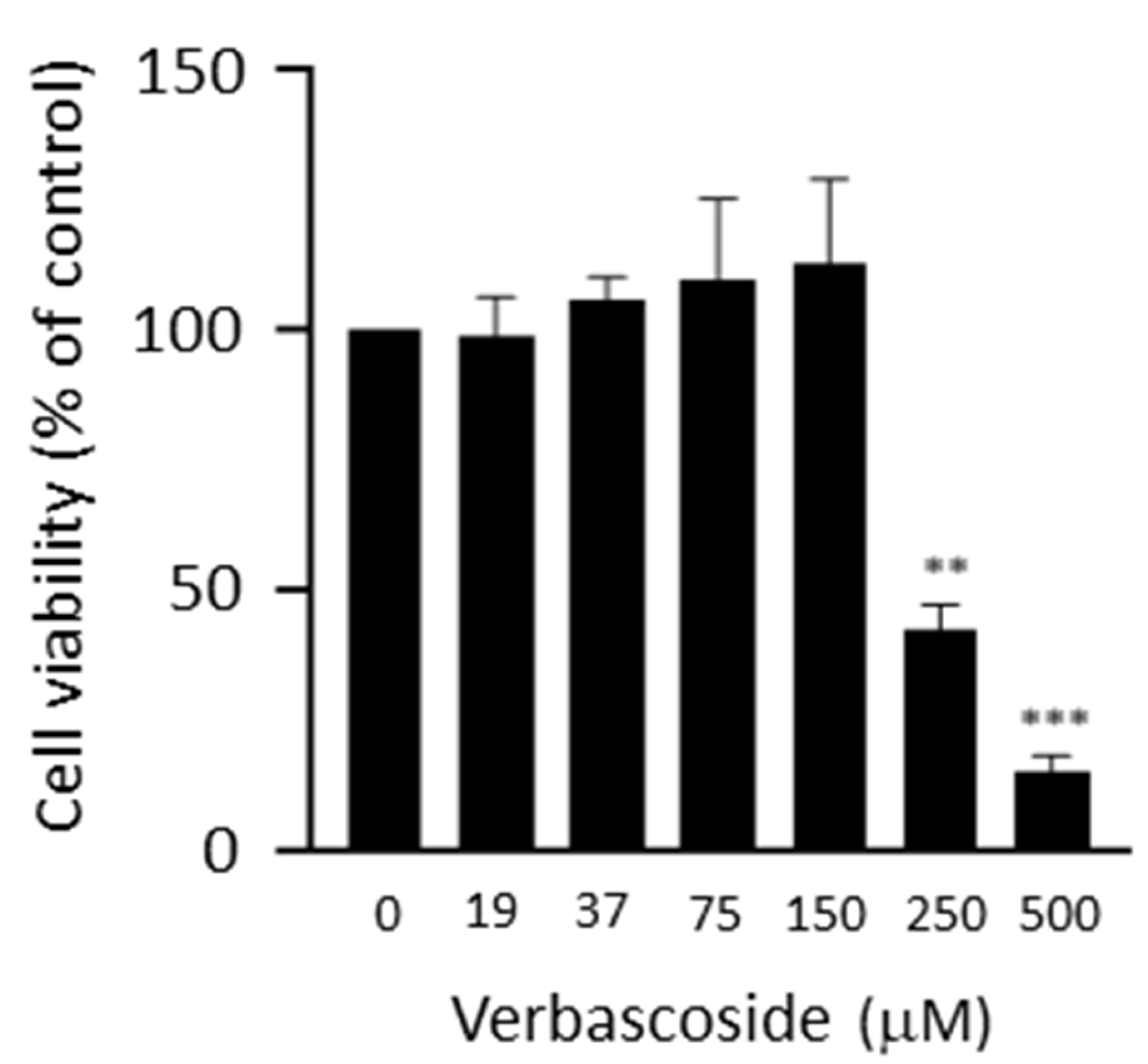
4.2. Cell Viability
4.3. High-Resolution Respirometry
4.4. Detection of Reactive Oxygen Species
4.5. Protein Extraction and Western Blotting Analysis
4.6. RNA Isolation and RT-PCR
4.7. Immunofluorescence
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signalling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular Basis of the Keap1-Nrf2 System. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-Sensing Mechanisms and the Physiological Roles of the Keap1-Nrf2 System during Cellular Stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Sinclair, D.A. Measuring PGC-1α and Its Acetylation Status in Mouse Primary Myotubes. Methods Mol. Biol. 2021, 2310, 301–309. [Google Scholar] [PubMed]
- Bellissimo, C.A.; Garibotti, M.C.; Perry, C.G.R. Mitochondrial stress responses in Duchenne muscular dystrophy: Metabolic dysfunction or adaptive reprogramming? Am. J. Physiol. Cell Physiol. 2022, 323, C718–C730. [Google Scholar] [CrossRef]
- Bernardi, P.; Bonaldo, P. Mitochondrial dysfunction and defective autophagy in the pathogenesis of collagen VI muscular dystrophies. Cold Spring Harb. Perspect. Biol. 2013, 5, a011387. [Google Scholar] [CrossRef]
- Fontes-Oliveira, C.C.; Steinz, M.; Schneiderat, P.; Mulder, H.; Durbeej, M. Bioenergetic Impairment in Congenital Muscular Dystrophy Type 1A and Leigh Syndrome Muscle Cells. Sci. Rep. 2017, 7, 45272. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Schuh, R.A.; Jackson, K.C.; Khairallah, R.J.; Ward, C.W.; Spangenburg, E.E. Measuring mitochondrial respiration in intact single muscle fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R712-9. [Google Scholar] [CrossRef]
