Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages
Abstract
1. Introduction
2. Results
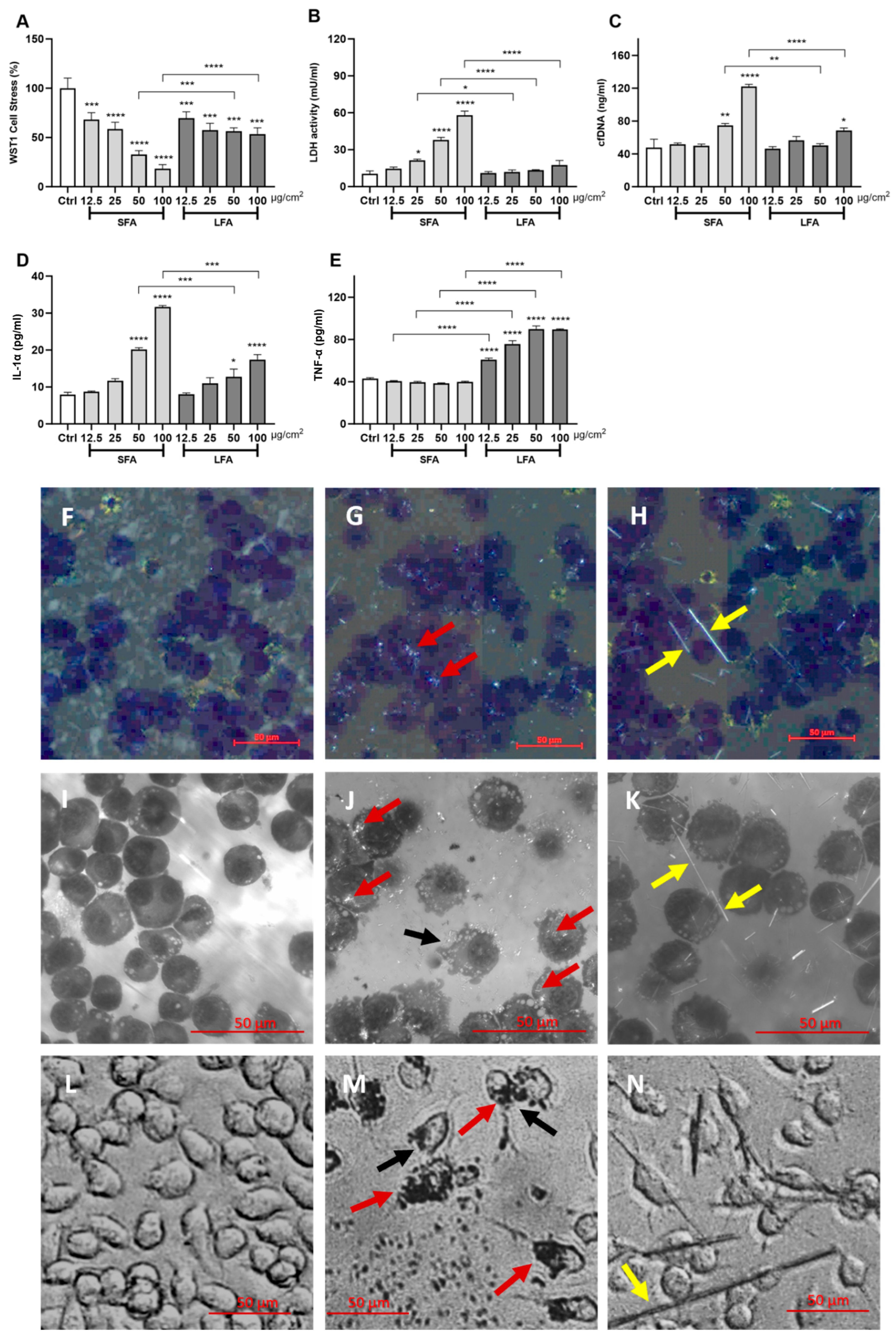
2.1. Short Fibers of Amosite (SFA) Induce Cytotoxicity, Membranolysis and IL-1α Release, While Long Fibers of Amosite (LFA) Only Trigger TNF-α Release
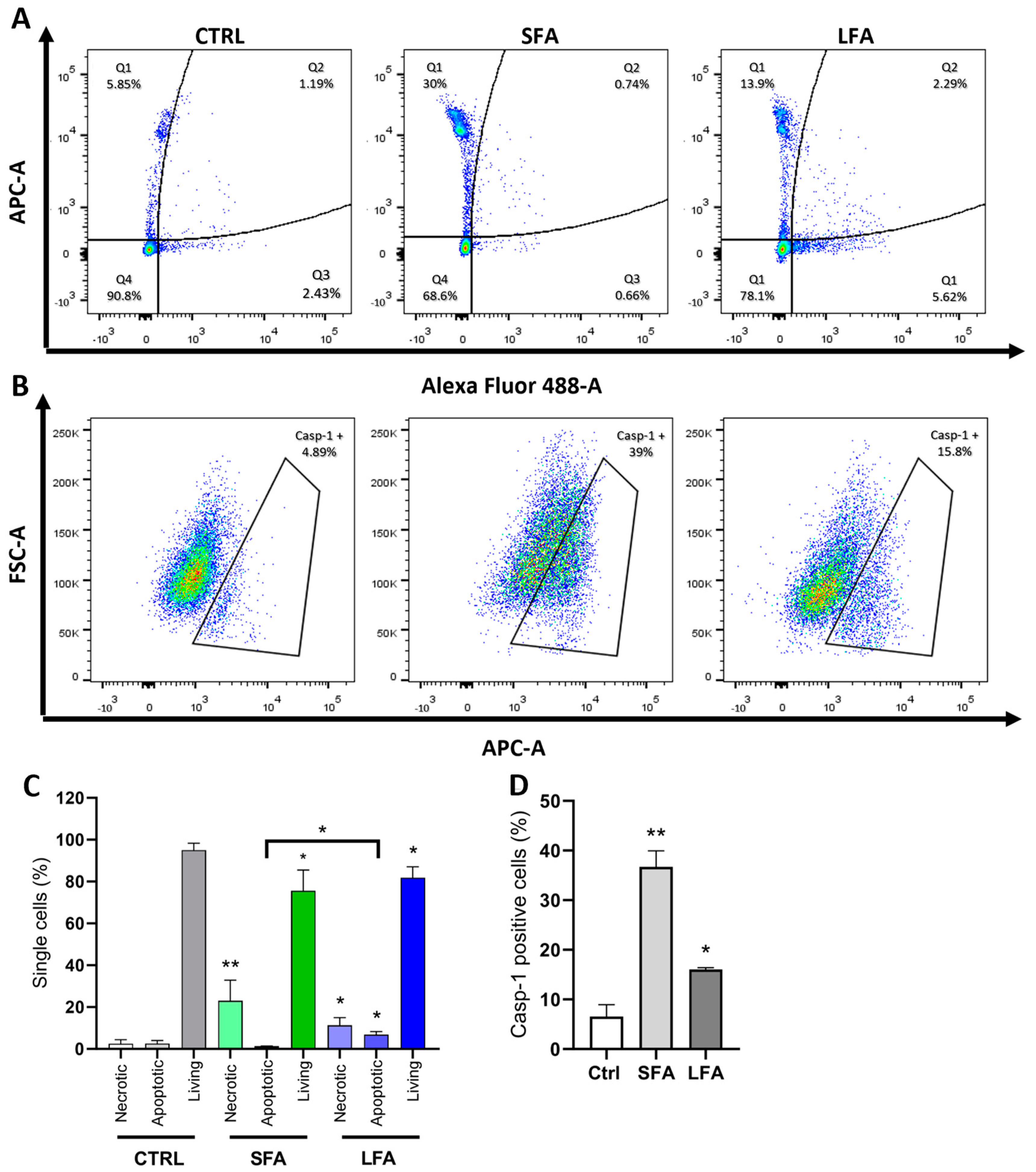
2.2. SFA Induces Casp-1 Activation and Pyroptotic Cell Death, While Proapoptotic Caspases Are Activated following LFA Treatment
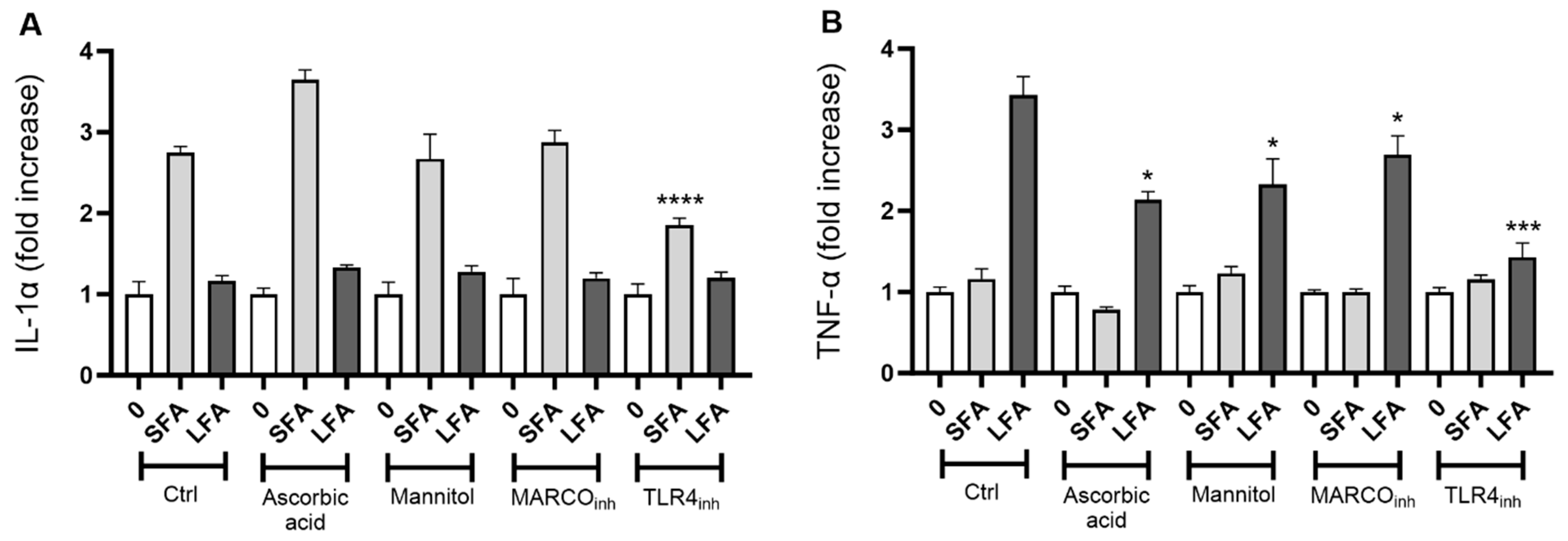
2.3. TLR4 Modulates Macrophage Activation and IL-1α upon SFA While MARCO, ROS and TLR4 Trigger LFA-Related Macrophage Responses
2.4. SFA-Related Pyroptotic Response Is Modulated by GSDMD Pores
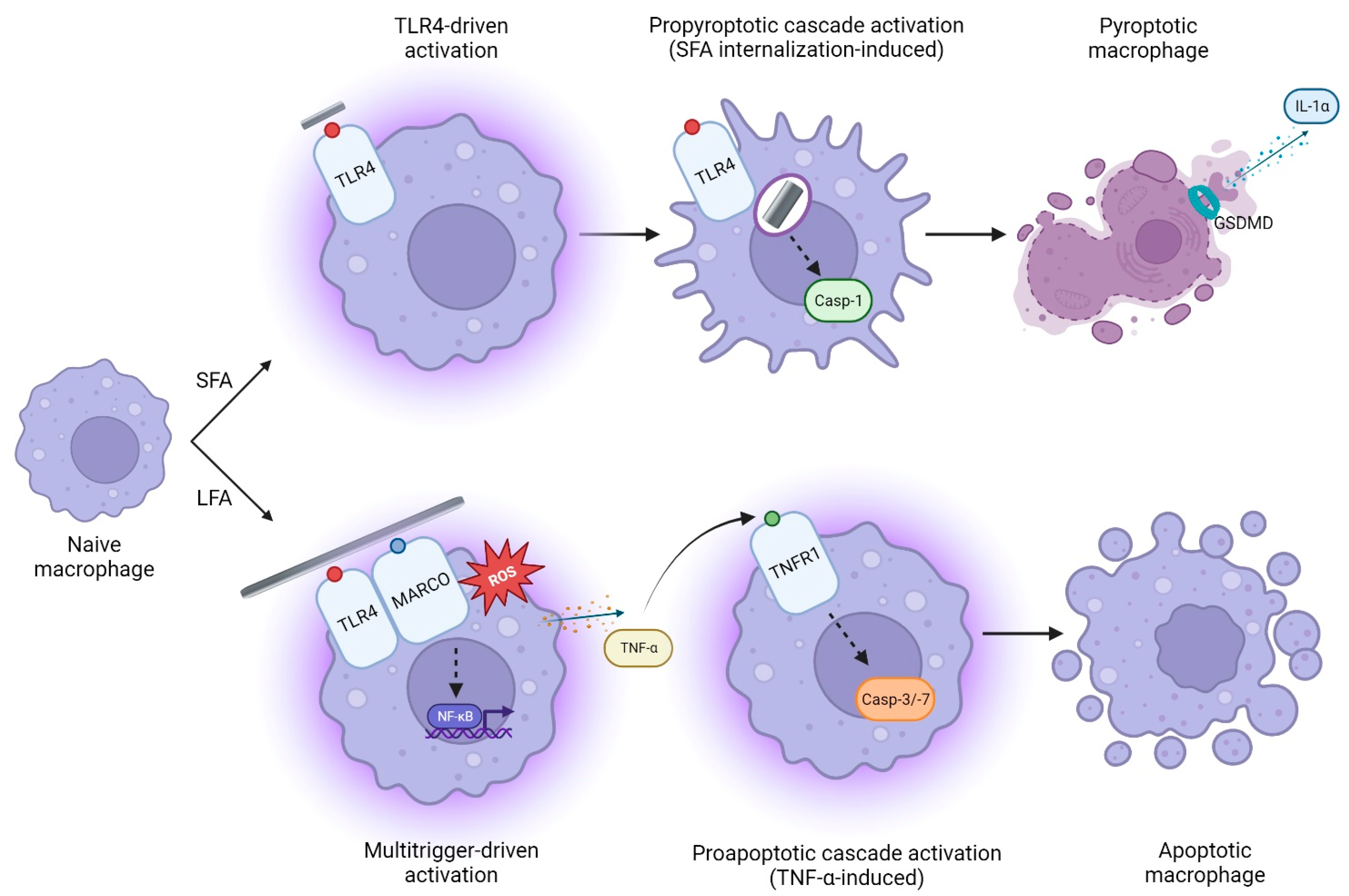
3. Discussion
4. Methods and Materials
4.1. Short (SFA) and Long (LFA) Fibers of Amosite Asbestos
4.2. Cell Culture and In Vitro Exposure
4.3. Peritoneal Macrophage Culture
4.4. In Vitro Cytotoxicity Assessment
4.5. Determination of Cytokines (IL-1α, TNF-α)
4.6. Cytological Analysis
4.7. Flow Cytometric Analysis of Caspases-3/-7 and Caspase-1 Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100 Pt C, 11–465. [Google Scholar]
- Bernstein, D.; Dunnigan, J.; Hesterberg, T.; Brown, R.; Velasco, J.A.; Barrera, R.; Hoskins, J.; Gibbs, A. Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Dell, L.; Adams, R.; Rose, T.; Van Orden, D. State-of-the-science assessment of non-asbestos amphibole exposure: Is there a cancer risk? Environ. Geochem. Health 2013, 35, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Case, B.W.; Abraham, J.L.; Meeker, G.; Pooley, F.D.; Pinkerton, K.E. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Craighead, J.E.; Abraham, J.L.; Churg, A.; Green, F.H.; Kleinerman, J.; Pratt, P.C.; Seemayer, T.A.; Vallyathan, V.; Weill, H. The pathology of asbestos-associated diseases of the lungs and pleural cavities: Diagnostic criteria and proposed grading schema. Report of the Pneumoconiosis Committee of the College of American Pathologists and the National Institute for Occupational Safety and Health. Arch. Pathol. Lab. Med. 1982, 106, 544–596. [Google Scholar]
