Potential Theranostic Roles of SLC4 Molecules in Human Diseases
Abstract
1. Introduction
2. Topological Structure, Structural Difference of SLC4 Proteins
3. Classification and Ion-Transporting Mechanism
4. The Roles of SLC4A Proteins in Human Tissues
4.1. Anion Exchangers
4.2. Sodium-Coupled SLC4 Proteins
4.3. The Other SLC4 Proteins
5. Molecules Interacted with SLC4 Proteins
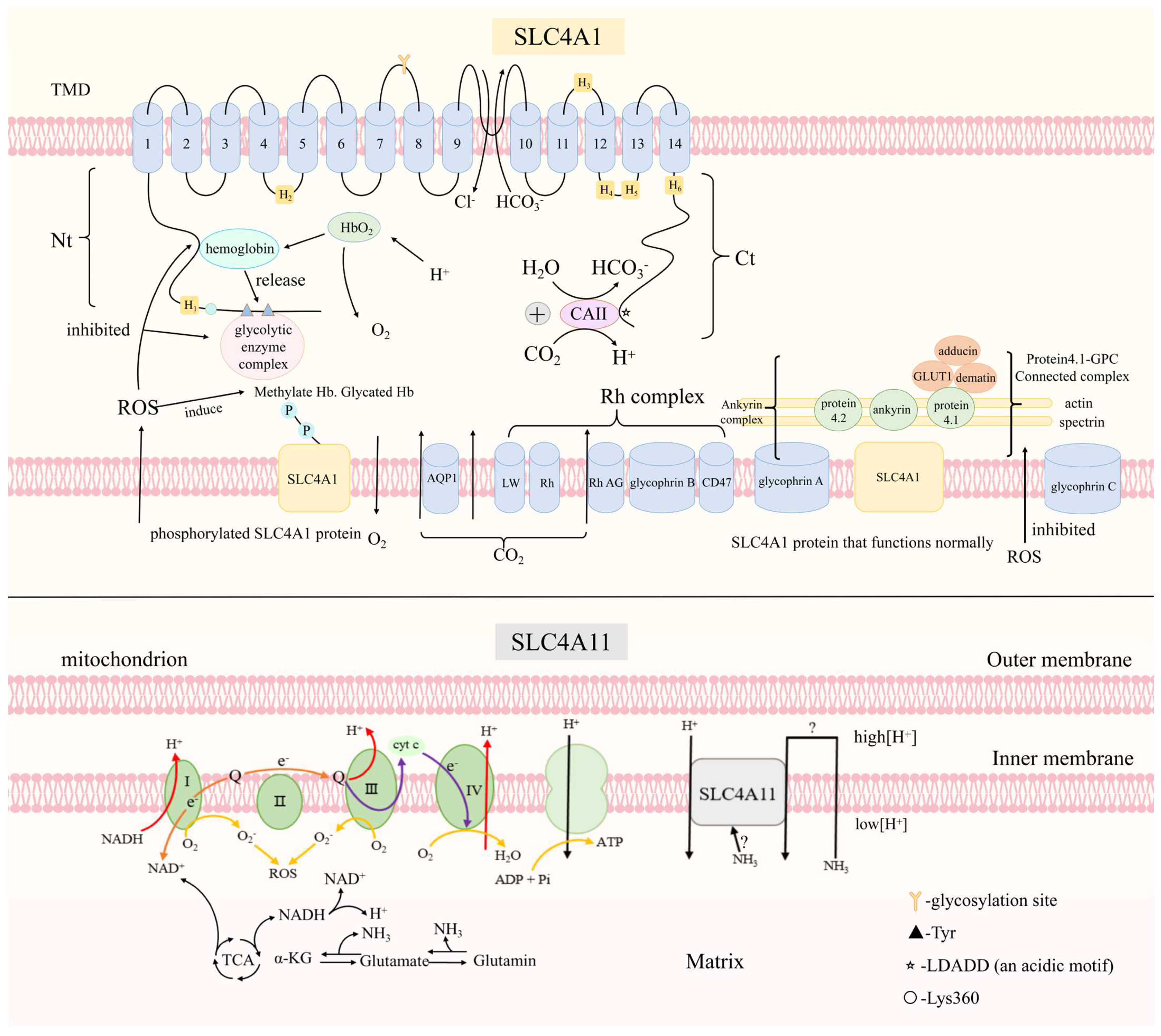
5.1. SLC4A1
5.2. SLC4A4
5.3. SLC4A7
5.4. SLC4A8
5.5. SLC4A10
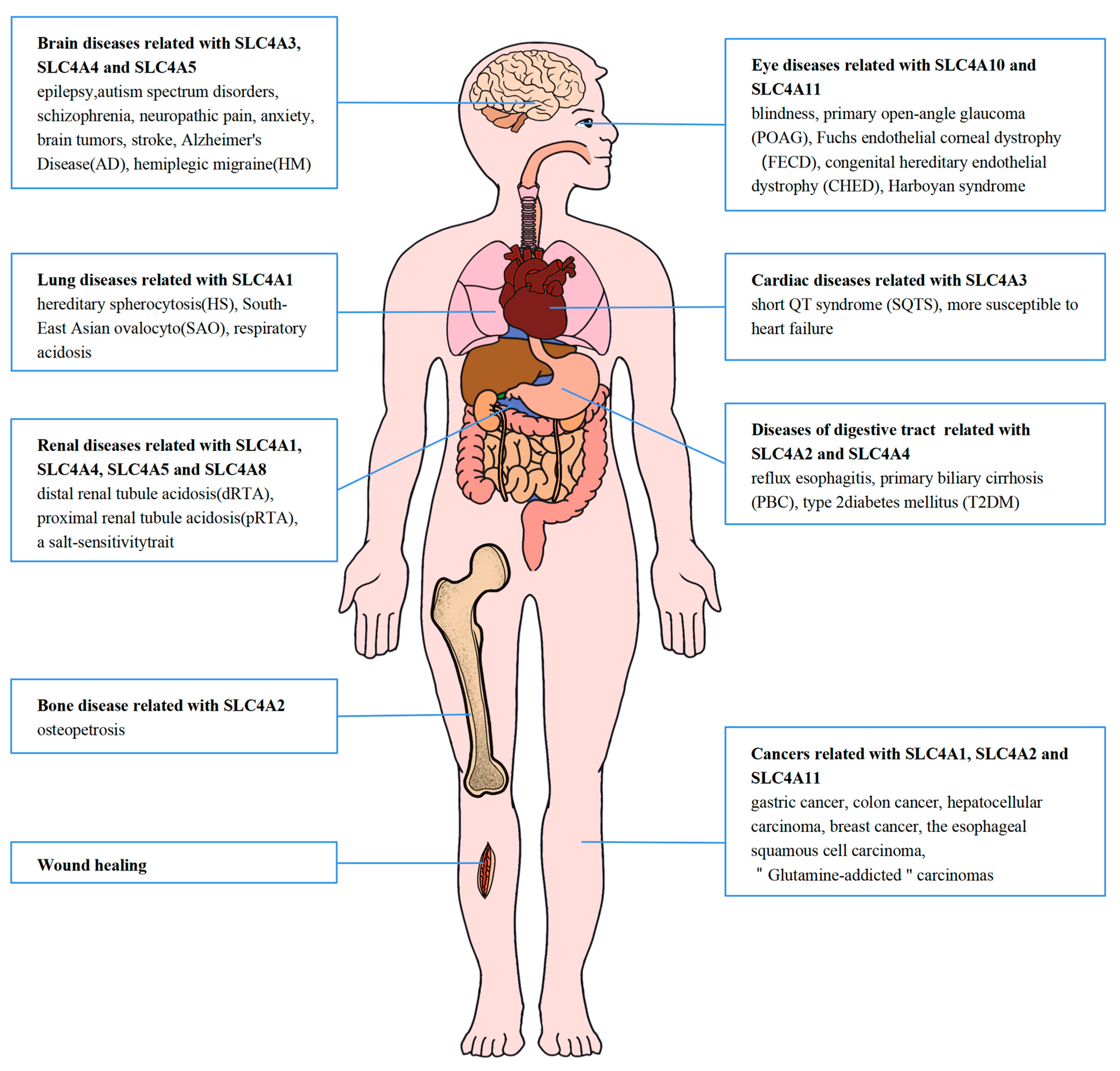
6. Associated Diseases and Potential Clinical Values of SLC4 Proteins in Human Tissues
6.1. SLC4A1-3
6.1.1. SLC4A1
6.1.2. SLC4A2
6.1.3. SLC4A3
6.2. Sodium-Coupled SLC4 Proteins
6.2.1. SLC4A4
6.2.2. SLC4A5
6.2.3. SLC4A7
6.2.4. SLC4A10
6.2.5. SLC4A8
6.3. The Other SLC4 Proteins
7. Homeostasis of Bicarbonate in the Body and Drugs Affecting Bicarbonate Transport
8. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gorbatenko, A.; Olesen, C.W.; Boedtkjer, E.; Pedersen, S.F. Regulation and Roles of Bicarbonate Transporters in Cancer. Front. Physiol. 2014, 5, 130. [Google Scholar] [CrossRef]
- Sinning, A.; Liebmann, L.; Hübner, C.A. Disruption of Slc4a10 Augments Neuronal Excitability and Modulates Synaptic Short-Term Plasticity. Front. Cell. Neurosci. 2015, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Chen, Y.; Vairamani, K.; Shull, G.E. Critical Role of Bicarbonate and Bicarbonate Transporters in Cardiac Function. World J. Biol. Chem. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zahra, A.; Jia, M.; Wang, Q.; Wu, J. Understanding the Functional Expression of Na+-Coupled SLC4 Transporters in the Renal and Nervous Systems: A Review. Brain Sci. 2021, 11, 1276. [Google Scholar] [CrossRef]
- Alexander, R.T.; Law, L.; Gil-Peña, H.; Greenbaum, L.A.; Santos, F. Hereditary Distal Renal Tubular Acidosis; University of Washington Seattle: Seattle, WA, USA, 2019. [Google Scholar]
- Kurtz, I. NBCe1 as a Model Carrier for Understanding the Structure-Function Properties of Na+ -Coupled SLC4 Transporters in Health and Disease. Pflugers Arch. 2014, 466, 1501–1516. [Google Scholar] [CrossRef]
- Tanner, M.J.A. Band 3 Anion Exchanger and Its Involvement in Erythrocyte and Kidney Disorders. Curr. Opin. Hematol. 2002, 9, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 Solute Carriers during Oxidative Stress. Acta Physiol. 2022, 235, e13796. [Google Scholar] [CrossRef]
- Gurnett, C.A.; Veile, R.; Zempel, J.; Blackburn, L.; Lovett, M.; Bowcock, A. Disruption of Sodium Bicarbonate Transporter SLC4A10 in a Patient with Complex Partial Epilepsy and Mental Retardation. Arch. Neurol. 2008, 65, 550–553. [Google Scholar] [CrossRef]
- Collin, G.B.; Shi, L.; Yu, M.; Akturk, N.; Charette, J.R.; Hyde, L.F.; Weatherly, S.M.; Pera, M.F.; Naggert, J.K.; Peachey, N.S.; et al. A Splicing Mutation in Slc4a5 Results in Retinal Detachment and Retinal Pigment Epithelium Dysfunction. Int. J. Mol. Sci. 2022, 23, 2220. [Google Scholar] [CrossRef]
- Heine, C.; Browning, C.J. Mental Health and Dual Sensory Loss in Older Adults: A Systematic Review. Front. Aging Neurosci. 2014, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Stock, C. Ion Channels and Transporters in Cancer: Pathophysiology, Regulation, and Clinical Potential. Cancer Res. 2013, 73, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Chen, L.M. Structure and Function of SLC4 Family [Formula: See Text] Transporters. Front. Physiol. 2015, 6, 355. [Google Scholar] [CrossRef]
- Badior, K.E.; Alka, K.; Casey, J.R. SLC4A11 Three-Dimensional Homology Model Rationalizes Corneal Dystrophy-Causing Mutations. Hum. Mutat. 2017, 38, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tsirulnikov, K.; Zhekova, H.R.; Kayık, G.; Khan, H.M.; Azimov, R.; Abuladze, N.; Kao, L.; Newman, D.; Noskov, S.Y.; et al. Cryo-EM Structure of the Sodium-Driven Chloride/Bicarbonate Exchanger NDCBE. Nat. Commun. 2021, 12, 5690. [Google Scholar] [CrossRef]
- Romero, M.F.; Chen, A.P.; Parker, M.D.; Boron, W.F. The SLC4 Family of Bicarbonate (HCO₃−) Transporters. Mol. Aspects Med. 2013, 34, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.; Zhu, Q. Proximal Renal Tubular Acidosis Mediated by Mutations in NBCe1-A: Unraveling the Transporter’s Structure-Functional Properties. Front. Physiol. 2013, 4, 350. [Google Scholar] [CrossRef][Green Version]
- Huynh, K.W.; Jiang, J.; Abuladze, N.; Tsirulnikov, K.; Kao, L.; Shao, X.; Newman, D.; Azimov, R.; Pushkin, A.; Zhou, Z.H.; et al. CryoEM Structure of the Human SLC4A4 Sodium-Coupled Acid-Base Transporter NBCe1. Nat. Commun. 2018, 9, 900. [Google Scholar] [CrossRef]
- Virkki, L.V.; Wilson, D.A.; Vaughan-Jones, R.D.; Boron, W.F. Functional Characterization of Human NBC4 as an Electrogenic Na+-HCO Cotransporter (NBCe2). Am. J. Physiol. Cell Physiol. 2002, 282, C1278–C1289. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, Y.; Boron, W.F. Role of an Extracellular Loop in Determining the Stoichiometry of Na+-HCO₃− Cotransporters. J. Physiol. 2011, 589, 877–890. [Google Scholar] [CrossRef]
- Reithmeier, R.A.F.; Casey, J.R.; Kalli, A.C.; Sansom, M.S.P.; Alguel, Y.; Iwata, S. Band 3, the Human Red Cell Chloride/Bicarbonate Anion Exchanger (AE1, SLC4A1), in a Structural Context. Biochim. Biophys. Acta 2016, 1858, 1507–1532. [Google Scholar] [CrossRef]
- Vilas, G.L.; Loganathan, S.K.; Liu, J.; Riau, A.K.; Young, J.D.; Mehta, J.S.; Vithana, E.N.; Casey, J.R. Transmembrane Water-Flux through SLC4A11: A Route Defective in Genetic Corneal Diseases. Hum. Mol. Genet. 2013, 22, 4579–4590. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Kurtz, I. Structural Determinants and Significance of Regulation of Electrogenic Na+-HCO3− Cotransporter Stoichiometry. Am. J. Physiol. Renal. Physiol. 2002, 283. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, S.; Su, P.; Xie, Z.D.; Gui, T.X.; Zhao, L.; Liu, Y.; Chen, L.M. Molecular Insight into Coordination Sites for Substrates and Their Coupling Kinetics in Na+/HCO3− Cotransporter NBCe1. J. Physiol. 2022, 600, 3083–3111. [Google Scholar] [CrossRef] [PubMed]
- Millar, I.D.; Brown, P.D. NBCe2 Exhibits a 3 HCO3−:1 Na+ Stoichiometry in Mouse Choroid Plexus Epithelial Cells. Biochem. Biophys. Res. Commun. 2008, 373, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Pushkin, A.; Abuladze, N.; Lee, I.; Newman, D.; Hwang, J.; Kurtz, I. Cloning, Tissue Distribution, Genomic Organization, and Functional Characterization of NBC3, a New Member of the Sodium Bicarbonate Cotransporter Family. J. Biol. Chem. 1999, 274, 16569–16575. [Google Scholar] [CrossRef]