- Timpani, C.A.; Hayes, A.; Rybalka, E. Revisiting the dystrophin-ATP connection: How half a century of research still implicates mitochondrial dysfunction in Duchenne Muscular Dystrophy aetiology. Med. Hypotheses 2015, 85, 1021–1033. [Google Scholar] [CrossRef]
- Desler, C.; Hansen, T.L.; Frederiksen, J.B.; Marcker, M.L.; Singh, K.K.; Rasmussen, L.J. Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? J. Aging Res. 2012, 2012, 192503. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Zhang, H.; Ropelle, E.R.; Sorrentino, V.; Mázala, D.A.; Mouchiroud, L.; Marshall, P.L.; Campbell, M.D.; Ali, A.S.; Knowels, G.M.; et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 2016, 8, 361ra139. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Lee, N.H.; Moon, J.Y.; Han, W.M.; Anderson, S.E.; Choi, J.J.; Shin, E.; Nakhai, S.A.; Tran, T.; Aliya, B.; et al. Critical Limb Ischemia Induces Remodeling of Skeletal Muscle Motor Unit, Myonuclear and Mitochondrial-Domains. Sci. Rep. 2019, 9, 9551. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside Protects Pancreatic β-Cells against ER-Stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Treml, J.; Večeřová, P.; Herczogová, P.; Šmejkal, K. Direct and Indirect Antioxidant Effects of Selected Plant Phenolics in Cell-Based Assays. Molecules 2021, 26, 2534. [Google Scholar] [CrossRef] [PubMed]
- Canovai, A.; Amato, R.; Melecchi, A.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Preventive Efficacy of an Antioxidant Compound on Blood Retinal Barrier Breakdown and Visual Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2022, 12, 811818. [Google Scholar] [CrossRef]