- Baumann, F.; Ambrosi, J.P.; Carbone, M. Asbestos is not just asbestos: An unrecognised health hazard. Lancet Oncol. 2013, 14, 576–578. [Google Scholar] [CrossRef]
- Kuroda, A. Recent progress and perspectives on the mechanisms underlying Asbestos toxicity. Genes Environ. 2021, 43, 46. [Google Scholar] [CrossRef]
- Toyokuni, S. Commentary on “Mechanisms of asbestos-induced carcinogenesis” published in 2009. Nagoya J. Med. Sci. 2023, 85, 13–15. [Google Scholar] [CrossRef]
- Barlow, C.A.; Grespin, M.; Best, E.A. Asbestos fiber length and its relation to disease risk. Inhal. Toxicol. 2017, 29, 541–554. [Google Scholar] [CrossRef]
- Bernstein, D.M. The health effects of short fiber chrysotile and amphibole asbestos. Crit. Rev. Toxicol. 2022, 52, 89–112. [Google Scholar] [CrossRef]
- Boulanger, G.; Andujar, P.; Pairon, J.C.; Billon-Galland, M.A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibers to assess asbestos exposure: A review of fiber size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, A.; Donaldson, K. Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo; frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part. Fibre Toxicol. 2012, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wu, L.J.; Santella, R.M.; Hei, T.K. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999, 59, 5922–5926. [Google Scholar]
- Blake, D.J.; Bolin, C.M.; Cox, D.P.; Cardozo-Pelaez, F.; Pfau, J.C. Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxicol. Sci. 2007, 99, 277–288. [Google Scholar] [CrossRef]
- Dodson, R.F.; Atkinson, M.A.; Levin, J.L. Asbestos fiber length as related to potential pathogenicity: A critical review. Am. J. Ind. Med. 2003, 44, 291–297. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yuen, S.R.; Ashley, R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: Pathological evidence. Int. J. Hyg. Environ. Health 2005, 208, 201–210. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Maurer, J.K.; Higgins, J.; Poynter, J. Alveolar macrophage cytokine and growth factor production in a rat model of crocidolite-induced pulmonary inflammation and fibrosis. J. Toxicol. Environ. Health 1995, 46, 155–169. [Google Scholar] [CrossRef]
