The Effects of Probiotic Bacillus Spores on Dexamethasone-Treated Rats
Abstract
:1. Introduction
2. Results
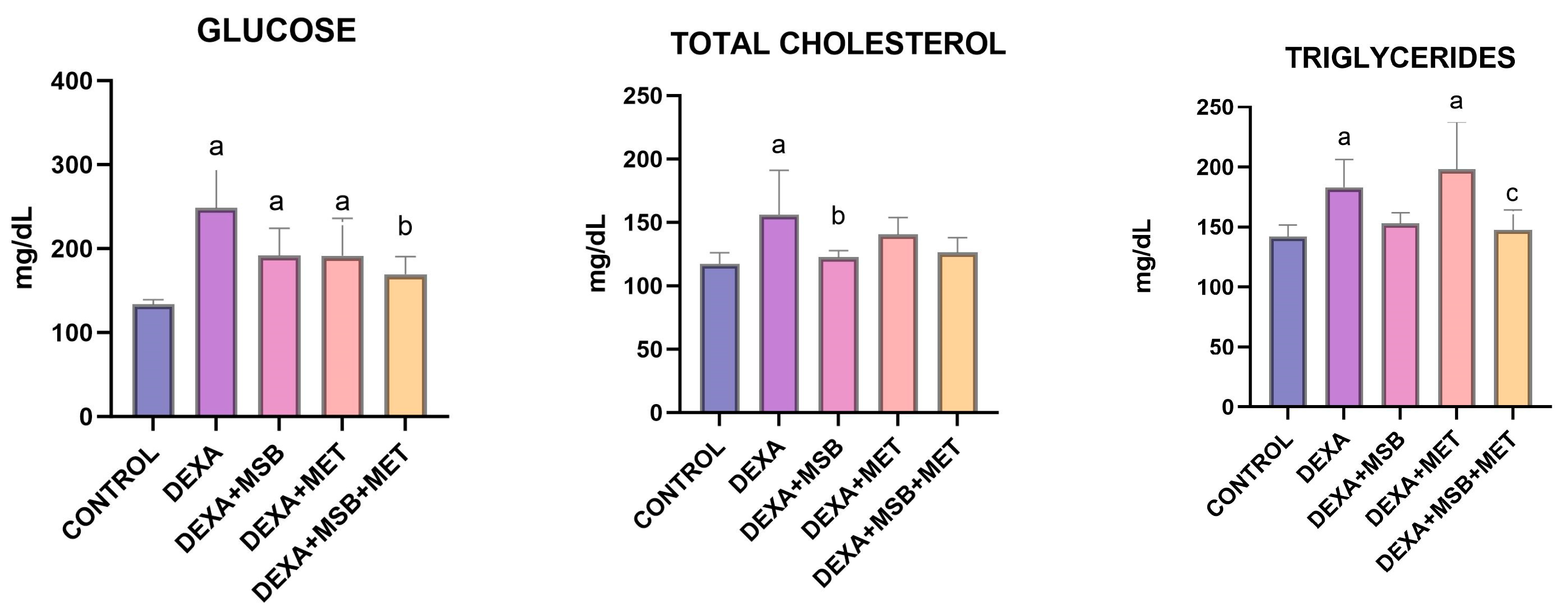
2.1. Biochemical Results
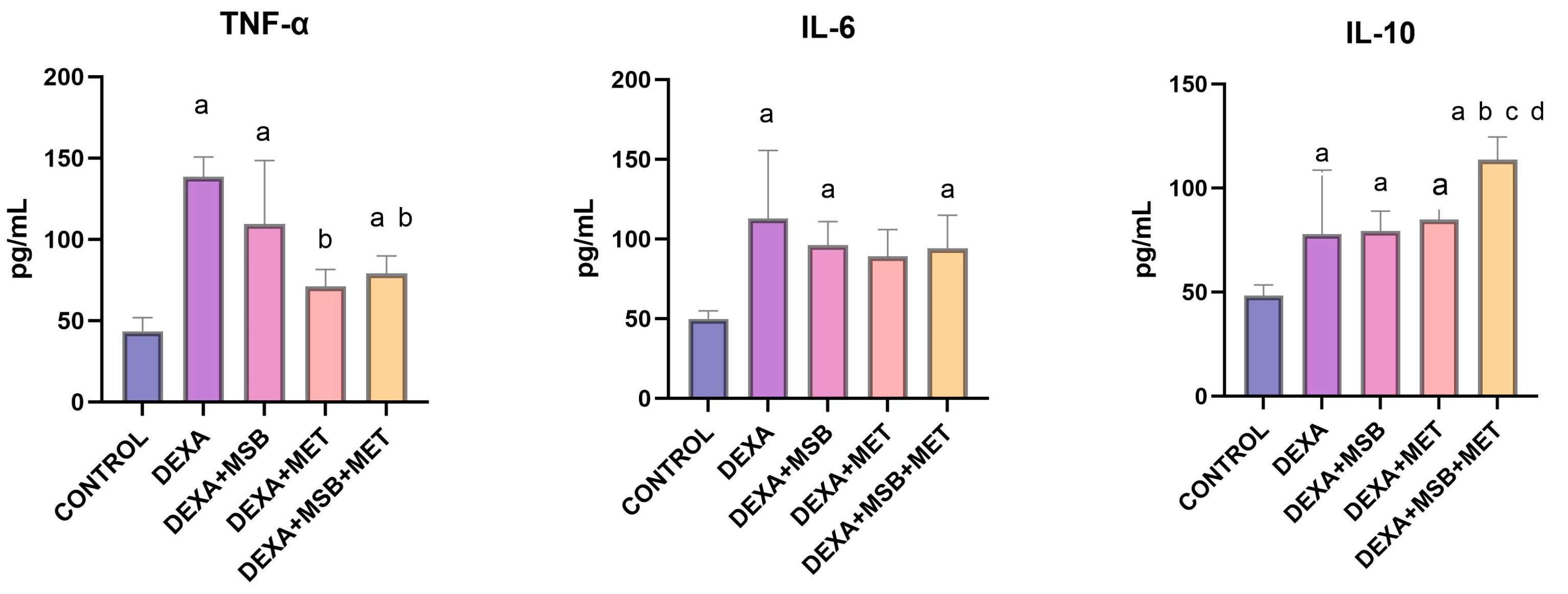
2.2. TNF-α, IL-6 and IL-10 Levels
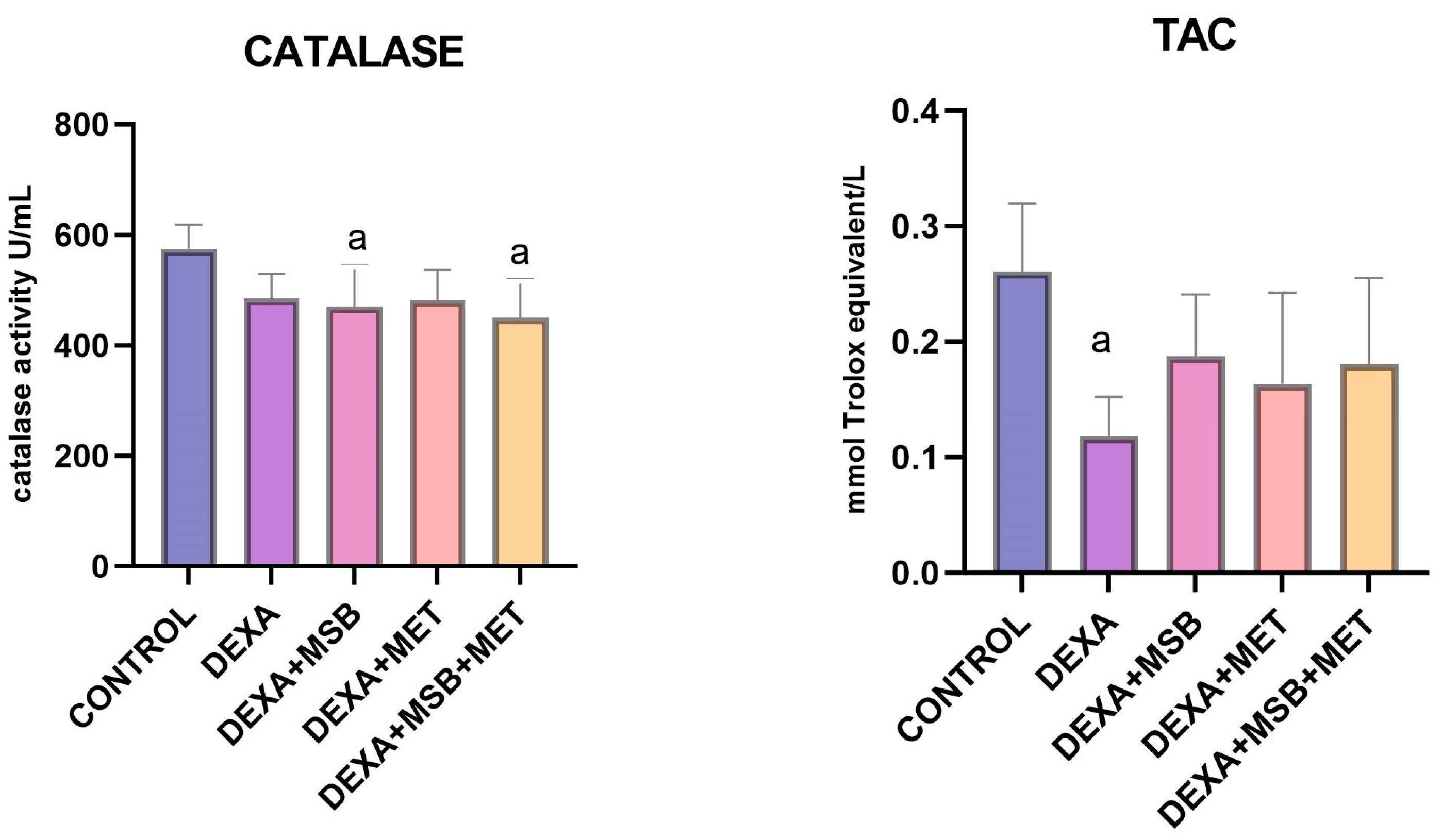
2.3. TAC and Catalase
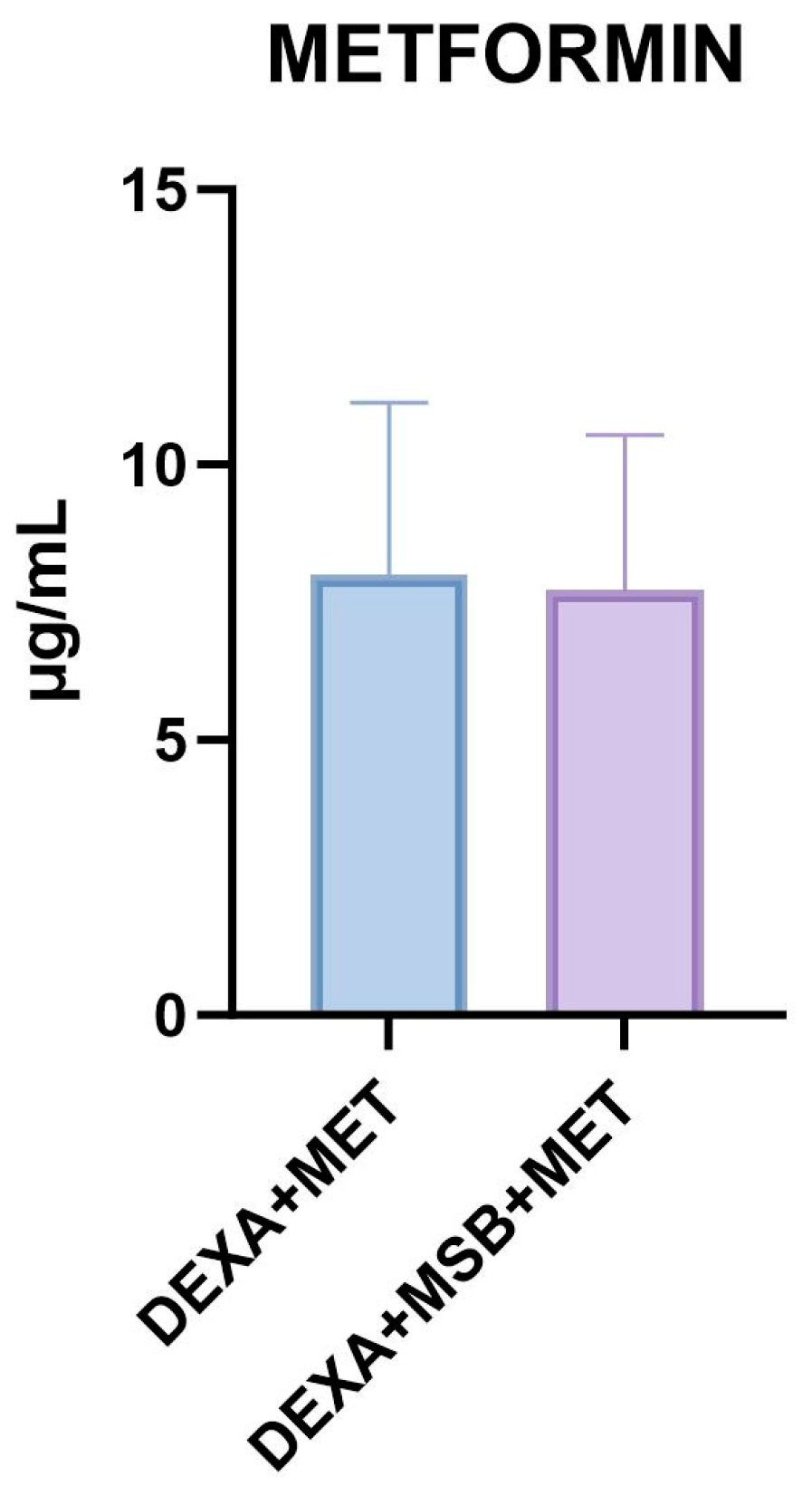
2.4. Metformin
2.5. Histopathology
3. Discussion
4. Materials and Methods
4.1. Agents and Chemicals
4.2. Animals
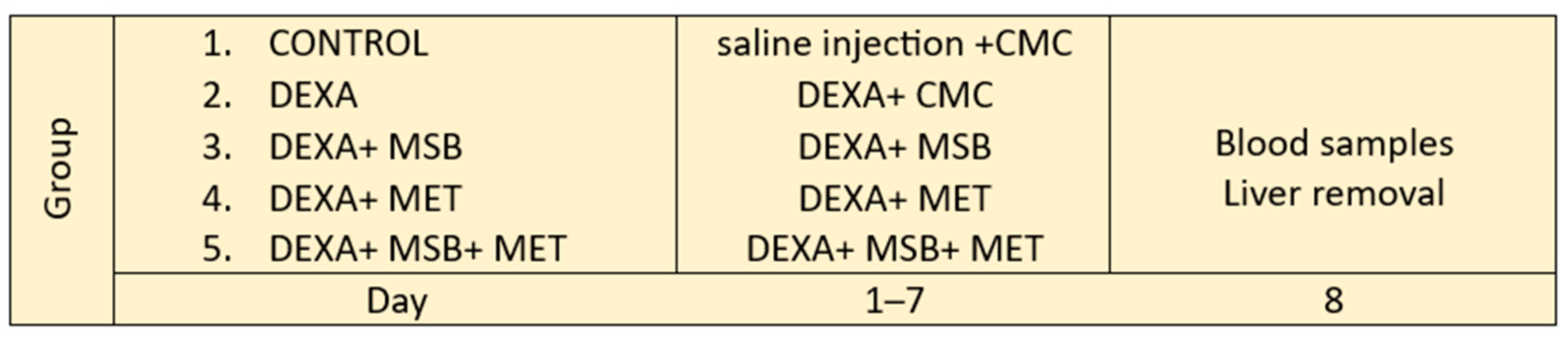
4.3. Experimental Design
4.4. Evaluation of Inflammatory and Biochemical Markers
4.5. Assessment of Oxidative Stress
4.6. Metformin Concentration
4.6.1. Chromatography Apparatus and Conditions
4.6.2. Sample Preparation
4.7. Histological Assessment
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Hao, L.; Zhu, S.; Yang, X.; Shi, W.; Zheng, K.; Wang, T.; Chen, H. Potential Adverse Effects of Dexamethasone Therapy on COVID-19 Patients: Review and Recommendations. Infect. Dis. Ther. 2021, 10, 1907–1931. [Google Scholar] [CrossRef]
- Li, J.-X.; Cummins, C.L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, W.; Xu, S.; Lou, J.; Wang, S.; Men, X. GSK-3β mediates dexamethasone-induced pancreatic β cell apoptosis. Life Sci. 2016, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, L.; Jiang, J.; Ni, Y.; Zhu, J.; Zheng, X.; Wang, Q.; Lu, X.; Fu, Z. Chronic glucocorticoid treatment induced circadian clock disorder leads to lipid metabolism and gut microbiota alterations in rats. Life Sci. 2018, 192, 173–182. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Charlton, J.; Latinovic, R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care 2006, 29, 2728–2729. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Liang, T.; Wu, L.; Xi, Y.; Li, Y.; Xie, X.; Fan, C.; Yang, L.; Yang, S.; Chen, X.; Zhang, J.; et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 1670–1688. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2020, 60, 670–683. [Google Scholar] [CrossRef]