- Parker, M.D.; Musa-Aziz, R.; Rojas, J.D.; Choi, I.; Daly, C.M.; Boron, W.F. Characterization of Human SLC4A10 as an Electroneutral Na/HCO3 Cotransporter (NBCn2) with Cl− Self-Exchange Activity. J. Biol. Chem. 2008, 283, 12777–12788. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Boedtkjer, E.; Choi, I.; Lee, S. Cation-Coupled Bicarbonate Transporters. Compr. Physiol. 2014, 4, 1605–1637. [Google Scholar] [CrossRef]
- Peña-Münzenmayer, G.; George, A.T.; Shull, G.E.; Melvin, J.E.; Catalán, M.A. Ae4 (Slc4a9) Is an Electroneutral Monovalent Cation-Dependent Cl−/HCO3− Exchanger. J. Gen. Physiol. 2016, 147, 423–436. [Google Scholar] [CrossRef]
- Parker, M.D.; Ourmozdi, E.P.; Tanner, M.J.A. Human BTR1, a New Bicarbonate Transporter Superfamily Member and Human AE4 from Kidney. Biochem. Biophys. Res. Commun. 2001, 282, 1103–1109. [Google Scholar] [CrossRef]
- Zhang, W.; Ogando, D.G.; Bonanno, J.A.; Obukhov, A.G. Human SLC4A11 Is a Novel NH3/H+ Co-Transporter. J. Biol. Chem. 2015, 290, 16894–16905. [Google Scholar] [CrossRef]
- Zail, S.S.; Van Den Hoek, A. Electrophoretic Analysis of the Major Polypeptides of Human Erythrocyte Membranes Prepared by Low and High Osmolarity Haemolysis. Clin. Chim. Acta 1975, 60, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Reithmeier, R.A.F. A Membrane Metabolon Linking Carbonic Anhydrase with Chloride/Bicarbonate Anion Exchangers. Blood Cells Mol. Dis. 2001, 27, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Brosius, F.C., III; Alper, S.L.; Garcia, A.M.; Lodish, H.F. The Major Kidney Band 3 Gene Transcript Predicts an Amino-Terminal Truncated Band 3 Polypeptide. J. Biol. Chem. 1989, 264, 7784–7787. Available online: https://pubmed.ncbi.nlm.nih.gov/2542243/ (accessed on 5 November 2022). [CrossRef] [PubMed]
- Loiselle, F.B.; Morgan, P.E.; Alvarez, B.V.; Casey, J.R. Regulation of the Human NBC3 Na+/HCO3− Cotransporter by Carbonic Anhydrase II and PKA. Am. J. Physiol. Cell Physiol. 2004, 286, C1423–C1433. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.; Jesus, T.; Martins, A.; Sousa, M.; Barros, A.; Cavaco, J.; Socorro, S.; Alves, M.; Oliveira, P. Molecular Basis of Bicarbonate Membrane Transport in the Male Reproductive Tract. Curr. Med. Chem. 2013, 20, 4037–4049. [Google Scholar] [CrossRef]
- Donà, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (AE1). Int. J. Mol. Sci. 2020, 21, 4063. [Google Scholar] [CrossRef]
- Wang, H.; An, J.; Jin, H.; He, S.; Liao, C.; Wang, J.; Tuo, B. Roles of Cl−/HCO3− Anion Exchanger 2 in the Physiology and Pathophysiology of the Digestive System (Review). Mol. Med. Rep. 2021, 24, 493. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Turner, M.J.; Saint-Criq, V.; Garnett, J.; Haq, I.J.; Brodlie, M.; Ward, C.; Borgo, C.; Salvi, M.; Venerando, A.; et al. CK2 Is a Key Regulator of SLC4A2-Mediated Cl−/HCO3− Exchange in Human Airway Epithelia. Pflugers. Arch. 2017, 469, 1073–1091. [Google Scholar] [CrossRef]
- Eladari, D.; Kumai, Y. Renal Acid-Base Regulation: New Insights from Animal Models. Pflugers. Arch. 2015, 467, 1623–1641. [Google Scholar] [CrossRef]
- Wu, J.; Glimcher, L.H.; Aliprantis, A.O. HCO3−/Cl− Anion Exchanger SLC4A2 Is Required for Proper Osteoclast Differentiation and Function. Proc. Natl. Acad. Sci. USA 2008, 105, 16934–16939. [Google Scholar] [CrossRef]
- Xue, J.Y.; Ikegawa, S.; Guo, L. SLC4A2, Another Gene Involved in Acid-Base Balancing Machinery of Osteoclasts, Causes Osteopetrosis. Bone 2023, 167, 116603. [Google Scholar] [CrossRef]
- Edwards, J.R.; Weivoda, M.M. Osteoclasts: Malefactors of Disease and Targets for Treatment. Discov. Med. 2012, 13, 201–210. Available online: https://pubmed.ncbi.nlm.nih.gov/22463796/ (accessed on 23 July 2023). [PubMed]
- Coury, F.; Zenger, S.; Stewart, A.K.; Stephens, S.; Neff, L.; Tsang, K.; Shull, G.E.; Alper, S.L.; Baron, R.; Aliprantis, A.O. SLC4A2-Mediated Cl−/HCO3− Exchange Activity Is Essential for Calpain-Dependent Regulation of the Actin Cytoskeleton in Osteoclasts. Proc. Natl. Acad. Sci. USA 2013, 110, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shan, J.; Kim, D.; Liao, J.; Evagelidis, A.; Alper, S.L.; Hanrahan, J.W. Basolateral Chloride Loading by the Anion Exchanger Type 2: Role in Fluid Secretion by the Human Airway Epithelial Cell Line Calu-3. J. Physiol. 2012, 590, 5299–5316. [Google Scholar] [CrossRef]
- Hwang, S.; Shin, D.M.; Hong, J.H. Intracellular Ca2+-Mediated AE2 Is Involved in the Vectorial Movement of HaCaT Keratinocyte. Int. J. Mol. Sci. 2020, 21, 8429. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of Ion Channels and Transporters in Cell Migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef] [PubMed]
- Kudrycki, K.E.; Newman, P.R.; Shull, G.E. CDNA Cloning and Tissue Distribution of MRNAs for Two Proteins That Are Related to the Band 3 Cl−/HCO3− Exchanger. J. Biol. Chem. 1990, 265, 462–471. Available online: https://pubmed.ncbi.nlm.nih.gov/2294114/ (accessed on 13 November 2022). [CrossRef]
- Koe, J.C.; Hewton, K.G.; Parker, S.J. SLC4A7 and MTORC1 Raise Nucleotide Synthesis with Bicarbonate. Mol. Cell 2022, 82, 3121–3123. [Google Scholar] [CrossRef]
- Samarel, A.M. Costameres, Focal Adhesions, and Cardiomyocyte Mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2291–H2301. [Google Scholar] [CrossRef]
- Sander, T.; Toliat, M.R.; Heils, A.; Leschik, G.; Becker, C.; Rüschendorf, F.; Rohde, K.; Mundlos, S.; Nürnberg, P. Association of the 867Asp Variant of the Human Anion Exchanger 3 Gene with Common Subtypes of Idiopathic Generalized Epilepsy. Epilepsy Res. 2002, 51, 249–255. [Google Scholar] [CrossRef]
- Hentschke, M.; Wiemann, M.; Hentschke, S.; Kurth, I.; Hermans-Borgmeyer, I.; Seidenbecher, T.; Jentsch, T.J.; Gal, A.; Hübner, C.A. Mice with a Targeted Disruption of the Cl−/HCO3− Exchanger AE3 Display a Reduced Seizure Threshold. Mol. Cell. Biol. 2006, 26, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.I.; Hübner, C.A.; Boron, W.F. Role of Cl−-HCO3− Exchanger AE3 in Intracellular PH Homeostasis in Cultured Murine Hippocampal Neurons, and in Crosstalk to Adjacent Astrocytes. J. Physiol. 2017, 595, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, J.V.; Richards, B.A.; Woodin, M.A. Neuronal Chloride and Excitability—The Big Impact of Small Changes. Curr. Opin. Neurobiol. 2017, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Downs, L.M.; Webster, A.R.; Moore, A.T.; Michaelides, M.; Ali, R.R.; Hardcastle, A.J.; Mellersh, C.S. Investigation of SLA4A3 as a Candidate Gene for Human Retinal Disease. J. Negat. Results Biomed. 2016, 15, 11. [Google Scholar] [CrossRef][Green Version]