- Acencio, M.M.; Soares, B.; Marchi, E.; Silva, C.S.; Teixeira, L.R.; Broaddus, V.C. Inflammatory Cytokines Contribute to Asbestos-Induced Injury of Mesothelial Cells. Lung 2015, 193, 831–837. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.A.; Hamilton, R.F., Jr.; Holian, A. Role of scavenger receptor a family in lung inflammation from exposure to environmental particles. J. Immunotoxicol. 2008, 5, 151–157. [Google Scholar] [CrossRef]
- Murthy, S.; Larson-Casey, J.L.; Ryan, A.J.; He, C.; Kobzik, L.; Carter, A.B. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 2015, 29, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef]
- Gross, O.; Yazdi, A.S.; Thomas, C.J.; Masin, M.; Heinz, L.X.; Guarda, G.; Quadroni, M.; Drexler, S.K.; Tschopp, J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012, 36, 388–400. [Google Scholar] [CrossRef]
- Chan, J.Y.W.; Tsui, J.C.C.; Law, P.T.W.; So, W.K.W.; Leung, D.Y.P.; Sham, M.M.K.; Tsui, S.K.W.; Chan, C.W.H. Regulation of TLR4 in silica-induced inflammation: An underlying mechanism of silicosis. Int. J. Med. Sci. 2018, 15, 986–991. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Bendtsen, K.M.; Knudsen, K.B.; Poulsen, S.S.; Stoeger, T.; Vogel, U. Nanomaterial- and shape-dependency of TLR2 and TLR4 mediated signaling following pulmonary exposure to carbonaceous nanomaterials in mice. Part. Fibre Toxicol. 2021, 18, 40. [Google Scholar] [CrossRef]
- Tomatis, M.; Turci, F.; Ceschino, R.; Riganti, C.; Gazzano, E.; Martra, G.; Ghigo, D.; Fubini, B. High aspect ratio materials: Role of surface chemistry vs. length in the historical “long and short amosite asbestos fibers”. Inhal. Toxicol. 2010, 22, 984–998. [Google Scholar] [CrossRef]
- Boyles, M.S.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.; Proudfoot, L.; Windle, A.H.; et al. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. In Vitro 2015, 29, 1513–1528. [Google Scholar] [CrossRef]