- Liao, F.; Zheng, R.L.; Gao, J.J.; Jia, Z.J. Retardation of skeletal muscle fatigue by the two phenylpropanoid glycosides: Verbascoside and martynoside from Pedicularis plicata maxim. Phytother. Res. 1999, 13, 621–623. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, H.; Tan, N.; Wang, H.; Chu, H.; Zhang, C. Central anti-fatigue activity of Verbascoside. Neurosci. Lett. 2016, 616, 75–79. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, F.; Liu, J.; Liu, T.; Yang, J.; Hu, J. A novel PHD2 inhibitor acteoside from Cistanche tubulosa induces skeletal muscle mitophagy to improve cancer-related fatigue. Biomed. Pharmacother. 2022, 150, 113004. [Google Scholar] [CrossRef]
- D’Imperio, M.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Minervini, F.; Redan, B.W.; Ferruzzi, M.G. Stability–activity of verbascoside, a known antioxidant compound, at different pH conditions. Food Res. Int. 2014, 66, 373–378. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Liu, T.; Lv, Y.F.; Zhao, J.L.; You, Q.D.; Jiang, Z.Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.A.; Jaiswal, A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003, 278, 44675–44682. [Google Scholar] [PubMed]
- Willi, L.; Abramovich, I.; Fernandez-Garcia, J.; Agranovich, B.; Shulman, M.; Milman, H.; Baskin, P.; Eisen, B.; Michele, D.E.; Arad, M.; et al. Bioenergetic and Metabolic Impairments in Induced Pluripotent Stem Cell-Derived Cardiomyocytes Generated from Duchenne Muscular Dystrophy Patients. Int. J. Mol. Sci. 2022, 23, 9808. [Google Scholar] [CrossRef]
- Vila, M.C.; Rayavarapu, S.; Hogarth, M.W.; Van der Meulen, J.H.; Horn, A.; Defour, A.; Takeda, S.; Brown, K.J.; Hathout, Y.; Nagaraju, K.; et al. Mitochondria mediate cell membrane repair and contribute to Duchenne muscular dystrophy. Cell Death Differ. 2017, 24, 330–342. [Google Scholar] [CrossRef]
- Moore, T.M.; Lin, A.J.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; Ho, T.; Lee, J.L.; Rucker, D.H.; Nguyen, C.Q.; et al. Mitochondrial Dysfunction Is an Early Consequence of Partial or Complete Dystrophin Loss in mdx Mice. Front. Physiol. 2020, 11, 690. [Google Scholar] [CrossRef]