- Bader, J.; Albin, A.; Stahl, U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Marzorati, M.; Abbeele, P.V.D.; Bubeck, S.; Bayne, T.; Krishnan, K.; Young, A. Treatment with a spore-based probiotic containing five strains of Bacillus induced changes in the metabolic activity and community composition of the gut microbiota in a SHIME® model of the human gastrointestinal system. Food Res. Int. 2021, 149, 110676. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Henning, A.L.; Bowman, E.M.; Gary, M.A.; Carbajal, K.M. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kelly, S.; Ajodha, S.; Sahdev, A.; Bestwick, J.P.; Gabrovska, P.; Akanle, O.; Ajjan, R.; Kola, B.; Stadler, M.; et al. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: A randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020, 8, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.P.; Hazlehurst, J.M.; Tomlinson, J.W. Glucocorticoids and non-alcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2015, 154, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Tzeng, C.; Chen, Y.; Chang, S.; Hsu, T.; Ho, W.; Kuo, Y.; Hung, P.; Chang, S. Improving insulin resistance with Antrodia cinnamomea mycelium powder to induce a hypoglycemic effect in dexamethasone-induced insulin-resistant rats. Mol. Med. Rep. 2017, 17, 3260–3266. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Ali, N.; Mostafa, I.; Hasan, R.A.; Sobeh, M. Coriander Oil Reverses Dexamethasone-Induced Insulin Resistance in Rats. Antioxidants 2022, 11, 441. [Google Scholar] [CrossRef]