- Riganti, C.; Aldieri, E.; Bergandi, L.; Tomatis, M.; Fenoglio, I.; Costamagna, C.; Fubini, B.; Bosia, A.; Ghigo, D. Long and short fiber amosite asbestos alters at a different extent the redox metabolism in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2003, 193, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, A.; Murphy, F.A.; Prina-Mello, A.; Poland, C.A.; Byrne, F.; Movia, D.; Glass, J.R.; Dickerson, J.C.; Schultz, D.A.; Jeffree, C.E.; et al. The threshold length for fiber-induced acute pleural inflammation: Shedding light on the early events in asbestos-induced mesothelioma. Toxicol. Sci. 2012, 128, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Hosojima, S.; Hara, H.; Kushiyama, H.; Mahib, M.R.; Kinoshita, T.; Suda, T. Gasdermin D mediates the maturation and release of IL-1alpha downstream of inflammasomes. Cell Rep. 2021, 34, 108887. [Google Scholar] [CrossRef] [PubMed]
- Leinardi, R.; Pochet, A.; Uwambayinema, F.; Yakoub, Y.; Quesniaux, V.; Ryffel, B.; Broz, P.; Pavan, C.; Huaux, F. Gasdermin D membrane pores orchestrate IL-1alpha secretion from necrotic macrophages after NFS-rich silica exposure. Arch. Toxicol. 2023, 97, 1001–1015. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Rabolli, V.; Wallemme, L.; Lo Re, S.; Uwambayinema, F.; Palmai-Pallag, M.; Thomassen, L.; Tyteca, D.; Octave, J.N.; Marbaix, E.; Lison, D.; et al. Critical role of aquaporins in interleukin 1beta (IL-1beta)-induced inflammation. J. Biol. Chem. 2014, 289, 13937–13947. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Rabolli, V.; Badissi, A.A.; Devosse, R.; Uwambayinema, F.; Yakoub, Y.; Palmai-Pallag, M.; Lebrun, A.; De Gussem, V.; Couillin, I.; Ryffel, B.; et al. The alarmin IL-1alpha is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part. Fibre Toxicol. 2014, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.; Ghio, A.J.; Dailey, L.A.; Bern, A.M.; Gibbs-Flournoy, E.A.; Padilla-Carlin, D.J.; Roggli, V.L.; Devlin, R.B. Effect of size fractionation on the toxicity of amosite and Libby amphibole asbestos. Toxicol. Sci. 2010, 118, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, J.; Valimaki, E.; Sund, J.; Vippola, M.; Clausen, P.A.; Jensen, K.A.; Savolainen, K.; Matikainen, S.; Alenius, H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 2011, 5, 6861–6870. [Google Scholar] [CrossRef] [PubMed]