- Garciarena, C.D.; Ma, Y.L.; Swietach, P.; Huc, L.; Vaughan-Jones, R.D. Sarcolemmal Localisation of Na+/H+ Exchange and Na+-HCO3− Co-Transport Influences the Spatial Regulation of Intracellular PH in Rat Ventricular Myocytes. J. Physiol. 2013, 591, 2287–2306. [Google Scholar] [CrossRef]
- Prasad, V.; Lorenz, J.N.; Miller, M.L.; Vairamani, K.; Nieman, M.L.; Wang, Y.; Shull, G.E. Loss of NHE1 Activity Leads to Reduced Oxidative Stress in Heart and Mitigates High-Fat Diet-Induced Myocardial Stress. J. Mol. Cell. Cardiol. 2013, 65, 33–42. [Google Scholar] [CrossRef]
- Wagner, C.A.; Imenez Silva, P.H.; Bourgeois, S. Molecular Pathophysiology of Acid-Base Disorders. Semin. Nephrol. 2019, 39, 340–352. [Google Scholar] [CrossRef]
- Jalali, R.; Guo, J.; Zandieh-Doulabi, B.; Bervoets, T.J.M.; Paine, M.L.; Boron, W.F.; Parker, M.D.; Bijvelds, M.J.C.; Medina, J.F.; DenBesten, P.K.; et al. NBCe1 (SLC4A4) a Potential PH Regulator in Enamel Organ Cells during Enamel Development in the Mouse. Cell Tissue Res. 2014, 358, 433–442. [Google Scholar] [CrossRef][Green Version]
- Barbuskaite, D.; Pedersen, F.D.; Christensen, H.L.; Johnsen, L.; Praetorius, J.; Damkier, H.H. NBCe2 (Slc4a5) Is Expressed in the Renal Connecting Tubules and Cortical Collecting Ducts and Mediates Base Extrusion. Front. Physiol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Chen, L.M.; Choi, I.; Haddad, G.G.; Boron, W.F. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2412–R2420. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Frische, S.; Leipziger, J.; Nielsen, S.; Praetorius, J. Sodium coupled bicarbonate transporters in the kidney, an update. Acta Physiol. Scand. 2004, 181, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Ha, H.T.T.; Nguyen, T.H.; Nguyen, L.N. The Role of SLC Transporters for Brain Health and Disease. Cell. Mol. Life Sci. 2021, 79, 20. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Cull-Candy, S.G. Pharmacological Properties and H+ Sensitivity of Excitatory Amino Acid Receptor Channels in Rat Cerebellar Granule Neurones. J Physiol 1991, 433, 727–763. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D. Mouse Models of SLC4-Linked Disorders of HCO3—Transporter Dysfunction. Am. J. Physiol. Cell Physiol. 2018, 314, C569–C588. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Praetorius, J.; Füchtbauer, E.M.; Aalkjaer, C. Antibody-Independent Localization of the Electroneutral Na+-HCO3− Cotransporter NBCn1 (Slc4a7) in Mice. Am. J. Physiol. Cell Physiol. 2008, 294, C591–C603. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Praetorius, J.; Matchkov, V.V.; Stankevicius, E.; Mogensen, S.; Füchtbauer, A.C.; Simonsen, U.; Füchtbauer, E.M.; Aalkjaer, C. Disruption of Na+,HCO₃− Cotransporter NBCn1 (Slc4a7) Inhibits NO-Mediated Vasorelaxation, Smooth Muscle Ca2+ Sensitivity, and Hypertension Development in Mice. Circulation 2011, 124, 1819–1829. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Aalkjaer, C. Acid-Base Transporters Modulate Cell Migration, Growth and Proliferation: Implications for Structure Development and Remodeling of Resistance Arteries? Trends Cardiovasc. Med. 2013, 23, 59–65. [Google Scholar] [CrossRef]
- Sedlyarov, V.; Eichner, R.; Girardi, E.; Essletzbichler, P.; Goldmann, U.; Nunes-Hasler, P.; Srndic, I.; Moskovskich, A.; Heinz, L.X.; Kartnig, F.; et al. The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification. Cell Host. Microbe 2018, 23, 766–774.e5. [Google Scholar] [CrossRef]
- Perry, C.; Quissell, D.O.; Reyland, M.E.; Grichtchenko, I.I. Electrogenic NBCe1 (SLC4A4), but Not Electroneutral NBCn1 (SLC4A7), Cotransporter Undergoes Cholinergic-Stimulated Endocytosis in Salivary ParC5 Cells. Am. J. Physiol. Cell Physiol. 2008, 295, C1385–C1398. [Google Scholar] [CrossRef]
- Pilling, L.C.; Joehanes, R.; Melzer, D.; Harries, L.W.; Henley, W.; Dupuis, J.; Lin, H.; Mitchell, M.; Hernandez, D.; Ying, S.X.; et al. Gene Expression Markers of Age-Related Inflammation in Two Human Cohorts. Exp. Gerontol. 2015, 70, 37–45. [Google Scholar] [CrossRef]
- Böger, C.A.; Gorski, M.; McMahon, G.M.; Xu, H.; Chang, Y.P.C.; Van Der Most, P.J.; Navis, G.; Nolte, I.M.; De Borst, M.H.; Zhang, W.; et al. NFAT5 and SLC4A10 Loci Associate with Plasma Osmolality. J. Am. Soc. Nephrol. 2017, 28, 2311–2321. [Google Scholar] [CrossRef]
- Purkerson, J.M.; Heintz, E.V.; Nakamori, A.; Schwartz, G.J. Insights into Acidosis-Induced Regulation of SLC26A4 (Pendrin) and SLC4A9 (AE4) Transporters Using Three-Dimensional Morphometric Analysis of β-Intercalated Cells. Am. J. Physiol. Renal Physiol. 2014, 307, F601–F611. [Google Scholar] [CrossRef] [PubMed]
- Peña-Münzenmayer, G.; Catalán, M.A.; Kondo, Y.; Jaramillo, Y.; Liu, F.; Shull, G.E.; Melvin, J.E. Ae4 (Slc4a9) Anion Exchanger Drives Cl- Uptake-Dependent Fluid Secretion by Mouse Submandibular Gland Acinar Cells. J. Biol. Chem. 2015, 290, 10677–10688. [Google Scholar] [CrossRef] [PubMed]
- Kurth, I.; Hentschke, M.; Hentschke, S.; Borgmeyer, U.; Gal, A.; Hübner, C.A. The Forkhead Transcription Factor Foxi1 Directly Activates the AE4 Promoter. Biochem. J. 2006, 393, 277–283. [Google Scholar] [CrossRef]
- Vera-Sigüenza, E.; Catalán, M.A.; Peña-Münzenmayer, G.; Melvin, J.E.; Sneyd, J. A Mathematical Model Supports a Key Role for Ae4 (Slc4a9) in Salivary Gland Secretion. Bull. Math. Biol. 2018, 80, 255–282. [Google Scholar] [CrossRef]
- Bonanno, J.A.; Shyam, R.; Choi, M.; Ogando, D.G. The H+ Transporter SLC4A11: Roles in Metabolism, Oxidative Stress and Mitochondrial Uncoupling. Cells 2022, 11, 197. [Google Scholar] [CrossRef]
- Choi, M.; Bonanno, J.A. Mitochondrial Targeting of the Ammonia-Sensitive Uncoupler SLC4A11 by the Chaperone-Mediated Carrier Pathway in Corneal Endothelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 4. [Google Scholar] [CrossRef]
- Guha, S.; Chaurasia, S.; Ramachandran, C.; Roy, S. SLC4A11 Depletion Impairs NRF2 Mediated Antioxidant Signaling and Increases Reactive Oxygen Species in Human Corneal Endothelial Cells during Oxidative Stress. Sci. Rep. 2017, 7, 4074. [Google Scholar] [CrossRef]