- El-Sonbaty, Y.A.; Suddek, G.M.; Megahed, N.; Gameil, N.M. Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie 2019, 167, 119–134. [Google Scholar] [CrossRef]
- Buzatto, A.Z.; Malkawi, A.; Sabi, E.M.; Mujamammi, A.H.; Li, L.; Rahman, A.M.A. Tissue Lipidomic Alterations Induced by Prolonged Dexamethasone Treatment. J. Proteome Res. 2021, 20, 1558–1570. [Google Scholar] [CrossRef]
- Dzinyela, R.; Asomaning, E.K.; Abdul-Baasit, A.-N.; Alhassan, A.R.; Movahedi, A. An in vivo Evaluation of Antihyperlipidaemic Activity of Ethanolic Extract of Amaranthus spinosus Leaves on Dexamethasone Induced Hyperlipidaemic Rats. Biochem. Mol. Biol. 2021, 6, 25–34. [Google Scholar] [CrossRef]
- Croci, S.; D’apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary strategies for management of metabolic syndrome: Role of gut microbiota metabolites. Nutrients 2021, 13, 1389. [Google Scholar] [CrossRef]
- Memarrast, F.; Ghafouri-Fard, S.; Kolivand, S.; Jafary-Nodooshan, S.; Neyazi, N.; Sadroddiny, E.; Motevaseli, E. Comparative evaluation of probiotics effects on plasma glucose, lipid, and insulin levels in streptozotocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2017, 33, e2912. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jing, Y.; Zhou, X.; Zhang, N.; Tai, J.; Cao, Y. Bacillus licheniformis Zhengchangsheng® Inhibits Obesity by Regulating the AMP-Activated Protein Kinase Signaling Pathway. Probiotics Antimicrob. Proteins 2021, 13, 1658–1667. [Google Scholar] [CrossRef]
- Aminlari, L.; Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of Probiotics Bacillus coagulans and Lactobacillus plantarum on Lipid Profile and Feces Bacteria of Rats Fed Cholesterol-Enriched Diet. Probiotics Antimicrob. Proteins 2018, 11, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Tovchiga, O.V. The influence of goutweed (Aegopodium podagraria L.) tincture and metformin on the carbohydrate and lipid metabolism in dexamethasone-treated rats. BMC Complement. Altern. Med. 2016, 16, 235. [Google Scholar] [CrossRef]