- Hillegass, J.M.; Miller, J.M.; MacPherson, M.B.; Westbom, C.M.; Sayan, M.; Thompson, J.K.; Macura, S.L.; Perkins, T.N.; Beuschel, S.L.; Alexeeva, V.; et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part. Fibre Toxicol. 2013, 10, 39. [Google Scholar] [CrossRef]
- Militello, G.M.; Gaggero, L.; La Maestra, S. Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects. Minerals 2021, 11, 525. [Google Scholar] [CrossRef]
- Scimeca, M.; Pietroiusti, A.; Milano, F.; Anemona, L.; Orlandi, A.; Marsella, L.T.; Bonanno, E. Elemental analysis of histological specimens: A method to unmask nano asbestos fibers. Eur. J. Histochem. 2016, 60, 2573. [Google Scholar] [CrossRef]
- Nishimura, T.; Ishida, T.; Funabashi, H.; Hirota, R.; Ikeda, T.; Kuroda, A. Detection of fine asbestos fibers using fluorescently labeled asbestos-binding proteins in talc. J. Hazard. Mater. Adv. 2023, 12, 100332. [Google Scholar] [CrossRef]
- Gamble, J.F.; Gibbs, G.W. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul. Toxicol. Pharmacol. 2008, 52, S154–S186. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Bondarenko, O.; Kohonen, P.; Andon, F.T.; Brzicova, T.; Gessner, I.; Mathur, S.; Bottini, M.; Calligari, P.; Stella, L.; et al. Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors. Sci. Rep. 2018, 8, 1115. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, M.S.; Palecanda, A.; Koziel, H.; Huang, Y.C.; Imrich, A.; Sulahian, T.H.; Ning, Y.Y.; Yang, Z.; Pikkarainen, T.; Sankala, M.; et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J. Immunol. 2005, 175, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Bauer, A.K.; Arredouani, M.; Soininen, R.; Tryggvason, K.; Kleeberger, S.R.; Kobzik, L. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J. Clin. Investig. 2007, 117, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Macrophage receptor with collagenous structure (MARCO) is a dynamic adhesive molecule that enhances uptake of carbon nanotubes by CHO-K1 cells. Toxicol. Appl. Pharmacol. 2012, 259, 96–103. [Google Scholar] [CrossRef]