- Kao, L.; Azimov, R.; Shao, X.M.; Abuladze, N.; Newman, D.; Zhekova, H.; Noskov, S.; Pushkin, A.; Kurtz, I. SLC4A11 Function: Evidence for H+(OH−) and NH3-H+ Transport. Am. J. Physiol. Cell Physiol. 2020, 318, C392–C405. [Google Scholar] [CrossRef]
- Malhotra, D.; Jung, M.; Fecher-Trost, C.; Lovatt, M.; Peh, G.S.L.; Noskov, S.; Mehta, J.S.; Zimmermann, R.; Casey, J.R. Defective Cell Adhesion Function of Solute Transporter, SLC4A11, in Endothelial Corneal Dystrophies. Hum. Mol. Genet. 2020, 29, 97–116. [Google Scholar] [CrossRef]
- Bevensee, M.O. Exchangers (Current Topics in Membranes, Volume 73), 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 73, ISBN 9780128002230. [Google Scholar]
- Thornell, I.M.; Bevensee, M.O. Regulators of Slc4 Bicarbonate Transporter Activity. Front. Physiol. 2015, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L. Cell Physiology and Molecular Mechanism of Anion Transport by Erythrocyte Band 3/AE1. Am. J. Physiol. Cell Physiol. 2021, 321, C1028–C1059. [Google Scholar] [CrossRef] [PubMed]
- Rungaldier, S.; Oberwagner, W.; Salzer, U.; Csaszar, E.; Prohaska, R. Stomatin Interacts with GLUT1/SLC2A1, Band 3/SLC4A1, and Aquaporin-1 in Human Erythrocyte Membrane Domains. Biochim. Biophys. Acta 2013, 1828, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Toye, A.M.; Ghosh, S.; Young, M.T.; Jones, G.K.; Sessions, R.B.; Ramaugé, M.; Leclerc, P.; Basu, J.; Delaunay, J.; Tanner, M.J.A. Protein-4.2 Association with Band 3 (AE1, SLCA4) in Xenopus Oocytes: Effects of Three Natural Protein-4.2 Mutations Associated with Hemolytic Anemia. Blood 2005, 105, 4088–4095. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, E.; Satchwell, T.J.; Williamson, R.C.; Toye, A.M. Band 3 Multiprotein Complexes in the Red Cell Membrane; of Mice and Men. Blood Cells Mol. Dis. 2010, 45, 1–8. [Google Scholar] [CrossRef]
- Su, Y.; Blake-Palmer, K.G.; Fry, A.C.; Best, A.; Brown, A.C.N.; Hiemstra, T.F.; Horita, S.; Zhou, A.; Toye, A.M.; Karet, F.E. Glyceraldehyde 3-Phosphate Dehydrogenase Is Required for Band 3 (Anion Exchanger 1) Membrane Residency in the Mammalian Kidney. Am. J. Physiol. Renal Physiol. 2011, 300, F157–F166. [Google Scholar] [CrossRef]
- Sowah, D.; Casey, J.R. An Intramolecular Transport Metabolon: Fusion of Carbonic Anhydrase II to the COOH Terminus of the Cl−/HCO3− Exchanger, AE1. Am. J. Physiol. Cell Physiol. 2011, 301, C336–C346. [Google Scholar] [CrossRef] [PubMed]
- Bevensee, M.O.; Schmitt, B.M.; Choi, I.; Romero, M.F.; Boron, W.F. An Electrogenic Na+-HCO3− Cotransporter (NBC) with a Novel COOH-Terminus, Cloned from Rat Brain. Am. J. Physiol. Cell Physiol. 2000, 278, C1200–C1211. [Google Scholar] [CrossRef]
- Lee, S.K.; Boron, W.F. Exploring the Autoinhibitory Domain of the Electrogenic Na+/HCO3− Transporter NBCe1-B, from Residues 28 to 62. J. Physiol. 2018, 596, 3637–3653. [Google Scholar] [CrossRef]
- Marino, C.R.; Jeanes, V.; Boron, W.F.; Schmitt, B.M. Expression and Distribution of the Na+-HCO3− Cotransporter in Human Pancreas. Am. J. Physiol. 1999, 277, G487–G494. [Google Scholar] [CrossRef]
- Ando, H.; Mizutani, A.; Matsu-ura, T.; Mikoshiba, K. IRBIT, a Novel Inositol 1,4,5-Trisphosphate (IP3) Receptor-Binding Protein, Is Released from the IP3 Receptor upon IP3 Binding to the Receptor. J. Biol. Chem. 2003, 278, 10602–10612. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kawaai, K.; Mikoshiba, K. IRBIT: A Regulator of Ion Channels and Ion Transporters. Biochim. Biophys. Acta 2014, 1843, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.H.; Disse-Nicodème, S.; Choate, K.A.; Ishikawa, K.; Nelson-Williams, C.; Desitter, I.; Gunel, M.; Milford, D.V.; Lipkin, G.W.; Achard, J.M.; et al. Human Hypertension Caused by Mutations in WNK Kinases. Science 2001, 293, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, H.S.; Kim, E.; Ju, E.J.; Kwon, M.H.; Dudley, R.K.; Smith, Y.; Yun, C.C.; Choi, I. PSD-95 Interacts with NBCn1 and Enhances Channel-like Activity without Affecting Na/HCO3 Cotransport. Cell. Physiol. Biochem. 2012, 30, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kwon, M.H.; Lee, S.; Hall, R.A.; Yun, C.C.; Choi, I. Systematic Family-Wide Analysis of Sodium Bicarbonate Cotransporter NBCn1/SLC4A7 Interactions with PDZ Scaffold Proteins. Physiol. Rep. 2014, 2, e12016. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D.; Bouyer, P.; Daly, C.M.; Boron, W.F. Cloning and Characterization of Novel Human SLC4A8 Gene Products Encoding Na+-Driven Cl−/HCO3− Exchanger Variants NDCBE-A, -C, and -D. Physiol. Genomics. 2008, 34, 265–276. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ouyang, Y.B.; Giffard, R.G. Regulation of the rat brain Na+-driven Cl−/HCO3− exchanger involves protein kinase A and a multiprotein signaling complex. FEBS Lett. 2006, 580, 4865–4871. [Google Scholar] [CrossRef]
- He, B.J.; Liao, L.; Deng, Z.F.; Tao, Y.F.; Xu, Y.C.; Lin, F.Q. Molecular Genetic Mechanisms of Hereditary Spherocytosis: Current Perspectives. Acta Haematol. 2018, 139, 60–66. [Google Scholar] [CrossRef]
- Karet, F.E. Hereditary Distal Renal Tubular Acidosis. J. Am. Soc. Nephrol. 2002, 13, 2178–2184. Available online: https://pubmed.ncbi.nlm.nih.gov/31600044/ (accessed on 2 August 2023). [CrossRef]
- Mohebbi, N.; Wagner, C.A. Pathophysiology, Diagnosis and Treatment of Inherited Distal Renal Tubular Acidosis. J. Nephrol. 2018, 31, 511–522. [Google Scholar] [CrossRef]
- Gueutin, V.; Vallet, M.; Jayat, M.; Peti-Peterdi, J.; Cornière, N.; Leviel, F.; Sohet, F.; Wagner, C.A.; Eladari, D.; Chambrey, R. Renal β-Intercalated Cells Maintain Body Fluid and Electrolyte Balance. J. Clin. Invest. 2013, 123, 4219–4231. [Google Scholar] [CrossRef] [PubMed]
- Deejai, N.; Wisanuyotin, S.; Nettuwakul, C.; Khositseth, S.; Sawasdee, N.; Saetai, K.; Yenchitsomanus, P.; Rungroj, N. Molecular Diagnosis of Solute Carrier Family 4 Member 1 (SLC4A1) Mutation-Related Autosomal Recessive Distal Renaltubular Acidosis. Lab. Med. 2019, 50, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Bordin, L.; Fiore, C.; Don, G.; Andrisani, A.; Ambrosini, G.; Faggian, D.; Plebani, M.; Clari, G.; Armanini, D. Evaluation of Erythrocyte Band 3 Phosphotyrosine Level, Glutathione Content, CA-125, and Human Epididymal Secretory Protein E4 as Combined Parameters in Endometriosis. Fertil. Steril. 2010, 94, 1616–1621. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Markers of Oxidative Stress in Erythrocytes and Plasma during Aging in Humans. Oxid. Med. Cell. Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L.; Sulima, A. The Structural and Functional Changes of Blood Cells and Molecular Components in Diabetes Mellitus. Biol. Chem. 2017, 398, 411–423. [Google Scholar] [CrossRef]