- Wulffele, M.G.; Kooy, A.; Zeeuw, D.; Stehouwer, C.D.A.; Gansevoort, R.T. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J. Intern. Med. 2004, 256, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Migliorati, G.; Bruscoli, S.; Riccardi, C. Defining the role of glucocorticoids in inflammation. Clin. Sci. 2018, 132, 1529–1543. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in TNF-α- and Obesity-Induced Insulin Resistance. Science 1996, 271, 665–670. [Google Scholar] [CrossRef]
- Stephens, J.; Pekala, P. Transcriptional repression of the C/EBP-α and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-α. Regulations is coordinate and independent of protein synthesis. J. Biol. Chem. 1992, 267, 13580–13584. [Google Scholar] [CrossRef]
- Piya, M.K.; McTernan, P.G.; Kumar, S. Adipokine inflammation and insulin resistance: The role of glucose, lipids and endotoxin. J. Endocrinol. 2013, 216, T1–T15. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Shen, X.; Wen, N.; Zhang, Y. Crocetin Prevents Dexamethasone-Induced Insulin Resistance in Rats. Planta Medica 2005, 71, 917–922. [Google Scholar] [CrossRef]
- Araujo, J.E.d.S.; Miguel-Dos-Santos, R.; Macedo, F.N.; Cunha, P.S.; Fontes, M.T.; Murata, G.M.; Lauton-Santos, S.; Santana-Filho, V.J.; Silva, A.M.d.O.; Antoniolli, A.R.; et al. Effects of high doses of glucocorticoids on insulin-mediated vasodilation in the mesenteric artery of rats. PLoS ONE 2020, 15, e0230514. [Google Scholar] [CrossRef]