- Benedetti, S.; Nuvoli, B.; Catalani, S.; Galati, R. Reactive oxygen species a double-edged sword for mesothelioma. Oncotarget 2015, 6, 16848–16865. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A., Jr.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Iyer, L.L.; Holian, A. Asbestos induces apoptosis in human alveolar macrophages. Am. J. Physiol. 1996, 271, L813–L819. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, T.; Feng, C.; Zeng, Y.; Zhang, X.; Dong, F.; Deng, J. Chrysotile asbestos induces apoptosis via activation of the p53-regulated mitochondrial pathway mediated by ROS in A549 cells. Appl. Clay Sci. 2019, 182, 105245. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Rothe, M.; Hu, Y.F.; Goeddel, D.V. Tumor necrosis factor’s cytotoxic activity is signaled by the p55 TNF receptor. Cell 1993, 73, 213–216. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Tephly, L.A.; Carter, A.B. Asbestos-induced MKP-3 expression augments TNF-alpha gene expression in human monocytes. Am. J. Respir. Cell Mol. Biol. 2008, 39, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F. Journey to the centre of the lung. The perspective of a mineralogist on the carcinogenic effects of mineral fibres in the lungs. J. Hazard. Mater. 2023, 442, 130077. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Addison, J.; Bolton, R.E.; Donaldson, K.; Jones, A.D.; Smith, T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 1986, 67, 415–430. [Google Scholar] [PubMed]
- International Programme on Chemical Safety & World Health Organization. Asbestos and Other Natural Mineral Fibres; World Health Organization: Geneva, Switzerland, 1986.
- Graham, A.; Higinbotham, J.; Allan, D.; Donaldson, K.; Beswick, P.H. Chemical differences between long and short amosite asbestos: Differences in oxidation state and coordination sites of iron, detected by infrared spectroscopy. Occup. Environ. Med. 1999, 56, 606–611. [Google Scholar] [CrossRef]
- Goodman, J.E.; Becich, M.J.; Bernstein, D.M.; Case, B.W.; Mandel, J.H.; Nel, A.E.; Nolan, R.; Odo, N.U.; Smith, S.R.; Taioli, E.; et al. Non-asbestiform elongate mineral particles and mesothelioma risk: Human and experimental evidence. Environ. Res. 2022, 230, 114578. [Google Scholar] [CrossRef] [PubMed]