- Iwasaki, S.; Shojaku, H.; Murofushi, T.; Seo, T.; Kitahara, T.; Origasa, H.; Watanabe, Y.; Suzuki, M.; Takeda, N. Diagnostic and Therapeutic Strategies for Meniere’s Disease of the Japan Society for Equilibrium Research. Auris Nasus Larynx 2021, 48, 15–22. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, D.; Sun, G.; Song, Y.; Cai, J.; Fan, Z.; Wang, H. Solute Carrier Family 4 Member 1 Might Participate in the Pathogenesis of Meniere’s Disease in a Murine Endolymphatic Hydrop Model. Acta Otolaryngol. 2019, 139, 966–976. [Google Scholar] [CrossRef]
- Huang, Z.; Du, G.; Huang, X.; Han, L.; Han, X.; Xu, B.; Zhang, Y.; Yu, M.; Qin, Y.; Xia, Y.; et al. The Enhancer RNA Lnc-SLC4A1-1 Epigenetically Regulates Unexplained Recurrent Pregnancy Loss (URPL) by Activating CXCL8 and NF-KB Pathway. EBioMedicine 2018, 38, 162–170. [Google Scholar] [CrossRef]
- Yang, L. Physcion 8-O-β-Glucopyranoside Exerts Carcinostasis Ability in Ishikawa Cells via Regulating Lnc-SLC4A1-1/H3K27ac/NF-ΚB Pathway. Pharmazie 2020, 75, 348–352. [Google Scholar]
- Zhu, M.; Ma, X.; Huang, J.; Lu, F.G.; Chen, Y.; Hu, J.; Cheng, L.; Zhang, B.; Liu, W.; Li, L. Extracellular Vesicle-Derived MiR-1249-5p Regulates Influenza A Virus-Induced Acute Lung Injury in RAW246.7 Cells through Targeting SLC4A1. Microbes Infect. 2022, 24, 104998. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Y.; Liu, B. Integrated Bioinformatics Analysis Reveals Marker Genes and Immune Infiltration for Pulmonary Arterial Hypertension. Sci. Rep. 2022, 12, 10154. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Trauner, M. When Lightning Strikes Twice: The Plot Thickens for a Dual Role of the Anion Exchanger 2 (AE2/SLC4A2) in the Pathogenesis and Treatment of Primary Biliary Cirrhosis. J. Hepatol. 2009, 50, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Arenas, F.; Hervías, I.; Sáez, E.; Melero, S.; Prieto, J.; Parés, A.; Medina, J.F. Promoter Hypermethylation of the AE2/SLC4A2 Gene in PBC. JHEP Rep. 2019, 1, 145–153. [Google Scholar] [CrossRef]
- Hisamoto, S.; Shimoda, S.; Harada, K.; Iwasaka, S.; Onohara, S.; Chong, Y.; Nakamura, M.; Bekki, Y.; Yoshizumi, T.; Ikegami, T.; et al. Hydrophobic Bile Acids Suppress Expression of AE2 in Biliary Epithelial Cells and Induce Bile Duct Inflammation in Primary Biliary Cholangitis. J. Autoimmun. 2016, 75, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Bernstein, D.; Shiffman, M.L.; Kwo, P.; Kim, W.R.; Kowdley, K.V.; Jacobson, I.M. Diagnosis and Management of Primary Biliary Cholangitis. Am. J. Gastroenterol. 2019, 114, 48–63. [Google Scholar] [CrossRef]
- Cazzagon, N.; Floreani, A. Primary Biliary Cholangitis: Treatment. Curr. Opin. Gastroenterol. 2021, 37, 99–104. [Google Scholar] [CrossRef]
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Agüero, R.; Fernandez-Irigoyen, J.; et al. MicroRNA-506 Promotes Primary Biliary Cholangitis-like Features in Cholangiocytes and Immune Activation. Hepatology 2018, 67, 1420–1440. [Google Scholar] [CrossRef]
- Xue, J.Y.; Grigelioniene, G.; Wang, Z.; Nishimura, G.; Iida, A.; Matsumoto, N.; Tham, E.; Miyake, N.; Ikegawa, S.; Guo, L. SLC4A2 Deficiency Causes a New Type of Osteopetrosis. J. Bone Miner. Res. 2022, 37, 226–235. [Google Scholar] [CrossRef]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet. J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Sun, R.; Qin, Y.; Liu, Z.; Yang, S.; Tang, T.; Zhu, Z.; Yu, D.; Liu, F. Targeting Anion Exchange of Osteoclast, a New Strategy for Preventing Wear Particles Induced- Osteolysis. Front. Pharmacol. 2018, 9, 1291. [Google Scholar] [CrossRef]
- Wu, T.; Hsieh, Y.; Wu, C.; Tsai, J.; Hsieh, Y.; Huang, C.; Liu, J. Overexpression of Anion Exchanger 2 in Human Hepatocellular Carcinoma. Chin. J. Physiol. 2006, 49, 192. Available online: https://pubmed.ncbi.nlm.nih.gov/17058451/ (accessed on 12 November 2022). [PubMed]
- Yang, Y.; Wu, P.P.; Wu, J.; Shen, W.W.; Wu, Y.L.; Fu, A.F.; Zheng, L.; Jin, X.L.; Fu, G.H. Expression of Anion Exchanger 2 in Human Gastric Cancer. Exp. Oncol. 2008, 30, 81–87. Available online: https://pubmed.ncbi.nlm.nih.gov/18438347/ (accessed on 12 November 2022). [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems Analysis of Intracellular PH Vulnerabilities for Cancer Therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Shiozaki, A.; Hikami, S.; Ichikawa, D.; Kosuga, T.; Shimizu, H.; Kudou, M.; Yamazato, Y.; Kobayashi, T.; Shoda, K.; Arita, T.; et al. Anion Exchanger 2 Suppresses Cellular Movement and Has Prognostic Significance in Esophageal Squamous Cell Carcinoma. Oncotarget 2018, 9, 25993–26006. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Hwang, J.M.; Wu, T.T.; Hsieh, Y.H.; Wu, C.C.; Hsieh, Y.S.; Tsai, C.H.; Wu, H.C.; Huang, C.Y.; Liu, J.Y. Anion Exchanger Inhibitor DIDS Induces Human Poorly-Differentiated Malignant Hepatocellular Carcinoma HA22T Cell Apoptosis. Mol. Cell. Biochem. 2008, 308, 117–125. [Google Scholar] [CrossRef]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic Anhydrase IX Interacts with Bicarbonate Transporters in Lamellipodia and Increases Cell Migration via Its Catalytic Domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef]
- Song, L.J.; Liu, R.J.; Zeng, Z.; Alper, S.L.; Cui, H.J.; Lu, Y.; Zheng, L.; Yan, Z.W.; Fu, G.H. Gastrin Inhibits a Novel, Pathological Colon Cancer Signaling Pathway Involving EGR1, AE2, and P-ERK. J. Mol. Med. 2012, 90, 707–718. [Google Scholar] [CrossRef]
- Wang, T.; Fei, H.J.; Yang, Y.; Jiang, X.S.; Yan, M.; Zeng, Z.; Wu, J.; Song, L.J.; Tian, H.; Fu, G.H. Expression of AE1/P16 Promoted Degradation of AE2 in Gastric Cancer Cells. BMC Cancer 2016, 16, 716. [Google Scholar] [CrossRef]
- Cui, Y.; Li, S.B.; Peng, X.C.; Wu, J.; Fu, G.H. Trastuzumab Inhibits Growth of HER2-Negative Gastric Cancer Cells Through Gastrin-Initialized CCKBR Signaling. Dig. Dis. Sci. 2015, 60, 3631–3641. [Google Scholar] [CrossRef]