- Han, M.; Liao, W.; Dong, Y.; Bai, C.; Gai, Z. Lacticaseibacillus rhamnosus Hao9 exerts antidiabetic effects by regulating gut microbiome, glucagon metabolism, and insulin levels in type 2 diabetic mice. Front. Nutr. 2023, 9, 1081778. [Google Scholar] [CrossRef] [PubMed]
- Catinean, A.; Neag, M.A.; Krishnan, K.; Muntean, D.M.; Bocsan, C.I.; Pop, R.M.; Mitre, A.O.; Melincovici, C.S.; Buzoianu, A.D. Probiotic bacillus spores together with amino acids and immunoglobulins exert protective effects on a rat model of ulcerative colitis. Nutrients 2020, 12, 3607. [Google Scholar] [CrossRef] [PubMed]
- Neag, M.A.; Catinean, A.; Muntean, D.M.; Pop, M.R.; Bocsan, C.I.; Botan, E.C.; Buzoianu, A.D. Probiotic bacillus spores protect against acetaminophen induced acute liver injury in rats. Nutrients 2020, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Abad-Jimenez, Z.; Lopez-Domenech, S.; Diaz-Rua, R.; Iannantuoni, F.; Gomez-Abril, S.A.; Perianez-Gomez, D.; Morillas, C.; Victor, V.M.; Rocha, M. Systemic oxidative stress and visceral adipose tissue mediators of NLRP3 inflammasome and autophagy are reduced in obese type 2 diabetic patients treated with metformin. Antioxidants 2020, 9, 892. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Franchimont, D.; Louis, E.; Dewe, W.; Martens, H.; Vrindts-Gevaert, Y.; De Groote, D.; Belaiche, J.; Geenen, V. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul. Pept. 1998, 73, 59–65. [Google Scholar] [CrossRef]
- Franchimont, D.; Martens, H.; Hagelstein, M.T.; Louis, E.; Dewe, W.; Chrousos, G.P.; Belaiche, J.; Geenen, V. Tumor Necrosis Factor Decreases, and Interleukin-10 Increases, the Sensitivity of Human Monocytes to Dexamethasone: Potential Regulation of the Glucocorticoid Receptor. J. Clin. Endocrinol. Metab. 1999, 84, 2834–2839. Available online: https://academic.oup.com/jcem/article/84/8/2834/2864429 (accessed on 15 September 2023).