- Scarfi, S.; Magnone, M.; Ferraris, C.; Pozzolini, M.; Benvenuto, F.; Benatti, U.; Giovine, M. Ascorbic acid pre-treated quartz stimulates TNF-alpha release in RAW 264.7 murine macrophages through ROS production and membrane lipid peroxidation. Respir. Res. 2009, 10, 25. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Beamer, G.L.; Seaver, B.P.; Jessop, F.; Shepherd, D.M.; Beamer, C.A. Acute Exposure to Crystalline Silica Reduces Macrophage Activation in Response to Bacterial Lipoproteins. Front. Immunol. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Heilig, R.; Dick, M.S.; Sborgi, L.; Meunier, E.; Hiller, S.; Broz, P. The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. Eur. J. Immunol. 2018, 48, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Huot-Marchand, S.; Nascimento, M.; Culerier, E.; Bourenane, M.; Savigny, F.; Panek, C.; Serdjebi, C.; Le Bert, M.; Quesniaux, V.F.J.; Ryffel, B.; et al. Cigarette smoke-induced gasdermin D activation in bronchoalveolar macrophages and bronchial epithelial cells dependently on NLRP3. Front. Immunol. 2022, 13, 918507. [Google Scholar] [CrossRef] [PubMed]
- Arras, M.; Louahed, J.; Simoen, V.; Barbarin, V.; Misson, P.; van den Brule, S.; Delos, M.; Knoops, L.; Renauld, J.C.; Lison, D.; et al. B lymphocytes are critical for lung fibrosis control and prostaglandin E2 regulation in IL-9 transgenic mice. Am. J. Respir. Cell Mol. Biol. 2006, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Trans-plasma membrane electron transport: A cellular assay for NADH- and NADPH-oxidase based on extracellular, superoxide-mediated reduction of the sulfonated tetrazolium salt WST-1. Protoplasma 1998, 205, 74–82. [Google Scholar] [CrossRef]
- Lison, D.; Thomassen, L.C.; Rabolli, V.; Gonzalez, L.; Napierska, D.; Seo, J.W.; Kirsch-Volders, M.; Hoet, P.; Kirschhock, C.E.; Martens, J.A. Nominal and effective dosimetry of silica nanoparticles in cytotoxicity assays. Toxicol. Sci. 2008, 104, 155–162. [Google Scholar] [CrossRef]