- Bozzi, M.; Sciandra, F. Molecular Mechanisms Underlying Muscle Wasting in Huntington’s Disease. Int. J. Mol. Sci. 2020, 21, 8314. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Adhihetty, P.; Shukla, S.; Hennessy, T.; Calingasan, N.; Yang, L.; Starkov, A.; Kiaei, M.; Cannella, M.; Sassone, J.; et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum. Mol. Genet. 2009, 18, 3048–3065. [Google Scholar] [CrossRef]
- Johri, A.; Calingasan, N.Y.; Hennessey, T.M.; Sharma, A.; Yang, L.; Wille, E.; Chandra, A.; Beal, M.F. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum. Mol. Genet. 2012, 21, 1124–1137. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis. In Mitochondr Physiol Network 19.12, 4th ed.; Oroboros MiPNet Publications: Innsbruck, Austria, 2014; p. 80. [Google Scholar]
- Wallace, K.B. Mitochondrial off targets of drug therapy. Trends Pharmacol. Sci. 2008, 29, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Pontoglio, A.; Scarà, S.; Pieroni, L.; Urbani, A.; Scatena, R. Mitochondrial Respiratory Complexes as Targets of Drugs: The PPAR Agonist Example. Cells 2022, 11, 1169. [Google Scholar] [CrossRef]
- Vazquez, F.; Lim, J.-H.; Chim, H.; Bhalla, K.; Girnun, G.; Pierce, K.; Clish, C.B.; Granter, S.R.; Widlund, H.R.; Spiegelman, B.M.; et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 2013, 23, 287–301. [Google Scholar] [CrossRef]
- Kashatus, J.A.; Nascimento, A.; Myers, L.J.; Sher, A.; Byrne, F.L.; Hoehn, K.L.; Counter, C.M.; Kashatus, D.F. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol. Cell 2015, 57, 537–551. [Google Scholar] [CrossRef]