- Thorsen, K.; Dam, V.S.; Kjaer-Sorensen, K.; Pedersen, L.N.; Skeberdis, V.A.; Jurevičius, J.; Treinys, R.; Petersen, I.M.B.S.; Nielsen, M.S.; Oxvig, C.; et al. Loss-of-Activity-Mutation in the Cardiac Chloride-Bicarbonate Exchanger AE3 Causes Short QT Syndrome. Nat. Commun. 2017, 8, 1696. [Google Scholar] [CrossRef]
- Dewi, I.P.; Dharmadjati, B.B. Short QT Syndrome: The Current Evidences of Diagnosis and Management. J. Arrhythm. 2020, 36, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.K.; Kjær-Sørensen, K.; Clavsen, N.C.; Dittmann, S.; Jensen, M.F.; Guldbrandsen, H.Ø.; Pedersen, L.N.; Sørensen, R.H.; Lildballe, D.L.; Müller, K.; et al. Genetic Analysis Identifies the SLC4A3 Anion Exchanger as a Major Gene for Short QT Syndrome. Heart Rhythm. 2023, 20, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Adler, A.; Amin, A.S.; Abiusi, E.; Care, M.; Bikker, H.; Amenta, S.; Feilotter, H.; Nannenberg, E.A.; Mazzarotto, F.; et al. Evaluation of Gene Validity for CPVT and Short QT Syndrome in Sudden Arrhythmic Death. Eur. Heart J. 2022, 43, 1500. [Google Scholar] [CrossRef]
- Al Moamen, N.J.; Prasad, V.; Bodi, I.; Miller, M.L.; Neiman, M.L.; Lasko, V.M.; Alper, S.L.; Wieczorek, D.F.; Lorenz, J.N.; Shull, G.E. Loss of the AE3 Anion Exchanger in a Hypertrophic Cardiomyopathy Model Causes Rapid Decompensation and Heart Failure. J. Mol. Cell Cardiol. 2011, 50, 137–146. [Google Scholar] [CrossRef]
- Prasad, V.; Lorenz, J.N.; Lasko, V.M.; Nieman, M.L.; Al Moamen, N.J.; Shull, G.E. Loss of the AE3 Cl(−)/HCO(−) 3 Exchanger in Mice Affects Rate-Dependent Inotropy and Stress-Related AKT Signaling in Heart. Front. Physiol. 2013, 4, 399. [Google Scholar] [CrossRef] [PubMed]
- Vilas, G.L.; Johnson, D.E.; Freund, P.; Casey, J.R. Characterization of an Epilepsy-Associated Variant of the Human Cl−/HCO3− Exchanger AE3. Am. J. Physiol. Cell Physiol. 2009, 297, C526–C536. [Google Scholar] [CrossRef]
- Cordat, E.; Reithmeier, R.A.F. Structure, Function, and Trafficking of SLC4 and SLC26 Anion Transporters. Curr. Top. Membr. 2014, 73, 1–67. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Chen, H.; Ge, L.; Xu, X.; Xiong, X.; He, J. Detection of CEA MRNA, P53 and AE1/AE3 in Haematoxylin-Eosin-Negative Lymph Nodes of Early-Stage Non-Small Cell Lung Cancer May Improve Veracity of N Staging and Indicate Prognosis. Jpn. J. Clin. Oncol. 2010, 40, 146–152. [Google Scholar] [CrossRef]
- Shorthouse, D.; Riedel, A.; Kerr, E.; Pedro, L.; Bihary, D.; Samarajiwa, S.; Martins, C.P.; Shields, J.; Hall, B.A. Exploring the Role of Stromal Osmoregulation in Cancer and Disease Using Executable Modelling. Nat. Commun. 2018, 9, 3011. Available online: https://pubmed.ncbi.nlm.nih.gov/30069015/ (accessed on 30 May 2023). [CrossRef]
- Palmer, B.F.; Kelepouris, E.; Clegg, D.J. Renal Tubular Acidosis and Management Strategies: A Narrative Review. Adv. Ther. 2021, 38, 949–968. [Google Scholar] [CrossRef]
- Igarashi, T.; Inatomi, J.; Sekine, T.; Cha, S.H.; Kanai, Y.; Kunimi, M.; Tsukamoto, K.; Satoh, H.; Shimadzu, M.; Tozawa, F.; et al. Mutations in SLC4A4 Cause Permanent Isolated Proximal Renal Tubular Acidosis with Ocular Abnormalities. Nat. Genet. 1999, 23, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Van Paesschen, W.; Stalmans, I.; Horita, S.; Yamada, H.; Bergmans, B.A.; Legius, E.; Riant, F.; De Jonghe, P.; Li, Y.; et al. Defective Membrane Expression of the Na+-HCO3− Cotransporter NBCe1 Is Associated with Familial Migraine. Proc. Natl. Acad. Sci. USA 2010, 107, 15963–15968. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, B.; Ortega-Pinto, A.; Morales-Bozo, I.; Rojas-Alcayaga, G.; Cifuentes, V. Defining a New Candidate Gene for Amelogenesis Imperfecta: From Molecular Genetics to Biochemistry. Biochem. Genet. 2011, 49, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Bulur, N.; Virreira, M.; Soyfoo, M.S.; Louchami, K.; Delporte, C.; Perret, J.; Beauwens, R.; Malaisse, W.J.; Sener, A. Expression of the Electrogenic Na+-HCO3—Cotransporter NBCe1 in Tumoral Insulin-Producing BRIN-BD11 Cells. Cell. Physiol. Biochem. 2009, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Holmes, H.; Rakshit, K.; Javeed, N.; Her, T.K.; Stiller, A.A.; Sen, S.; Shull, G.E.; Prakash, Y.S.; Romero, M.F.; et al. Electrogenic Sodium Bicarbonate Cotransporter NBCe1 Regulates Pancreatic β Cell Function in Type 2 Diabetes. J. Clin. Investig. 2021, 131, e142365. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; Verkerk, R.; Aerts, T.; De Surgeloose, D.; Wauters, A.; Scharpé, S.; De Deyn, P.P. The Role of Tryptophan Catabolism along the Kynurenine Pathway in Acute Ischemic Stroke. Neurochem. Res. 2010, 35, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Otani, H.; Mishima, K.; Imamura, H.; Inagaki, C. Involvement of Anion Exchange in the Hypoxia/Reoxygenation-Induced Changes in PH(i) And. Eur. J. Pharmacol. 2001, 411, 35–43. [Google Scholar] [CrossRef]
- Pignataro, G.; Sirabella, R.; Anzilotti, S.; Di Renzo, G.; Annunziato, L. Does Na+/Ca2+ Exchanger, NCX, Represent a New Druggable Target in Stroke Intervention? Transl. Stroke Res. 2014, 5, 145–155. [Google Scholar] [CrossRef]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, P.; Zhuang, Y.; Du, L. Hsa_circRNA_001587 Upregulates SLC4A4 Expression to Inhibit Migration, Invasion, and Angiogenesis of Pancreatic Cancer Cells via Binding to MicroRNA-223. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G703–G717. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Zhai, G.; Ke, S.; Yu, X.; Guo, J. SLC4A4 Promotes Prostate Cancer Progression in Vivo and in Vitro via AKT-Mediated Signalling Pathway. Cancer. Cell Int. 2022, 22, 127. [Google Scholar] [CrossRef]
- Cappellesso, F.; Orban, M.P.; Shirgaonkar, N.; Berardi, E.; Serneels, J.; Neveu, M.A.; Di Molfetta, D.; Piccapane, F.; Caroppo, R.; Debellis, L.; et al. Targeting the Bicarbonate Transporter SLC4A4 Overcomes Immunosuppression and Immunotherapy Resistance in Pancreatic Cancer. Nat. Cancer 2022, 3, 1464–1483. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Feng, Y.; Guan, W. Prognostic Value of SLC4A4 and Its Correlation with Immune Infiltration in Colon Adenocarcinoma. Med. Sci. Monit. 2020, 26, e925016-1. [Google Scholar] [CrossRef]