- Mozo, L.; Suarez, A.; Gutierrez, C. Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin. Exp. Allergy 2004, 34, 406–412. [Google Scholar] [CrossRef]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Cota, S.d.J.; Aguilar-Medina, E.M.; Ramos-Payán, R.; Maldonado, J.G.R.; Romero-Quintana, J.G.; Montes-Avila, J.; Sarmiento-Sánchez, J.I.; Plazas-Guerrero, C.G.; Vergara-Jiménez, M.J.; Sánchez-López, A.; et al. Therapeutic effect of treatment with metformin and/or 4-hydroxychalcone in male Wistar rats with nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 863, 172699. [Google Scholar] [CrossRef]
- Brandt, A.; Hernández-Arriaga, A.; Kehm, R.; Sánchez, V.; Jin, C.J.; Nier, A.; Baumann, A.; Camarinha-Silva, A.; Bergheim, I. Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci. Rep. 2019, 9, 6668. [Google Scholar] [CrossRef]
- Dutta, R.K.; Lee, J.N.; Maharjan, Y.; Park, C.; Choe, S.-K.; Ho, Y.-S.; Park, R. Catalase deficiency facilitates the shuttling of free fatty acid to brown adipose tissue through lipolysis mediated by ROS during sustained fasting. Cell Biosci. 2021, 11, 201. [Google Scholar] [CrossRef]
- Zerin, M.; Karakilçik, A.Z.; Bitiren, M.; Musa, D.; Özgönül, A.; Selek, Ş.; Nazligül, Y.; Uzunköy, A. Vitamin C modulates oxidative stress-induced colitis in rats. Turk. J. Med. Sci. 2010, 40, 871–879. [Google Scholar] [CrossRef]
- Ghaisas, M.; Navghare, V.; Takawale, A.; Zope, V.; Tanwar, M.; Deshpande, A. Effect of Tectona grandis Linn. on dexamethasone-induced insulin resistance in mice. J. Ethnopharmacol. 2009, 122, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Nguelefack-Mbuyo, E.P.; Peyembouo, F.P.; Fofié, C.K.; Nguelefack, T.B. Dose-dependent and time-dependent metabolic, hemodynamic, and redox disturbances in dexamethasone-treated Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 457–469. [Google Scholar] [CrossRef]
- Ghaisas, M.; Zope, V.; Takawale, A.; Navghare, V.; Tanwar, M.; Deshpande, A. Preventive effect of Sphaeranthus indicus during progression of glucocorticoid-induced insulin resistance in mice. Pharm. Biol. 2010, 48, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, M.; Ai, Q.; Lin, L.; Wu, K.; Deng, X.; Jing, Y.; Jia, M.; Wan, J.; Zhang, L. Involvement of catalase in the protective benefits of metformin in mice with oxidative liver injury. Chem. Interact. 2014, 216, 34–42. [Google Scholar] [CrossRef]
- Shen, W.-Y.; Fu, L.-L.; Li, W.-F.; Zhu, Y.-R. Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aquac. Res. 2010, 41, 1691–1698. [Google Scholar] [CrossRef]
- Hu, T.; Shuai, X.; Chen, J.; Wei, Y.; Zheng, R. Protective effect of a Potentilla anserine polysaccharide on oxidative damages in mice. Int. J. Biol. Macromol. 2009, 45, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef]
- Zheng, L.P.; Zou, T.; Ma, Y.J.; Wang, J.W.; Zhang, Y.Q. Antioxidant and DNA damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus cereus SZ1. Molecules 2016, 21, 174. [Google Scholar] [CrossRef]
- Kotowicz, N.; Bhardwaj, R.; Ferreira, W.; Hong, H.; Olender, A.; Ramirez, J.; Cutting, S. Safety and probiotic evaluation of two Bacillus strains producing antioxidant compounds. Benef. Microbes 2019, 10, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Elmaghraby, A.M.; Ali, N.; Mostafa, I.; El-Shazly, A.M.; Abdelfattah, M.A.; Sobeh, M. Black pepper oil (Piper nigrum L.) mitigates dexamethasone induced pancreatic damage via modulation of oxidative and nitrosative stress. Biomed. Pharmacother. 2022, 153, 113456. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox. Biol. 2020, 34, 101517. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M.; Tsai, R.; Lin, V.; Bookout, A.L.; Zhang, Y.; Magomedova, L.; Li, T.; Chan, J.F.; Budd, C.; et al. LXRβ is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in mice. J. Clin. Investig. 2011, 121, 431–441. [Google Scholar] [CrossRef]
- Xiang, L.; Jiao, Y.; Qian, Y.; Li, Y.; Mao, F.; Lu, Y. Comparison of hepatic gene expression profiles between three mouse models of Nonalcoholic Fatty Liver Disease. Genes Dis. 2022, 9, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.-S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.-K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Louis, H.; Le Moine, O.; Goldman, M.; Devière, J. Modulation of liver injury by interleukin-10. Acta Gastroenterol. Belg. 2003, 66, 7–14. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12812143 (accessed on 15 September 2023).
- Thompson, K.; Maltby, J.; Fallowfield, J.; McAulay, M.; Millward-Sadler, H.; Sheron, N. Interleukin-10 Expression and Function in Experimental Murine Liver Inflammation and Fibrosis. Hepatology 1998, 28, 1597–1606. [Google Scholar] [CrossRef]
- Bruno, C.M.; Valenti, M.; Bertino, G.; Ardiri, A.; Amoroso, A.; Consolo, M.; Mazzarino, C.M.; Neri, S. Relationship between circulating interleukin-10 and histological features in patients with chronic C hepatitis. Ann. Saudi Med. 2011, 31, 360–364. [Google Scholar] [CrossRef]
- Campbell, A.W.; Sinatra, D.; Zhang, Z.; Sinatra, S.T. Efficacy of Spore Forming Bacilli Supplementation in Patients with Mild to Moderate Elevation of Triglycerides: A 12 week, Randomized, Double-Blind, Placebo Controlled Trial. Integr. Med. 2020, 19, 22–27. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33041703 (accessed on 15 September 2023).