- Stevens, D.A.; Lee, Y.; Kang, H.C.; Lee, B.D.; Lee, Y.I.; Bower, A.; Jiang, H.; Kang, S.U.; Andrabi, S.A.; Dawson, V.L.; et al. Parkin loss leads to PARIS dependent declines in mitochondrial mass and respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 11696–11701. [Google Scholar] [CrossRef]
- Costa, A.C.; Loh, S.H.Y.; Martins, L.M. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis. 2019, 4, e467. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signalling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef]
- Chan, M.C.; Rowe, G.C.; Raghuram, S.; Patten, I.S.; Farrell, C.; Arany, Z. Post-natal induction of PGC-1α protects against severe muscle dystrophy independently of utrophin. Skelet. Muscle 2014, 4, 2. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Xu, Y.; Yan, J.; Zhao, X.; Sun, X.; Zhang, Y.; Guo, J.; Zhu, C. Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res. 2009, 1283, 139–147. [Google Scholar] [CrossRef]
- Sheng, G.Q.; Zhang, J.R.; Pu, X.P.; Ma, J.; Li, C.L. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur. J. Pharmacol. 2002, 451, 119–124. [Google Scholar] [CrossRef]
- Koo, K.A.; Kim, S.H.; Oh, T.H.; Kim, Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006, 79, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Xu, Y.X.; Zhu, C.Q. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox. Res. 2012, 21, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.N.; Zhuo, J.Y.; Nie, J.; Liu, Y.L.; Chen, B.Y.; Wu, A.Z.; Li, Y.C. Phenylethanoid Glycosides from Callicarpa kwangtungensis Chun Attenuate TNF-α-Induced Cell Damage by Inhibiting NF-κB Pathway and Enhancing Nrf2 Pathway in A549 Cells. Front. Pharmacol. 2021, 12, 693983. [Google Scholar] [CrossRef]