- Gröger, N.; Vitzthum, H.; Fröhlich, H.; Krüger , M.; Ehmke, H.; Braun, T.; Boettger, T. Targeted Mutation of SLC4A5 Induces Arterial Hypertension and Renal Metabolic Acidosis. Hum. Mol. Genet. 2012, 21, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.L.; Barbuskaite, D.; Rojek, A.; Malte, H.; Christensen, I.B.; Füchtbauer, A.C.; Füchtbauer, E.M.; Wang, T.; Praetorius, J.; Damkier, H.H. The Choroid Plexus Sodium-Bicarbonate Cotransporter NBCe2 Regulates Mouse Cerebrospinal Fluid PH. J. Physiol. 2018, 596, 4709–4728. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.; Kurtz, L.M.; Shao, X.; Papadopoulos, M.C.; Liu, L.; Bok, D.; Nusinowitz, S.; Chen, B.; Stella, S.L.; Andre, M.; et al. Severe Neurologic Impairment in Mice with Targeted Disruption of the Electrogenic Sodium Bicarbonate Cotransporter NBCe2 (Slc4a5 Gene). J. Biol. Chem. 2011, 286, 32563–32574. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Lin, X.; Huang, J.; Charles Gu, C.; He, M.; Shen, H.; He, J.; Zhu, J.; Li, H.; et al. Genome-Wide Association Study in Chinese Identifies Novel Loci for Blood Pressure and Hypertension. Hum. Mol. Genet. 2015, 24, 865–874. [Google Scholar] [CrossRef]
- Ito, K.; Hirooka, Y.; Kishi, T.; Kimura, Y.; Kaibuchi, K.; Shimokawa, H.; Takeshita, A. Rho/Rho-Kinase Pathway in the Brainstem Contributes to Hypertension Caused by Chronic Nitric Oxide Synthase Inhibition. Hypertension 2004, 43, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Schank, J.R.; Lee, S.; Gonzalez-Islas, C.E.; Nennig, S.E.; Fulenwider, H.D.; Chang, J.; Li, J.M.; Kim, Y.; Jeffers, L.A.; Chung, J.; et al. Increased Alcohol Consumption in Mice Lacking Sodium Bicarbonate Transporter NBCn1. Sci. Rep. 2020, 10, 11017. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Rajbhandari, I.; Yang, H.S.; Lee, S.; Cucoranu, D.; Cooper, D.S.; Klein, J.D.; Sands, J.M.; Choi, I. Neuronal Expression of Sodium/Bicarbonate Cotransporter NBCn1 (SLC4A7) and Its Response to Chronic Metabolic Acidosis. Am. J. Physiol. Cell Physiol. 2010, 298, C1018–C1028. [Google Scholar] [CrossRef]
- Park, H.J.; Gonzalez-Islas, C.E.; Kang, Y.; Li, J.M.; Choi, I. Deletion of the Na/HCO3 Transporter NBCn1 Protects Hippocampal Neurons from NMDA-Induced Seizures and Neurotoxicity in Mice. Sci. Rep. 2019, 9, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Galbraith, G.; Lopez, I.; Woodruff, M.; Nusinowitz, S.; BeltrandelRio, H.; Huang, W.; Zhao, S.; Geske, R.; Montgomery, C.; et al. Blindness and Auditory Impairment Caused by Loss of the Sodium Bicarbonate Cotransporter NBC3. Nat. Genet. 2003, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Choong, L.Y.; Lin, Q.; Philp, R.; Wong, C.H.; Ang, B.K.; Tan, Y.L.; Loh, M.C.S.; Hew, C.L.; Shah, N.; et al. Differential Expression of Novel Tyrosine Kinase Substrates during Breast Cancer Development. Mol. Cell. Proteom. 2007, 6, 2072–2087. [Google Scholar] [CrossRef]
- Hu, J.; Li, G.; Liu, Z.; Ma, H.; Yuan, W.; Lu, Z.; Zhang, D.; Ling, H.; Zhang, F.; Liu, Y.; et al. Bicarbonate Transporter SLC4A7 Promotes EMT and Metastasis of HNSCC by Activating the PI3K/AKT/MTOR Signaling Pathway. Mol. Carcinog. 2023, 62, 628–640. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Qin, X.J.; Schmidt, S.; Hauser, M.A.; Allingham, R.R. AQP1 and SLC4A10 as Candidate Genes for Primary Open-Angle Glaucoma. Mol. Vis. 2010, 16, 93. Available online: https://pubmed.ncbi.nlm.nih.gov/20101282/ (accessed on 26 May 2023). [PubMed]
- Aldave, A.J.; Han, J.; Frausto, R.F. Genetics of the Corneal Endothelial Dystrophies: An Evidence-Based Review. Clin. Genet. 2013, 84, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Parker, M.D. SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy. Biomed. Res. Int. 2015, 2015, 475392. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Tsujikawa, M.; Kawasaki, S.; Nishida, K. Homeostasis of SLC4A11 Protein Is Mediated by Endoplasmic Reticulum-Associated Degradation. Exp. Eye Res. 2019, 188, 107782. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Ogando, D.G.; Bonanno, J.A. Mitochondrial ROS in Slc4a11 KO Corneal Endothelial Cells Lead to ER Stress. Front. Cell Dev. Biol. 2022, 10, 878395. [Google Scholar] [CrossRef]
- Alka, K.; Casey, J.R. Ophthalmic Nonsteroidal Anti-Inflammatory Drugs as a Therapy for Corneal Dystrophies Caused by SLC4A11 Mutation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4258–4267. [Google Scholar] [CrossRef]
- Siddiqui, S.; Zenteno, J.C.; Rice, A.; Chacón-Camacho, O.; Naylor, S.G.; Rivera-De La Parra, D.; Spokes, D.M.; James, N.; Toomes, C.; Inglehearn, C.F.; et al. Congenital Hereditary Endothelial Dystrophy Caused by SLC4A11 Mutations Progresses to Harboyan Syndrome. Cornea 2014, 33, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Still, E.R.; Yuneva, M.O. Hopefully Devoted to Q: Targeting Glutamine Addiction in Cancer. Br. J. Cancer 2017, 116, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Dong, Q.; Hall, M.; Jeyaneethi, J.; Silva, E.; Karteris, E.; Sisu, C. Identification of Potential Bisphenol A (BPA) Exposure Biomarkers in Ovarian Cancer. J. Clin. Med. 2021, 10, 1979. [Google Scholar] [CrossRef]
- Shinto, E.; Yoshida, Y.; Kajiwara, Y.; Okamoto, K.; Mochizuki, S.; Yamadera, M.; Shiraishi, T.; Nagata, K.; Tsuda, H.; Hase, K.; et al. Clinical Significance of a Gene Signature Generated from Tumor Budding Grade in Colon Cancer. Ann. Surg. Oncol. 2020, 27, 4044–4054. [Google Scholar] [CrossRef]
- Alka, K.; Casey, J.R. Bicarbonate Transport in Health and Disease. IUBMB Life 2014, 66, 596–615. [Google Scholar] [CrossRef]
- Martínez-Crespo, L.; Valkenier, H. Transmembrane Transport of Bicarbonate by Anion Receptors. Chempluschem 2022, 87, e202200266. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, M.; Kim, Y.; Jun, I.; Jung, J.; Nam, J.H.; Cheng, M.H.; Lee, M.G. Bicarbonate Permeation through Anion Channels: Its Role in Health and Disease. Pflugers Arch. 2020, 472, 1003–1018. [Google Scholar] [CrossRef]
- Larsen, A.M.; Krogsgaard-Larsen, N.; Lauritzen, G.; Olesen, C.W.; Honoréhansen, S.; Boedtkjer, E.; Pedersen, S.F.; Bunch, L. Gram-Scale Solution-Phase Synthesis of Selective Sodium Bicarbonate Co-Transport Inhibitor S0859: In Vitro Efficacy Studies in Breast Cancer Cells. ChemMedChem 2012, 7, 1808–1814. [Google Scholar] [CrossRef]
- Lee, S.; Axelsen, T.V.; Jessen, N.; Pedersen, S.F.; Vahl, P.; Boedtkjer, E. Na+,HCO3−-Cotransporter NBCn1 (Slc4a7) Accelerates ErbB2-Induced Breast Cancer Development and Tumor Growth in Mice. Oncogene 2018, 37, 5569–5584. [Google Scholar] [CrossRef]