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
 ); large lipid droplets within the hepatocytes (
); large lipid droplets within the hepatocytes ( ); degenerated eosinophilic hepatocytes with pyknotic and hyperchromatic nuclei (
); degenerated eosinophilic hepatocytes with pyknotic and hyperchromatic nuclei ( ); loss of cellular borders; vascular congestion in the central vein (
); loss of cellular borders; vascular congestion in the central vein ( ); moderately dilated sinusoids with mild inflammatory infiltrate (polymorphonuclears neutrophils) within the sinusoids (
); moderately dilated sinusoids with mild inflammatory infiltrate (polymorphonuclears neutrophils) within the sinusoids ( ). (C): DEXA + MSB treated rat group; ((C1), 20×) and ((C2), 40×). Partial reduction in lipid accumulation with small perinuclear lipid droplets within the hepatocytes (
). (C): DEXA + MSB treated rat group; ((C1), 20×) and ((C2), 40×). Partial reduction in lipid accumulation with small perinuclear lipid droplets within the hepatocytes ( ); moderate improvement in hepatocytes structure; mild central vein ectasia (
); moderate improvement in hepatocytes structure; mild central vein ectasia ( ); sinusoidal stasis and dilatation with few inflammatory cells (
); sinusoidal stasis and dilatation with few inflammatory cells ( ). (D): DEXA + MET-treated rat group; ((D1), 20×); and ((D2), 40×). Important restoration of the hepatic architecture and hepatocyte structure, with a significant reduction of intra-hepatocyte lipid content (
). (D): DEXA + MET-treated rat group; ((D1), 20×); and ((D2), 40×). Important restoration of the hepatic architecture and hepatocyte structure, with a significant reduction of intra-hepatocyte lipid content ( ); congestion within the portal vessels (
); congestion within the portal vessels ( ) and sinusoids (
) and sinusoids ( ); mild central vein congestion and stasis (
); mild central vein congestion and stasis ( ). (E): DEXA + MSB + MET-treated rat group; ((E1), 20×); and ((E2), 40×). Poor improvement in hepatic lobular architecture and hepatocyte structure with still disorganized cords of cells and high intracellular lipid accumulation (intra-hepatocyte small (
). (E): DEXA + MSB + MET-treated rat group; ((E1), 20×); and ((E2), 40×). Poor improvement in hepatic lobular architecture and hepatocyte structure with still disorganized cords of cells and high intracellular lipid accumulation (intra-hepatocyte small ( ) and large lipid droplets) (
) and large lipid droplets) ( ); central vein congestion with intraluminal lymphocytes; (
); central vein congestion with intraluminal lymphocytes; ( ); moderate inflammatory infiltrate within the dilated sinusoids (
); moderate inflammatory infiltrate within the dilated sinusoids ( ). Abbreviations: H&E, hematoxylin, and eosin.
). Abbreviations: H&E, hematoxylin, and eosin.
 ); large lipid droplets within the hepatocytes (
); large lipid droplets within the hepatocytes ( ); degenerated eosinophilic hepatocytes with pyknotic and hyperchromatic nuclei (
); degenerated eosinophilic hepatocytes with pyknotic and hyperchromatic nuclei ( ); loss of cellular borders; vascular congestion in the central vein (
); loss of cellular borders; vascular congestion in the central vein ( ); moderately dilated sinusoids with mild inflammatory infiltrate (polymorphonuclears neutrophils) within the sinusoids (
); moderately dilated sinusoids with mild inflammatory infiltrate (polymorphonuclears neutrophils) within the sinusoids ( ). (C): DEXA + MSB treated rat group; ((C1), 20×) and ((C2), 40×). Partial reduction in lipid accumulation with small perinuclear lipid droplets within the hepatocytes (
). (C): DEXA + MSB treated rat group; ((C1), 20×) and ((C2), 40×). Partial reduction in lipid accumulation with small perinuclear lipid droplets within the hepatocytes ( ); moderate improvement in hepatocytes structure; mild central vein ectasia (
); moderate improvement in hepatocytes structure; mild central vein ectasia ( ); sinusoidal stasis and dilatation with few inflammatory cells (
); sinusoidal stasis and dilatation with few inflammatory cells ( ). (D): DEXA + MET-treated rat group; ((D1), 20×); and ((D2), 40×). Important restoration of the hepatic architecture and hepatocyte structure, with a significant reduction of intra-hepatocyte lipid content (
). (D): DEXA + MET-treated rat group; ((D1), 20×); and ((D2), 40×). Important restoration of the hepatic architecture and hepatocyte structure, with a significant reduction of intra-hepatocyte lipid content ( ); congestion within the portal vessels (
); congestion within the portal vessels ( ) and sinusoids (
) and sinusoids ( ); mild central vein congestion and stasis (
); mild central vein congestion and stasis ( ). (E): DEXA + MSB + MET-treated rat group; ((E1), 20×); and ((E2), 40×). Poor improvement in hepatic lobular architecture and hepatocyte structure with still disorganized cords of cells and high intracellular lipid accumulation (intra-hepatocyte small (
). (E): DEXA + MSB + MET-treated rat group; ((E1), 20×); and ((E2), 40×). Poor improvement in hepatic lobular architecture and hepatocyte structure with still disorganized cords of cells and high intracellular lipid accumulation (intra-hepatocyte small ( ) and large lipid droplets) (
) and large lipid droplets) ( ); central vein congestion with intraluminal lymphocytes; (
); central vein congestion with intraluminal lymphocytes; ( ); moderate inflammatory infiltrate within the dilated sinusoids (
); moderate inflammatory infiltrate within the dilated sinusoids ( ). Abbreviations: H&E, hematoxylin, and eosin.