- Li, M.; Zhou, F.; Xu, T.; Song, H.; Lu, B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signalling pathway. Food Chem. Toxicol. 2018, 119, 6–13. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, S.; Hwang, E.; Yi, T.H.; Wang, Y.S. Effects of phenylethanol glycosides from Orobanche cernua Loefling on UVB-Induced skin photodamage: A comparative study. Photochem. Photobiol. Sci. 2021, 20, 599–614. [Google Scholar] [CrossRef]
- Yang, J.; Hua, Z.; Zheng, Z.; Ma, X.; Zhu, L.; Li, Y. Acteoside inhibits high glucose-induced oxidative stress injury in RPE cells and the outer retina through the Keap1/Nrf2/ARE pathway. Exp. Eye Res. 2023, 232, 109496. [Google Scholar] [CrossRef]
- Urish, K.L.; Vella, J.B.; Okada, M.; Deasy, B.M.; Tobita, K.; Keller, B.B.; Cao, B.; Piganelli, J.D.; Huard, J. Antioxidant Levels Represent a Major Determinant in the Regenerative Capacity of Muscle Stem Cells. Mol. Biol. Cell 2009, 20, 509–520. [Google Scholar] [CrossRef]
- Laumonier, T.; Yang, S.; Konig, S.; Chauveau, C.; Anegon, I.; Hoffmeyer, P.; Menetrey, J. Lentivirus Mediated HO-1 Gene Transfer Enhances Myogenic Precursor Cell Survival after Autologous Transplantation in Pig. Mol. Ther. 2008, 16, 404–410. [Google Scholar] [CrossRef]
- Aggeli, I.-K.; Kefaloyianni, E.; Beis, I.; Gaitanaki, C. HOX-1 and COX-2: Two Differentially Regulated Key Mediators of Skeletal Myoblast Tolerance under Oxidative Stress. Free Radic. Res. 2010, 44, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Perez-Terzic, C.; Behfar, A.; Terzic, A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Terzic, A. Developmental restructuring of the creatine kinase system integrates mitochondrial energetics with stem cell cardiogenesis. Ann. N. Y. Acad. Sci. 2008, 1147, 254–263. [Google Scholar] [CrossRef]