- Benmerzoug, S.; Rose, S.; Bounab, B.; Gosset, D.; Duneau, L.; Chenuet, P.; Mollet, L.; Le Bert, M.; Lambers, C.; Geleff, S.; et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018, 9, 5226. [Google Scholar] [CrossRef]
- Koh, C.M. Preparation of cells for microscopy using cytospin. Methods Enzymol. 2013, 533, 235–240. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef]


| SFA | LFA | ||
|---|---|---|---|
| Particle size distribution + (quartiles: 25/50/75%) (μm) | P25 | 0.5 | 3.0 |
| P50 | 1.0 | 5.0 | |
| P75 | 1.5 | 9.0 | |
| Specific surface area * (m2/g) | 11 | 6 | |
| Surface Fe/Si ° (at. %) | 0.91 ± 0.03 | 0.89 ± 0.03 | |
| Average surface oxidation state # | SFA > LFA | ||
| Surface oxidation state—Binding energy $ (eV) | 56.6 | 55.8 | |
| Leachable Fe at 24 h (Fe3+ + Fe2+, ng Fe/mg amosite) § | Fetot = 20.4 ± 0.8 | Fetot = 13.6 ± 1.3 | |
| Fe3+ = 16.6 ± 0.6 | Fe3+ = 7 ± 1.2 | ||
| Fe2+ = 3.8 ± 0.2 | Fe2+ = 6.6 ± 0.2 | ||
| Particle-derived free radicals £ | Moderate | Very high | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leinardi, R.; Petriglieri, J.R.; Pochet, A.; Yakoub, Y.; Lelong, M.; Lescoat, A.; Turci, F.; Lecureur, V.; Huaux, F. Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages. Int. J. Mol. Sci. 2023, 24, 15145. https://doi.org/10.3390/ijms242015145
Leinardi R, Petriglieri JR, Pochet A, Yakoub Y, Lelong M, Lescoat A, Turci F, Lecureur V, Huaux F. Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages. International Journal of Molecular Sciences. 2023; 24(20):15145. https://doi.org/10.3390/ijms242015145
Chicago/Turabian StyleLeinardi, Riccardo, Jasmine Rita Petriglieri, Amandine Pochet, Yousof Yakoub, Marie Lelong, Alain Lescoat, Francesco Turci, Valérie Lecureur, and François Huaux. 2023. "Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages" International Journal of Molecular Sciences 24, no. 20: 15145. https://doi.org/10.3390/ijms242015145
APA StyleLeinardi, R., Petriglieri, J. R., Pochet, A., Yakoub, Y., Lelong, M., Lescoat, A., Turci, F., Lecureur, V., & Huaux, F. (2023). Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages. International Journal of Molecular Sciences, 24(20), 15145. https://doi.org/10.3390/ijms242015145