- Ch’en, F.F.T.; Villafuerte, F.C.; Swietach, P.; Cobden, P.M.; Vaughan-Jones, R.D. S0859, an N-Cyanosulphonamide Inhibitor of Sodium-Bicarbonate Cotransport in the Heart. Br. J. Pharmacol. 2008, 153, 972–982. [Google Scholar] [CrossRef]

| Protein Name | Expression Sites | Physiological Functions | Pathological Processes |
|---|---|---|---|
| SLC4A1 | Erythrocytes, renal intercalated-A cells, epididymis | Participation in gas exchange, regulation of pH in the blood, involvement in sperm capacitation and rearrangement | Instability of the erythrocyte lipid bilayer (HS), changed concentration of bicarbonate and chloride in the kidney and blood (dRTA), phosphorylation of SLC4A1 and deformability of erythrocytes (oxidative stress), initiation of inflammation (unexplained recurrent pregnancy loss), activation of the NF-κB signaling pathway (acute lung injury) |
| SLC4A2 | Esophagus, stomach, small intestine, pancreas, cholangiocytes, airway epitheliums, osteoclasts, keratinocytes | Regulation of pH in digestive tract, involvements in osteoclast differentiation, apoptosis and maturation, participation in cytoskeletal organization of osteoclasts, mediation in bicarbonate resorption of TAL, regulation of keratinocyte migration | Immune disorders and broken bicarbonate umbrella of the bile duct (primary biliary cholangitis), affected differentiation of osteoclasts and increased bone mineral density (osteopetrosis), facilitation of intracellular alkalinization and promotion of cancer cell metabolism (esophageal squamous cell carcinoma) |
| SLC4A3 | Cardiomyocytes, neurons, glial cells | Participation in recovering pHi of myocardial cells, involvements in cardiac mechanical conduction, maintenance of pH in nervous cells, signal transmission of astrocytes, regulation of Cl− at the neurotransmitter receptor | Association with some heart diseases, epilepsy |
| SLC4A4 | Heart, proximal renal tubule, ameloblasts, corneal epithelial cells | Impact on myocardial contractility, participation in bicarbonate absorption of proximal renal tubule, secretion of bicarbonate in ameloblasts, regulation of pH in corneal epithelial cells | Defect of bicarbonate resorption (proximal renal tubule acidosis), dysregulation of brain local pH (primary headache), abnormal NMD-mediated neuronal hyperactivity (migraine), dysregulation of pH during amelogenesis (amelogenesis imperfecta), perturbation of the β cell’s transcriptional regulation (type 2 diabetes mellitus), regulation of pH (ischemia), promoting role (prostate cancer), regulation of pH and impact of efficacy in some immune cells (pancreatic ductal adenocarcinoma), association with lymph node invasion and distant metastasis (colon adenocarcinoma) |
| SLC4A5 | Isolated connecting tubules (CNT), cortical collecting ducts (CCD), Golgi apparatus | Mediation of ion exchange on the membrane of kidney and RPE | Increased blood pressure and hypoaldosteronism(hypertension), changed CSF production (Alzheimer’s disease) |
| SLC4A7 | Nervous system, cardiac cells, renal cells | Modulation of neurons, impact on the activity of endothelial NO synthase (eNOS), maintenance of vasomotor responsiveness and arterial structure, neutralization of gastric acid, maintenance of acidification of phagosome, production of bicarbonate in the saliva, maintenance of brain function, association with cellular growth and tumor proliferation | Modulation of neurons, impact on the activity of endothelial NO synthase, maintenance of vasomotor responsiveness and arterial structure, neutralization of gastric acid, maintenance of acidification of phagosome, production of bicarbonate in the saliva, maintenance of brain function, association with cellular growth and tumor proliferation, vascular change and the inhibition of NO synthase and rho kinase (hypertension), intracellular acidosis and decreased nerve excitability (chronic alcohol consumption and susceptibility to alcohol-induced sedation), alteration in exploratory behaviors or emotional ability, deficits in visual and acoustic faculty, the altered perception in sensory cue (reduced locomotor activity), regulation of pH (breast carcinoma), activation of PI3K/AKT/mTOR signaling pathway (head and neck squamous cell carcinoma) |
| SLC4A8 | Brain, pituitary gland, testis, the trachea, thyroid, kidney, and pancreas | Mediation of electroneutral NaCl absorption in type B intercalated cells of CCD | Dysregulation of ion transport in renal cortical collecting ducts (salt-dependent hypertension) |
| SLC4A9 | Renal β-intercalated cells, submandibular acinar cells | Uptake of Cl− in the submandibular gland (SMG), contribution to absorption of NaCl in CCD, maintenance of fluid homeostasis | |
| SLC4A10 | Brain | Association with plasma osmolality and systemic water balance, maintenance of brain function | Alteration of acid-base equilibrium, impact on neuronal excitability (epilepsy), alteration of CSF production, impact on the translaminar pressure (primary open-angle glaucoma) |
| SLC4A11 | Mitochondrion, corneal endothelial cells | Function in nitrogen homeostasis and ammonia detoxification, mediation of corneal transport, adhesion action of CEC to DM | Misfolded protein, oxidative stress, endoplasmic reticulum stress, the lost function of adhesion (congenital hereditary endothelial dystrophy), the lost function of adhesion (Fuchs’ endothelial corneal dystrophy), upregulated activity (“glutamine-addicted” cancers) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Dong, J.; Ruan, W.; Duan, X. Potential Theranostic Roles of SLC4 Molecules in Human Diseases. Int. J. Mol. Sci. 2023, 24, 15166. https://doi.org/10.3390/ijms242015166
Zhong J, Dong J, Ruan W, Duan X. Potential Theranostic Roles of SLC4 Molecules in Human Diseases. International Journal of Molecular Sciences. 2023; 24(20):15166. https://doi.org/10.3390/ijms242015166
Chicago/Turabian StyleZhong, Jingwen, Jing Dong, Wenyan Ruan, and Xiaohong Duan. 2023. "Potential Theranostic Roles of SLC4 Molecules in Human Diseases" International Journal of Molecular Sciences 24, no. 20: 15166. https://doi.org/10.3390/ijms242015166
APA StyleZhong, J., Dong, J., Ruan, W., & Duan, X. (2023). Potential Theranostic Roles of SLC4 Molecules in Human Diseases. International Journal of Molecular Sciences, 24(20), 15166. https://doi.org/10.3390/ijms242015166