- Folmes, C.D.L.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Z.; Sun, Z.; Wang, L.; Zhao, G.; Hassan, H.M.; Fan, S.; Zhou, W.; Han, S.; Zhang, L.; et al. Mitochondrial dysfunction and inhibition of myoblast differentiation in mice with high-fat-diet-induced pre-diabetes. J. Cell Physiol. 2019, 234, 7510–7523. [Google Scholar] [CrossRef]
- Marigo, L.; Spagnuolo, G.; Malara, F.; Martorana, G.E.; Cordaro, M.; Lupi, A.; Nocca, G. Relation between conversion degree and cytotoxicity of a flowable bulk-fill and three conventional flowable resin-composites. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4469–4480. [Google Scholar]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Dott, W.; Mistry, P.; Wright, J.; Cain, K.; Herbert, K.E. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2014, 2, 224–233. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palmieri, V.; Bozzi, M.; Signorino, G.; Papi, M.; De Spirito, M.; Brancaccio, A.; Maulucci, G.; Sciandra, F. α-Dystroglycan hypoglycosylation affects cell migration by influencing β-dystroglycan membrane clustering and filopodia length: A multiscale confocal microscopy analysis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2182–2191. [Google Scholar] [CrossRef]
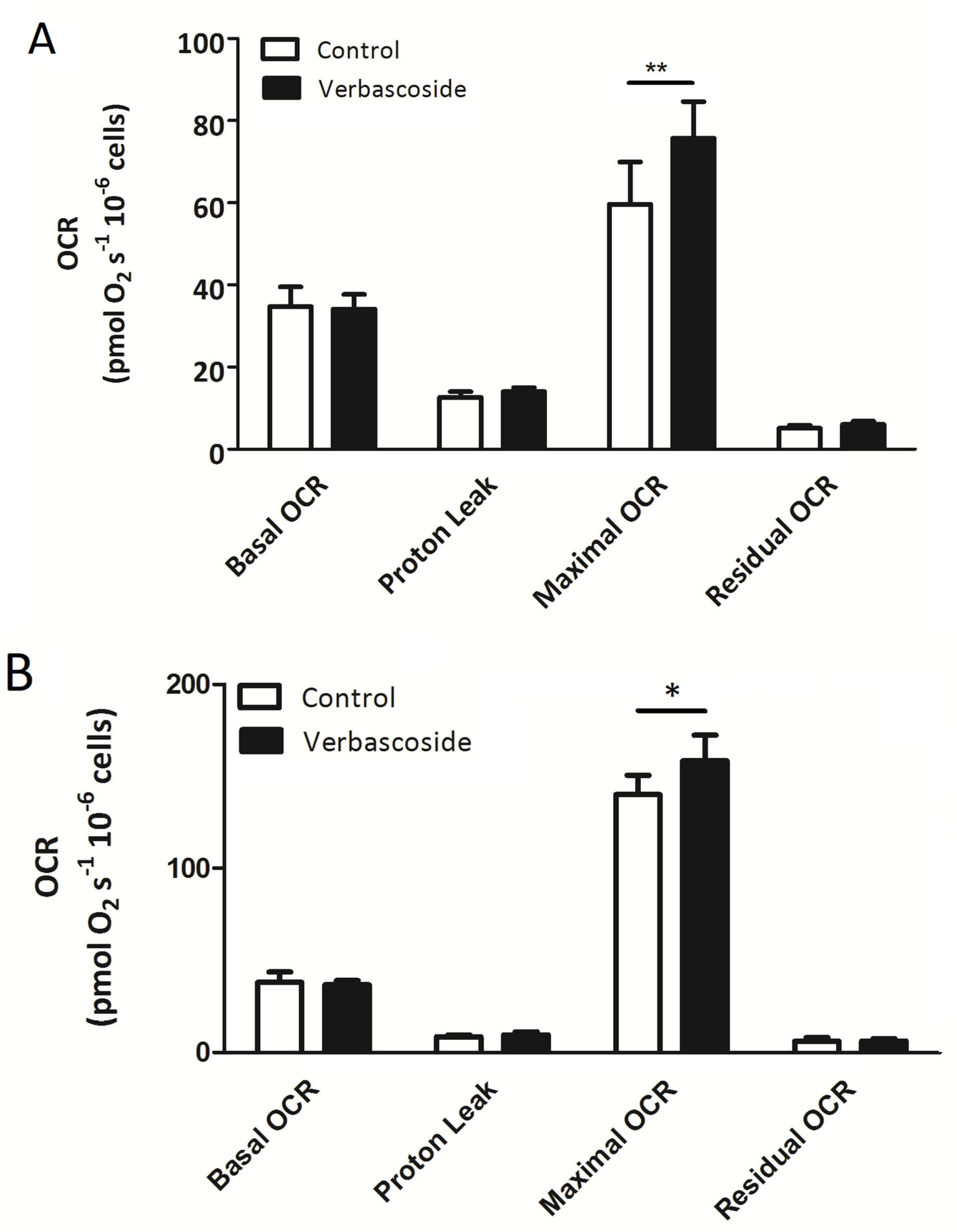
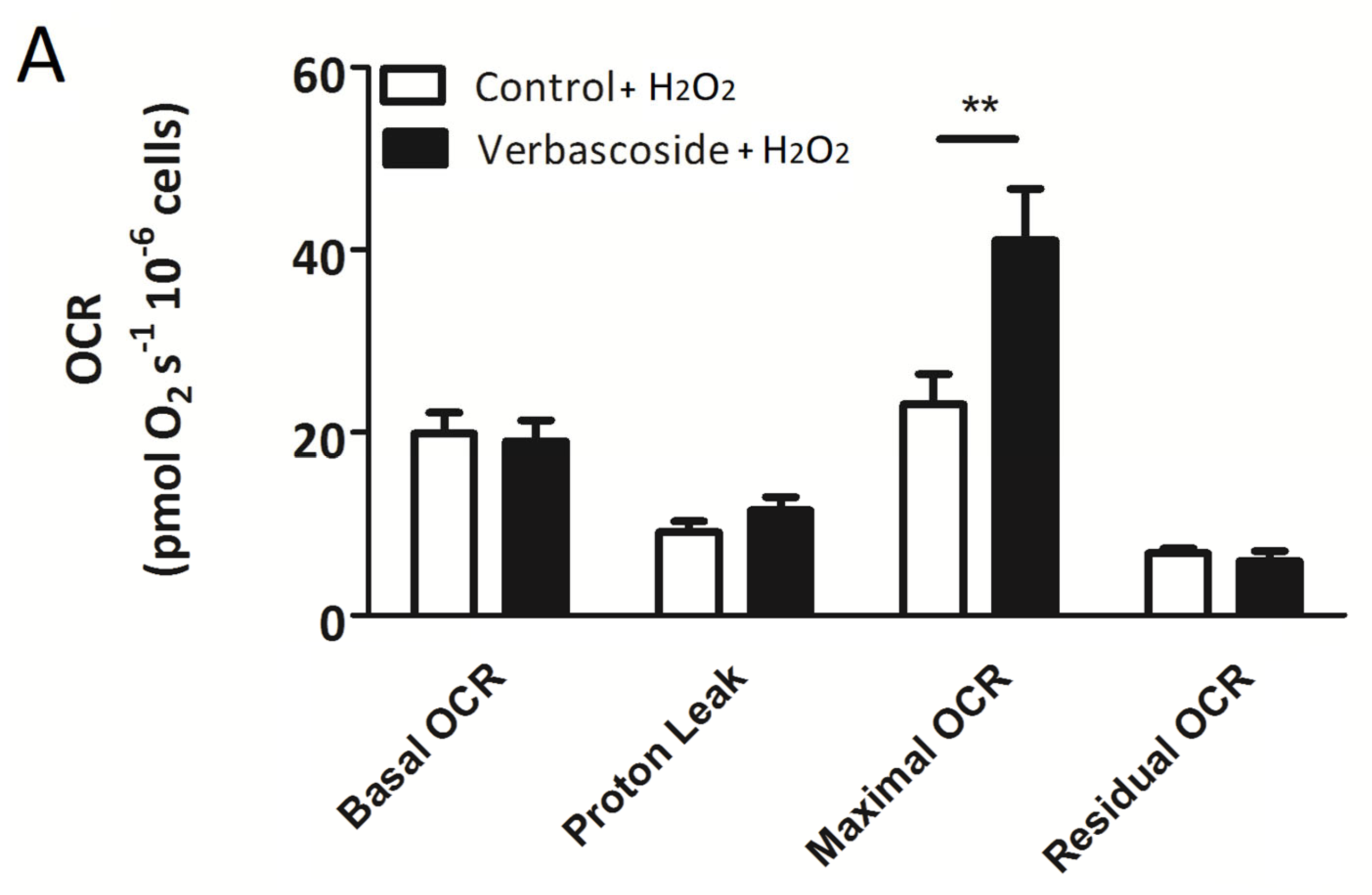
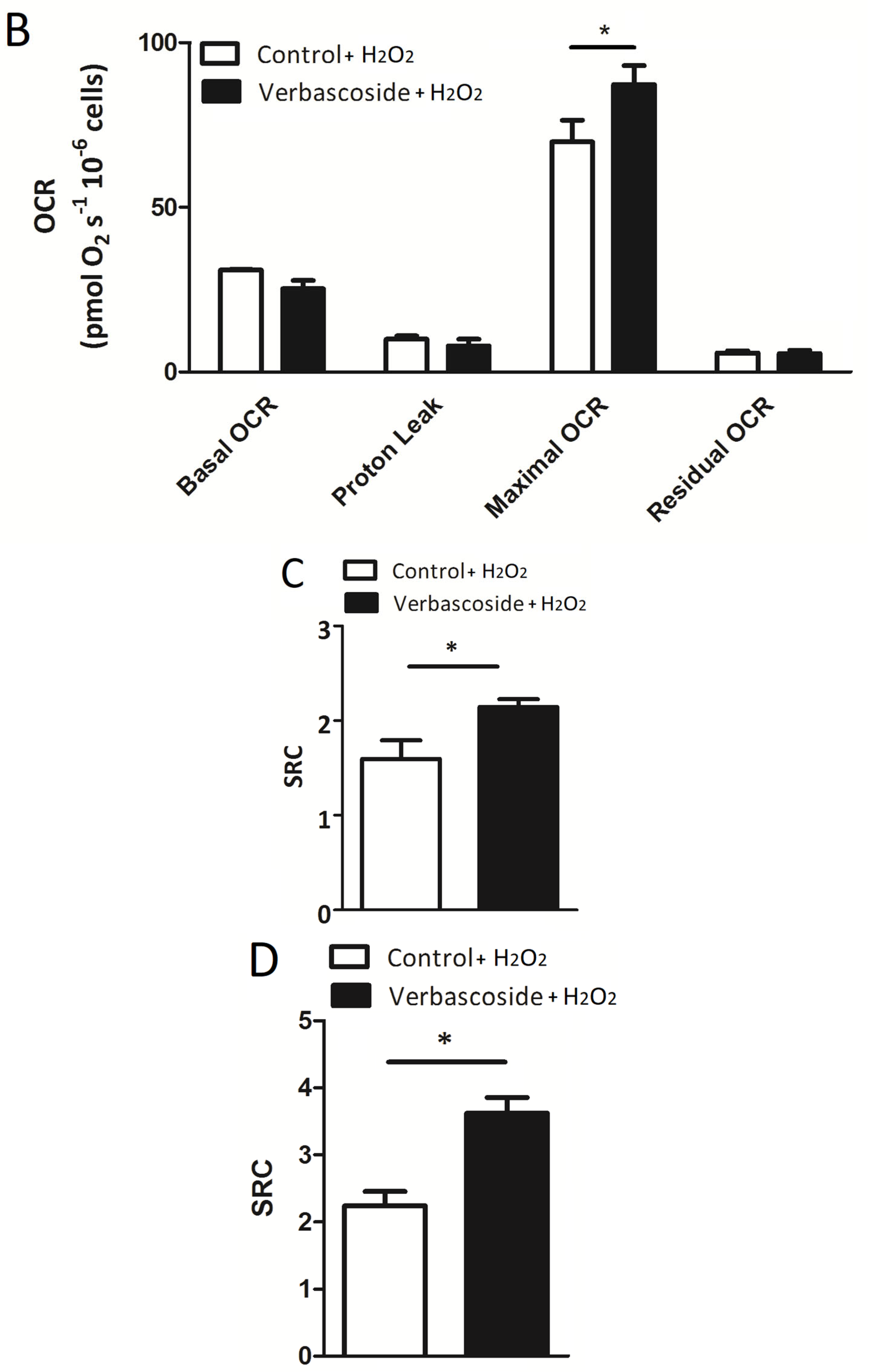




| Myoblasts | OCR (pmol/(s × 106) −V | OCR (pmol/(s × 106) +V | OCR (pmol/(s × 106) −V/+H2O2 | OCR (pmol/(s × 106) +V/+H2O2 |
| Basal OCR | 34.7 ± 4.7 | 34.1 ± 3.5 | 19.9 ± 2.3 | 19.0 ± 2.3 |
| Proton Leak | 12.6 ± 1.4 | 14.01 ± 0.99 | 9.1 ± 1.2 | 11.5 ± 1.4 |
| Maximal OCR | 59.6 ± 10.3 | 75.7 ± 8.9 ** | 23.1 ± 3.3 | 41.0 ± 5.6 ** |
| Residual OCR | 5.10 ± 0.69 | 6.03 ± 0.77 | 6.77 ± 0.56 | 5.93 ± 1.08 |
| Myotubes |
OCR
(pmol/(s × 106) −V |
OCR
(pmol/(s × 106) +V |
OCR
(pmol/(s × 106) −V/+H2O2 |
OCR
(pmol/(s × 106) +V/+H2O2 |