). Abbreviations: H&E, hematoxylin, and eosin.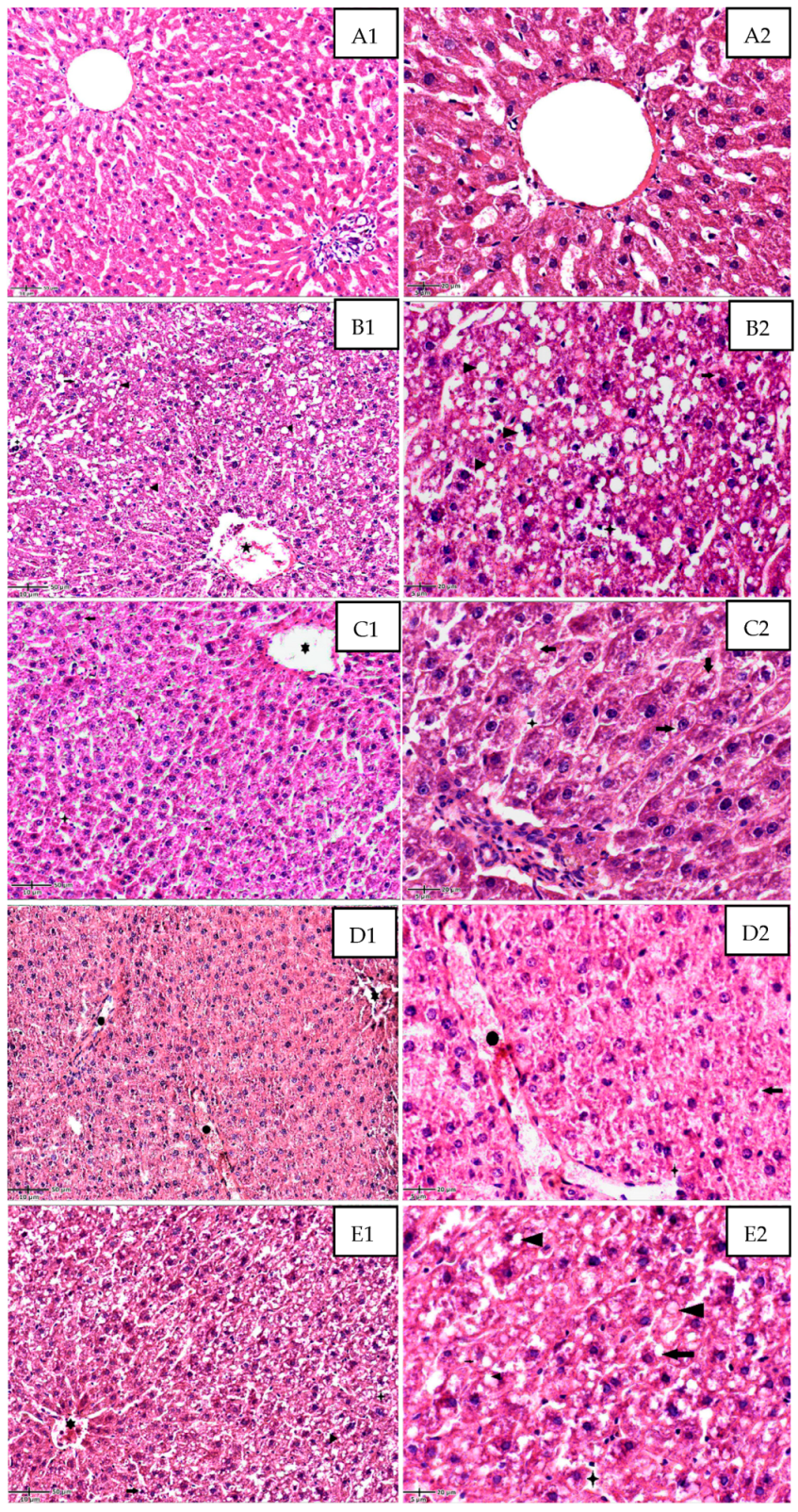
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inceu, A.I.; Neag, M.A.; Catinean, A.; Bocsan, C.I.; Craciun, C.I.; Melincovici, C.S.; Muntean, D.M.; Onofrei, M.M.; Pop, R.M.; Buzoianu, A.D. The Effects of Probiotic Bacillus Spores on Dexamethasone-Treated Rats. Int. J. Mol. Sci. 2023, 24, 15111. https://doi.org/10.3390/ijms242015111
Inceu AI, Neag MA, Catinean A, Bocsan CI, Craciun CI, Melincovici CS, Muntean DM, Onofrei MM, Pop RM, Buzoianu AD. The Effects of Probiotic Bacillus Spores on Dexamethasone-Treated Rats. International Journal of Molecular Sciences. 2023; 24(20):15111. https://doi.org/10.3390/ijms242015111
Chicago/Turabian StyleInceu, Andreea Ioana, Maria Adriana Neag, Adrian Catinean, Corina Ioana Bocsan, Cristian Ioan Craciun, Carmen Stanca Melincovici, Dana Maria Muntean, Mădălin Mihai Onofrei, Raluca Maria Pop, and Anca Dana Buzoianu. 2023. "The Effects of Probiotic Bacillus Spores on Dexamethasone-Treated Rats" International Journal of Molecular Sciences 24, no. 20: 15111. https://doi.org/10.3390/ijms242015111
APA StyleInceu, A. I., Neag, M. A., Catinean, A., Bocsan, C. I., Craciun, C. I., Melincovici, C. S., Muntean, D. M., Onofrei, M. M., Pop, R. M., & Buzoianu, A. D. (2023). The Effects of Probiotic Bacillus Spores on Dexamethasone-Treated Rats. International Journal of Molecular Sciences, 24(20), 15111. https://doi.org/10.3390/ijms242015111