| Basal OCR | 38.0 ± 5.5 | 36.5 ± 2.3 | 30.9 ± 0.2 | 25.3 ± 2.5 |
| Proton Leak | 8.6 ± 1.1 | 9.6 ± 1.6 | 9.9 ± 1.1 | 8.0 ± 1.9 |
| Maximal OCR | 140.1 ± 13.6 | 158.6 ± 18.1 * | 69.8 ± 6.6 | 87.3 ± 5.7 * |
| Residual OCR | 6.0 ± 2.1 | 6.3 ± 1.1 | 5.8 ± 0.6 | 5.6 ± 1.0 |
| Myoblasts | −V | +V | −V/+H2O2 | +V/+H2O2 |
| SRC | 1.72 ± 0.15 | 2.60 ± 0.22 ** | 1.60 ± 0.20 | 2.14 ± 0.08 * |
| RCR | 5.33 ± 0.59 | 5.53 ± 0.78 | 3.24 ± 0.17 | 4.11 ± 0.54 |
| CE | 0.61 ± 0.05 | 0.57 ± 0.07 | 0.53 ± 0.06 | 0.50 ± 0.02 |
| Myotubes | −V | + V | −V/+H2O2 | +V/+H2O2 |
| SRC | 3.8 ± 0.3 | 4.3 ± 0.3 * | 2.24 ± 0.2 | 3.6 ± 0.2 * |
| RCR | 16.5 ± 1.0 | 16.9 ± 1.1 | 7.0 ± 0.3 | 11.6 ± 1.6 * |
| CE | 0.76 ± 0.03 | 0.78 ± 0.005 | 0.67 ± 0.04 | 0.77 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciandra, F.; Bottoni, P.; De Leo, M.; Braca, A.; Brancaccio, A.; Bozzi, M. Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line. Int. J. Mol. Sci. 2023, 24, 15276. https://doi.org/10.3390/ijms242015276
Sciandra F, Bottoni P, De Leo M, Braca A, Brancaccio A, Bozzi M. Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line. International Journal of Molecular Sciences. 2023; 24(20):15276. https://doi.org/10.3390/ijms242015276
Chicago/Turabian StyleSciandra, Francesca, Patrizia Bottoni, Marinella De Leo, Alessandra Braca, Andrea Brancaccio, and Manuela Bozzi. 2023. "Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line" International Journal of Molecular Sciences 24, no. 20: 15276. https://doi.org/10.3390/ijms242015276
APA StyleSciandra, F., Bottoni, P., De Leo, M., Braca, A., Brancaccio, A., & Bozzi, M. (2023). Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line. International Journal of Molecular Sciences, 24(20), 15276. https://doi.org/10.3390/ijms242015276












